Current Trends in the Treatment of Cervical Pregnancy: A Narrative Review
Abstract
1. Introduction
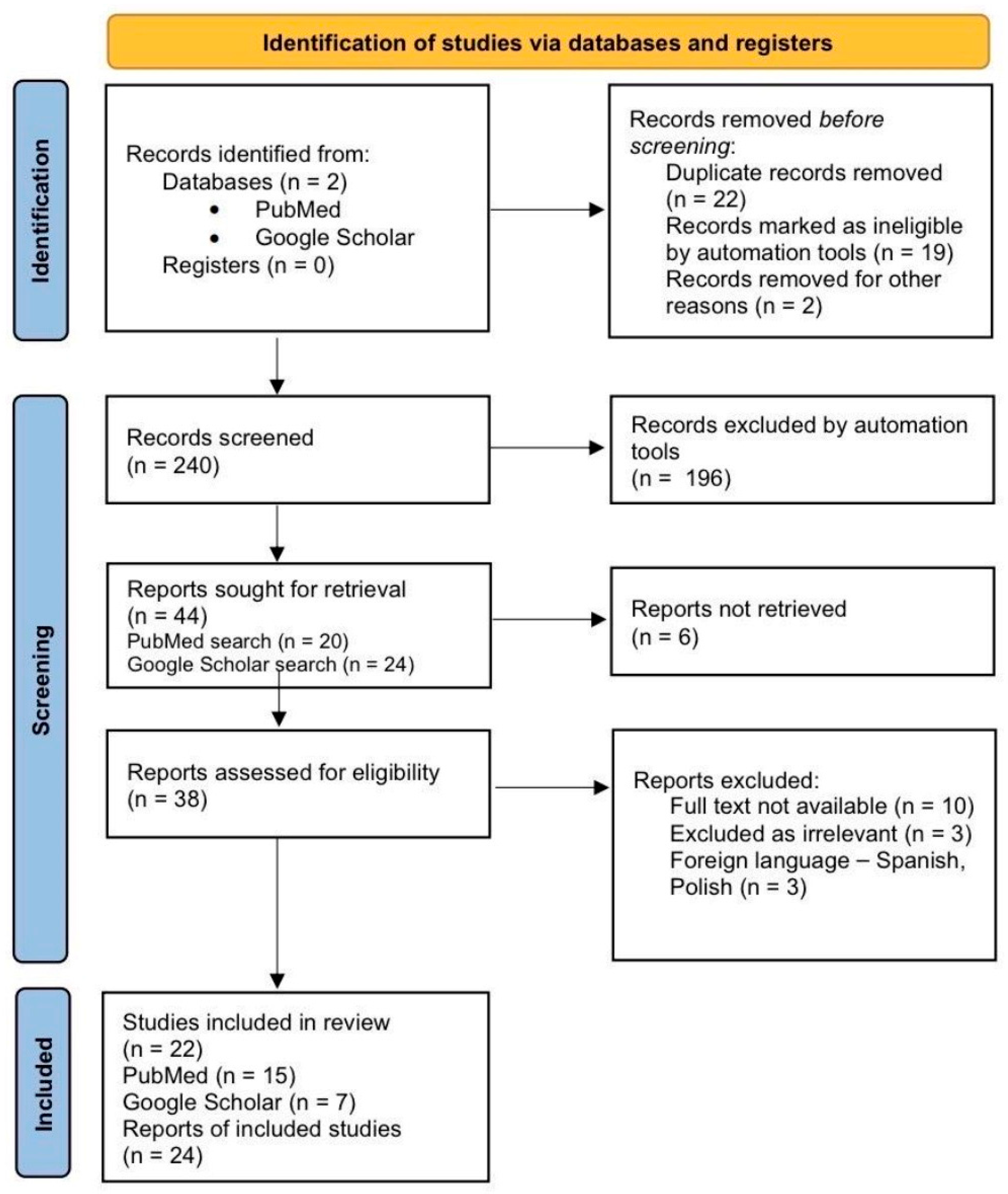
2. Methodology
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Data Items and Outcomes
Risk of Bias and Level of Evidence
2.5. Data Synthesis
3. Results
3.1. Patient Characteristics
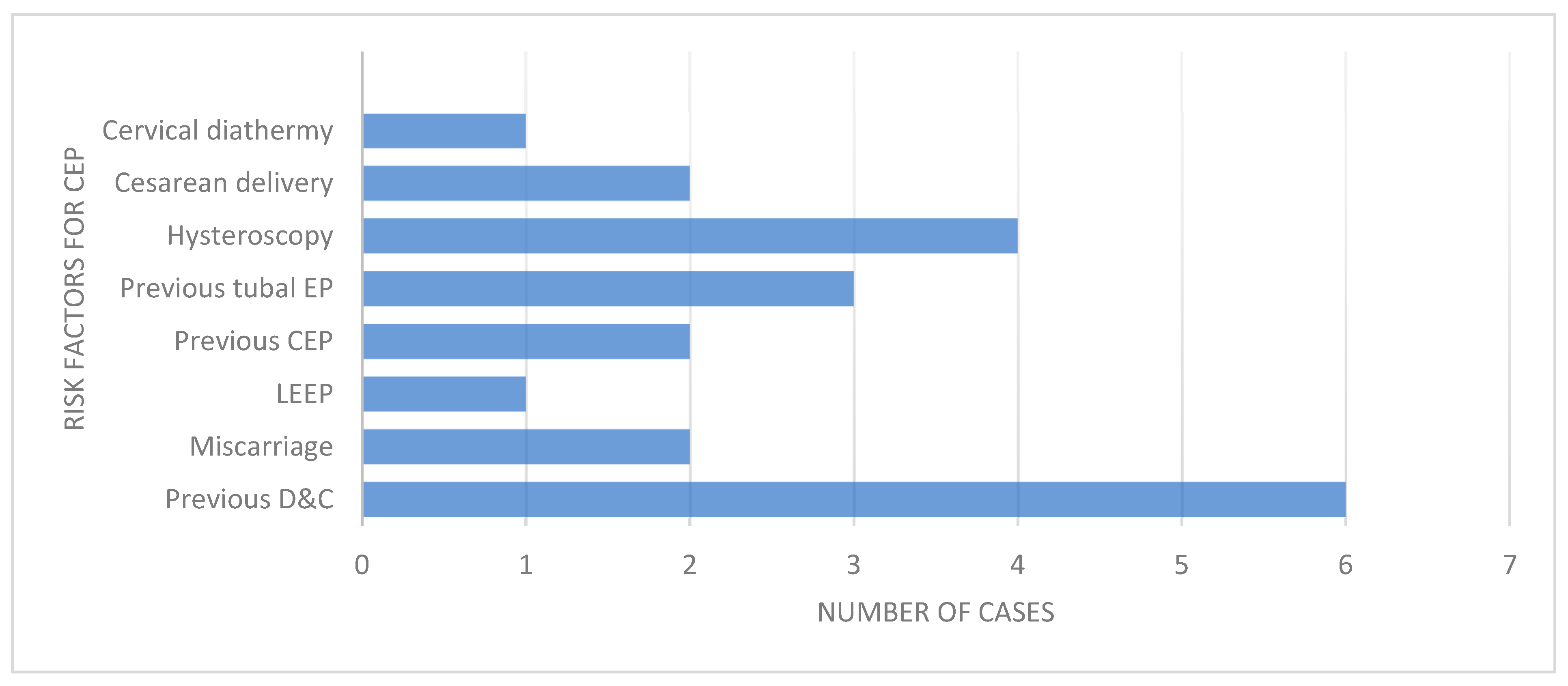
3.2. Risk Factors
3.3. Diagnostic Methods
3.4. Treatment Modalities
3.5. Complications
3.6. Outcomes
4. Discussion
4.1. Clinical Significance and Risk Factors
4.2. Diagnosis: Advances and Challenges
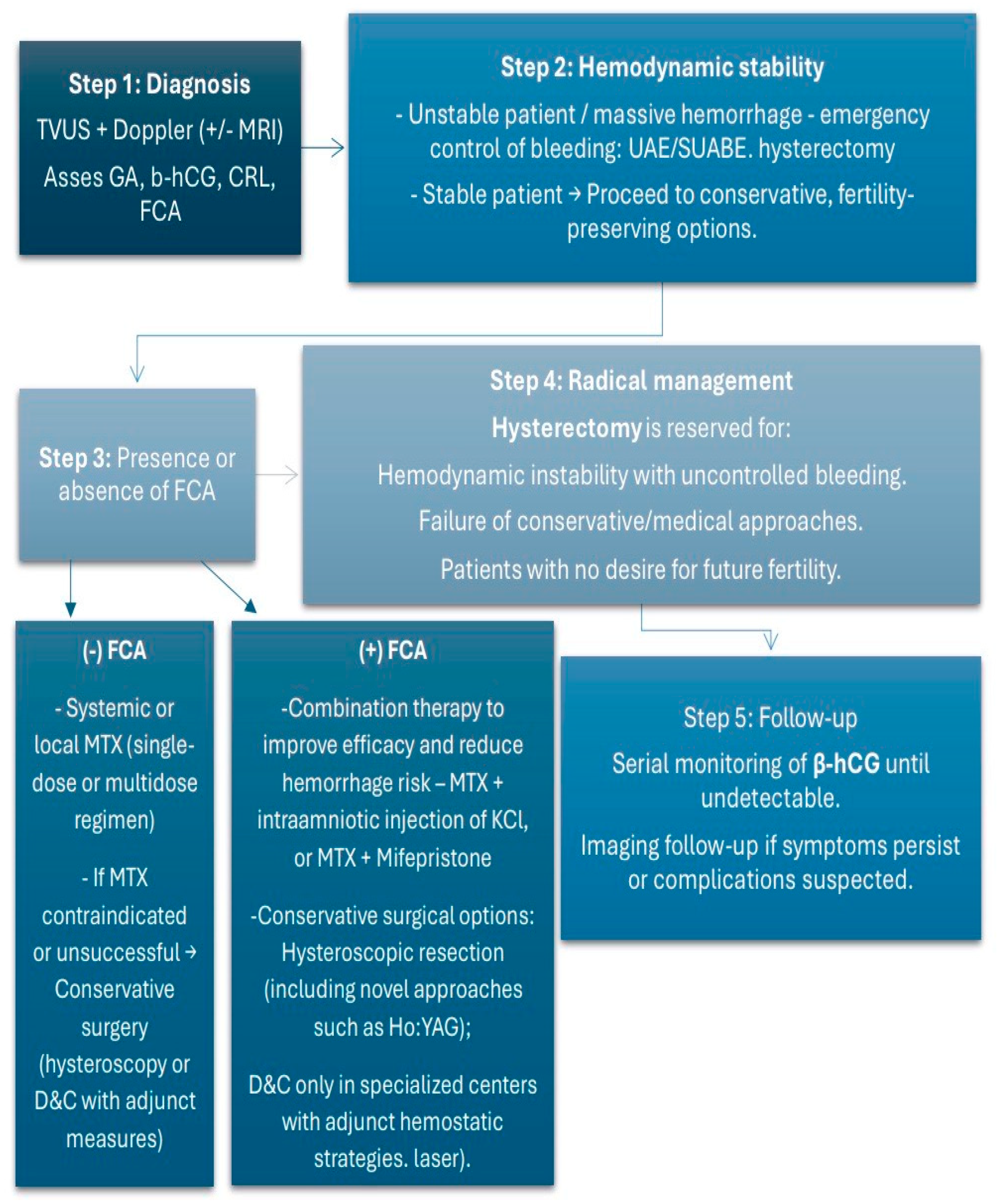
4.3. Treatment Approaches
4.4. Critical Appraisal, Limitations, and Implications for Practice
4.4.1. Critical Appraisal and Limitations
4.4.2. Implications for Clinical Practice
4.4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| n.a. | not available |
| CEP | cervical ectopic pregnancy |
| GA | gestational age |
| g.w. | gestational weeks |
| TVUS | transvaginal ultrasound |
| TAUS | transabdominal ultrasound |
| UAE | Angiographic uterine artery embolization |
| EP | endometrial polyps |
| ET | embryo transfer |
| PROM | premature rupture of membranes |
| SUABE | super-selective embolization of the pathological uterine arteries branch |
| IUI | intrauterine insemination |
| CRL | crown-rump length |
| FCA | fetal cardiac activity |
References
- Stabile, G.; Mangino, F.P.; Romano, F.; Zinicola, G.; Ricci, G. Ectopic Cervical Pregnancy: Treatment Route. Medicina 2020, 56, 293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoyos, L.R.; Tamakuwala, S.; Rambhatla, A.; Brar, H.; Vilchez, G.; Allsworth, J.; Rodriguez-Kovacs, J.; Awonuga, A. Risk factors for cervical ectopic pregnancy. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101665. [Google Scholar] [CrossRef]
- Matorras, R.; Zallo, A.; Hernandez-Pailos, R.; Ferrando, M.; Quintana, F.; Remohi, J.; Malaina, I.; Laínz, L.; Exposito, A. Cervical pregnancy in assisted reproduction: An analysis of risk factors in 91,067 ongoing pregnancies. Reprod. Biomed. Online 2020, 40, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, S.; Fu, J.; Song, Y.; Xiao, L.; Huang, W.; Zhang, H. Outcomes of Bilateral Uterine Artery Chemoembolization in Combination with Surgical Evacuation or Systemic Methotrexate for Cervical Pregnancy. J. Minim. Invasive Gynecol. 2015, 22, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Aroche Gutierrez, L.L.; Bunn, J.; Duvernois, G.; Baker, C. Cervical Ectopic Pregnancy: Combination Treatment with Multi-Dose Methotrexate Regimen, Uterine Artery Embolization, and Suction Curettage. Cureus 2024, 16, e52125. [Google Scholar] [CrossRef]
- Pascual, M.A.; Ruiz, J.; Tresserra, F.; Sanuy, C.; Grases, P.J.; Tur, R.; Barri, P.N. Cervical ectopic twin pregnancy: Diagnosis and conservative treatment: Case report. Hum. Reprod. 2001, 16, 584–586. [Google Scholar] [CrossRef]
- Astruc, A.; Paulus, A.; Jouffray, C.; Bouet, P.E.; Legendre, G. Cervical ectopic pregnancy: A case report of a massive pregnancy with a minimally invasive blood-free treatment and a review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2024, 53, 102837. [Google Scholar] [CrossRef] [PubMed]
- Mininni, C.; Garibaldi, S.; Fornari, L.; Domenici, L.; Cattani, R.; Bottone, P. Effective combined treatment in ectopic cervical pregnancy preserving fertility: A case report and literature review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4190–4197. [Google Scholar] [CrossRef] [PubMed]
- Dilday, E.; Douglas, C.; Brennan, K. Single-dose intramuscular methotrexate for treatment of cervical ectopic pregnancy: A case report. Case Rep. Womens Health 2021, 31, e00340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asante, A.; Verma, U.; Coddington, C.C.; Stewart, E.A. Single-dose intramuscular methotrexate treatment of cervical ectopic pregnancy. A case report. J. Reprod. Med. 2015, 60, 78–82. [Google Scholar] [PubMed]
- Sheng, S.M.; Zhang, H.M.; Pan, Z.M.; Li, T.; Wang, X.M.; Shi, M.; Wang, F. Treatment of heterotopic cervical pregnancy by ultrasound-guided hysteroscopy: A case report and literature review. Medicine 2022, 101, e32177. [Google Scholar] [CrossRef]
- Fan, Y.; Du, A.; Zhang, Y.; Xiao, N.; Zhang, Y.; Ma, J.; Meng, W.; Luo, H. Heterotopic cervical pregnancy: Case report and literature review. J. Obstet. Gynaecol. Res. 2022, 48, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolaños-Bravo, H.H.; Ricaurte-Fajardo, A.; Zarama-Márquez, F.; Ricaurte-Sossa, A.; Fajardo-Rivera, R.; Chicaiza-Maya, R.; Guerrero-Mejía, C.A. Conservative management in a patient with cervical ectopic pregnancy in Nariño, Colombia: Case report and review of the literature. Rev. Colomb. Obstet. Ginecol. 2019, 70, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.E.F.F.; Giordano, L.A.; Giordano, M.V.; Sá, R.A.M.; Campos, F.; Yadid, I.M.; Pinto, F.O. Heterotopic cervical pregnancy after in-vitro fertilization-case report and literature review. JBRA Assist. Reprod. 2019, 23, 290–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gun, M.; Mavrogiorgis, M. Cervical ectopic pregnancy: A case report and literature review. Ultrasound Obstet. Gynecol. 2002, 19, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.; Abele, H.; Hahn, M.; Wallwiener, D.; Rajab, T.K.; Hornung, R. Cervical ectopic pregnancy on the portio: Conservative case management and clinical review. Fertil. Steril. 2008, 90, 2011.e1–2011.e4. [Google Scholar] [CrossRef]
- Kouyoumdjian, A. Cervical pregnancy: Case report and literature review. J. Natl. Med. Assoc. 1984, 76, 791–796. [Google Scholar] [PubMed] [PubMed Central]
- Oleksik, T.P.; Pluta, K.; Issat, T.; Jakimiuk, A.; Wierzba, W. The use of super-selective uterine artery branch embolization and methotrexate in cervical pregnancy-case reports and literature review. Ann. Agric. Environ. Med. 2021, 28, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Faschingbauer, F.; Mueller, A.; Voigt, F.; Beckmann, M.W.; Goecke, T.W. Treatment of heterotopic cervical pregnancies. Fertil. Steril. 2011, 95, 1787.e9–1787.e13. [Google Scholar] [CrossRef]
- Tsakos, E.; Tsagias, N.; Dafopoulos, K. Suggested method for the management of heterotopic cervical pregnancy leading to term delivery of the intrauterine pregnancy: Case report and literature review. J. Minim. Invasive Gynecol. 2015, 22, 896–901. [Google Scholar] [CrossRef]
- Moragianni, V.A.; Hamar, B.D.; McArdle, C.; Ryley, D.A. Management of a cervical heterotopic pregnancy presenting with first-trimester bleeding: Case report and review of the literature. Fertil. Steril. 2012, 98, 89–94. [Google Scholar] [CrossRef]
- Corticelli, A.; Grimaldi, M.; Caporale, E. Conservative management of cervical ectopic pregnancy: Case report and review of literature. Clin. Exp. Obstet. Gynecol. 2008, 35, 297–298. [Google Scholar]
- Mantalenakis, S.; Tsalikis, T.; Grimbizis, G.; Aktsalis, A.; Mamopoulos, M.; Farmakides, G. Successful pregnancy after treatment of cervical pregnancy with methotrexate and curettage: A case report. J. Reprod. Med. 1995, 40, 409–414. [Google Scholar]
- Davis, L.B.; Lathi, R.B.; Milki, A.A.; Dahan, M.H. Transvaginal ligation of the cervical branches of the uterine artery and injection of vasopressin in a cervical pregnancy as an initial step to controlling hemorrhage: A case report. J. Reprod. Med. 2008, 53, 365–368. [Google Scholar] [PubMed]
- De La Vega, G.A.; Avery, C.; Nemiroff, R.; Marchiano, D. Treatment of early cervical pregnancy with cerclage, carboprost, curettage, and balloon tamponade. Obstet. Gynecol. 2007, 109 Pt 2, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Yıldızhan, B. Diagnosis and treatment of early cervical pregnancy: A case report and literature review. Clin. Exp. Obstet. Gynecol. 2005, 32, 254–256. [Google Scholar]
- Cvetkov, D.; Lukanovic, D.; Yordanov, A. Outpatient Hysteroscopic Treatment of Cervical Ectopic Pregnancy in a Primigravida Using the Ho:YAG Laser: A Case Report and Operative Protocol Evaluation. Reprod. Med. 2025, 6, 21. [Google Scholar] [CrossRef]
- Bader-Armstrong, B.; Shah, Y.; Rubens, D. Use of ultrasound and magnetic resonance imaging in the diagnosis of cervical pregnancy. J. Clin. Ultrasound 1989, 17, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.T.; Chau Su Levy, J.; Legatt, E. Cervical pregnancy analysis: A review and report of five cases. Obstet. Gynecol. 1983, 62, 79. [Google Scholar] [PubMed]
- Samal, S.; Ghose, S.; Pallavee, P.; Porkkodi, P. Successful Management of Live Cervical Ectopic Pregnancy: A Case Report. J. Clin. Diagn. Res. 2015, 9, QD03–QD04. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raskin Michael, M. Diagnosis of cervical pregnancy by ultrasound: A Case report. Am. J. Obstet. Gynecol. 1978, 130, 234–235. [Google Scholar] [CrossRef]
- Jurkovic, D.; Hacket, E.; Campbell, S. Diagnosis and treatment of early cervical pregnancy. Ultrasound Obstet. Gynecol. 1996, 8, 373–380. [Google Scholar] [CrossRef]
- Ortiz, G.; Kameyama, N.; Sulaiman, J.P.; Lopez-Bayghen, E. Successful management of cervical ectopic pregnancy with embryo reduction: Report of three cases. J. Surg. Case Rep. 2021, 2021, rjab216. [Google Scholar] [CrossRef]
- Yavanasuriya, J.; Keepanasseril, A.; Ns, K.; Maurya, D.K.; Dorairajan, G. Management of cervical pregnancy with a combination of systemic methotrexate and ultrasound-guided local instillation of methotrexate and potassium chloride: A case report and review of the literature. J. Gynecol. Surg. 2016, 32, 290–292. [Google Scholar] [CrossRef]
- Petousis, S.; Margioula-Siarkou, C.; Kalogiannidis, I.; Karavas, G.; Palapelas, V.; Prapas, N.; Rousso, D. Conservative management of cervical pregnancy with intramuscular administration of methotrexate and KCl injection: Case report and review of the literature. World J. Clin. Cases 2015, 3, 81–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García, M.T.G.; Benitez, G.A.; Belda, B.B.; Rodríguez, C.C.; Merlo, G.G. Medical therapy (methotrexate and mifepristone) alone or in combination with another type of therapy for the management of cervical or interstitial ectopic pregnancy. Eur. J. Obstet. Gynecol. Reprod. Boil. 2012, 165, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Nikolettos, K.; Oikonomou, E.; Kotanidou, S.; Kritsotaki, N.; Kyriakou, D.; Tsikouras, P.; Kontomanolis, E.; Gerede, A.; Nikolettos, N. A Systematic Review about Cervical Pregnancy and our Experience. Acta Med. Litu. 2024, 31, 92–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maglic, R.; Rakic, A.; Nikolic, B.; Maglic, D.; Jokanovic, P.; Mihajlovic, S. Management of Cervical Ectopic Pregnancy with Small-Caliber Hysteroscopy. J. Second. Lang. Stud. 2021, 25, e202100016. [Google Scholar] [CrossRef]
- Takeda, K.; Mackay, J.; Watts, S. Successful Management of Cervical Ectopic Pregnancy with Bilateral Uterine Artery Embolization and Methotrexate. Case Rep. Emerg. Med. 2018, 2018, 9593824. [Google Scholar] [CrossRef]
- Hosni, M.M.; Herath, R.P.; Mumtaz, R. Diagnostic and therapeutic dilemmas of cervical ectopic pregnancy. Obstet. Gynecol. Surv. 2014, 69, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Alammari, R.; Thibodeau, R.; Harmanli, O. Vaginal Hysterectomy for Treatment of Cervical Ectopic Pregnancy. Obstet. Gynecol. 2017, 129, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-Q.; Gao, T.-T.; Lu, B.; Zhang, Q.; Jin, M.-Y.; Cheng, H.-J. Cervical pregnancy management by lauromacrogol combined with intrauterine visualization system and vacuum aspiration: Two case studies. Clin. Exp. Obstet. Gynecol. 2022, 49, 93. [Google Scholar] [CrossRef]
- Albahlol Ibrahim, A. Cervical pregnancy management: An updated stepwise approach and algorithm. J. Obstet. Gynaecol. Res. 2021, 47, 469–475. [Google Scholar] [CrossRef] [PubMed]
| N° | Maternal Age | Parity | Risk Factors | Method of Conception | GA at Diagnosis | Initial β-hCG Levels | Diagnosis | Treatment | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Pascual MA et al. [6] | n.a. | n.a. | n.a. | n.a. | 8th g.w. | n.a. | TVUS—cervical twin pregnancy | Intra-amniotic administration of methotrexate under ultrasonographic guidance followed by curettage | Favorable outcome followed by a subsequent intrauterine pregnancy |
| Astruc A et al. [7] | 25 | G3P0A2 | Induced abortion by D&C, one miscarriage | Spontaneous | 9th g.w. | 109,850 mIU/mL | TVUS | Uterine artery embolization + in situ methotrexate | β-hCG normalized by day 104; full recovery by 6 months. |
| Mininni C et al. [8] | 43 | Nulliparous | n.a. | Spontaneous | 9th g.w. | 85,220 mIU/mL | TVUS | Methotrexate i.m. + intra-amniotic chloride potassium installation | Rehospitalization due to massive vaginal bleeding 3 months later followed by UAE |
| Dilday E [9] | 45 | G3P0A2 | LEEP for CIN II | IVF | 5 + 1 g.w. | 3217 mIU/mL | TVUS | Single dose of intramuscular methotrexate | Nonpregnant levels of β-hCG by day 28 |
| Asante A [10] | n.a. | G2P0 | Previous CEP treated with MTX i.m. | Spontaneous | n.a. | n.a. | n.a. | Single dose of intramuscular methotrexate | Uneventful outcome |
| Sheng [11] | 31 | G2P0 | Left salpingectomy for tubal pregnancy, hysteroscopy for multiple EP | IVF | 6 g.w. | 119,885 mIU/L | TVUS—heterotopic CEP | US-guided hysteroscopy | Preserving the intrauterine pregnancy, Cesarean section in 38th g.w. |
| Fan Y [12]—case 1 | 29 | Nulliparous | n.a. | ICSI | 6 g.w. | 819 mIU/mL | TVUS—heterotopic CEP | Ultrasound-guided aspiration | Preserving the intrauterine pregnancy, Cesarean section in 39 g.w. |
| Fan Y [12]—case 2 | 27 | G2P1 | Right salpingectomy | IVF | 6 g.w. | 910 mIU/mL 14 days after ET | TVUS—heterotopic CEP | Ultrasound-guided aspiration | Preserving the intrauterine pregnancy, vaginal delivery at 27th g.w. due to PROM |
| Bolaños-Bravo HH et al. [13] | 30 | G2P1 | Previous Cesarean section | Spontaneous | 5 + 4 g.w. | 16,189 mIU/mL | TVUS | MTX i.m. 1, 3, 5, 7 day, followed by D&C due to doubled values of β-hCG (35,199 mIU/mL) | Uneventful postoperative period, β-hCG 16 mIU/mL on the 17th day |
| Terra MEFF et al. [14] | 39 | G7P0A5 | Right tubal pregnancy, treated with MTX, hysteroscopic myomectomy | IVF | 10 g.w. | n.a. | TVUS—heterotoic CEP | Spontaneous expulsion of CEP, followed by cervical curettage | Preserving the intrauterine pregnancy, Cesarean section in 39 g.w. |
| Gun M [15] | 39 | G2P0A1 | D&C of pregnancy at 8th g.w., diathermy of the cervix due to erosion | Spontaneous | 6 g.w. | 23,060 mIU/mL | TAUS | MTX i.m. on 1 and 7 day | Massive vaginal bleeding on day 11 required surgical intervention—bilateral UAE |
| Kraemer B et al. [16] | 38 | G3P2A1 | D&C of pregnancy at 9 g.w. | Spontaneous | n.a. | 231.4 mIU/mL | Speculum examination—CP on the portio; histological confirmation | Completely excision with biopsy forceps under local anesthesia | Normalization of β-hCG after 7 days |
| Kouyoumdjian A [17] | 22 | G2P1A0 | Irrelevant | Spontaneous | 10 g.w. | n.a. | - | Curettage followed by heavy bleeding and second curettage | Uterine perforation; Total hysterectomy |
| Oleksik TP et al. [18]—case 1 | 32 | Nulliparous | Irrelevant | Spontaneous | 8 g.w. | 20,760 mIU/mL | TVUS | MTX i.m. on 1 and 7 day | Increased vascularization detected by Doppler; SUABE followed by decreased vascularization and normalization of β-hCG |
| Oleksik TP et al. [18]—case 2 | 31 | Nulliparous | Irrelevant | IVF | 6 g.w. | 13,600 mIU/mL | TVUS | MTX i.m. on 1 and 7 day | Increased vascularization detected by Doppler; SUABE followed by decreased vascularization and normalization of β-hCG |
| Faschingbauer F et al. [19] | 25 | G1P0A0 | Irrelevant | Conceiving after induction of ovulation with clomiphene citrate | 9 g.w. | n.a. | TVUS—heterotopic CEP | Extraction with curettage + Shirodkar cerclage | Uncomplicated postoperative period; Vaginal delivery at 39 g.w. |
| Tsakos [20] | 41 | G2P0 | CEP after IVF treated with D&C in the past | IVF | 7 g.w. | n.a. | TVUS—heterotopic CEP | Aspiration of CEP, followed by cervical Foley catheter placement and Shirodkar cerclage | Uneventful full-term delivery |
| Moragianni VA et al. [21] | 40 | G3P1A1 | 6-week spontaneous miscarriage treated with D&E | IUI | 7 + 3 g.w. | n.a. | TVUS | US-guided removal with ring forceps led to heavy vaginal bleeding—US-guided placement of an endocervical Foley catheter | Cesarean section at 39 g.w. |
| Corticelli A. et al. [22] | 34 | G2P1 | Previous Cesarean section | Spontaneous | 5 + 5 g.w. | 12,396 mIU/mL | TVUS | MTX i.m. on day 1 and 4, two days later severe vaginal bleeding acquired D&C followed by a Foley ballon tamponade | Uneventful |
| Mantalenakis, S. et al. [23] | 28 | Nulliparous | Irrelevant | Spontaneous | 12 g.w. | n.a. | TVUS | MTX intraamniotically + i.m. —persistent fetal viability on the 7th day—uneventful curettage | Favorable outcome followed by a subsequent intrauterine pregnancy 5 months later |
| Davis LB et al. [24] | 43 | Nulliparous | Irrelevant | IVF | 6 g.w. | n.a. | TVUS | Transvaginal ligation of the cervical branches of the uterine artery and injection of vasopressin, followed by D&C | Uneventful |
| De La Vega GA et al. [25] | 35 | Nulliparous | Irrelevant | Spontaneous | 8 g.w. | n.a. | TVUS | Intracervical infiltration of carboprost, suction curettage of cervix and Foley balloon tamponade | Uneventful |
| Yıldızhan, B. [26] | 43 | G2P0A2 | Two induced abortions with D&C | Spontaneous | 7 g.w. | 46,000 mIU/mL | TVUS | Single-dose MTX i.m. + US-guided intra-amniotic administration of MTX | Nonpregnant levels of β-hCG on day 83 |
| Cvetkov D. et al. [27] | 32 | G1P0 | Hysteroscopy for removing a uterine septum | Spontaneous | 6 g.w. | 5119 mIU/mL | TVUS | Ho:YAG Laser hysteroscopy | Uneventful |
| Treatment Modality | Number of Cases (n) | Successful (n, %) | Failed/Required Additional Intervention (n, %) |
|---|---|---|---|
| MTX (local or systemic) | 11 | 5 (45%) | 6 (55%) |
| Uterine artery embolization (UAE/SUABE) | 4 | 4 (100%) | 0 (0%) |
| Dilation and curettage (D&C) | 6 | 4 (67%) | 2 (33%) |
| Hysterectomy | 3 | 3 (100%) | - |
| Complication | Number of Cases (n) | Percentage (% of Total) |
|---|---|---|
| Severe vaginal bleeding | 4 | 17% |
| Hysterectomy | 3 | 12% |
| Uterine perforation | 1 | 4% |
| Rehospitalization (delayed complications) | 1 | 4% |
| Diagnostic Ultrasound Criteria for CEP | |
|---|---|
| Ultrasound Findings | Additional Doppler Findings |
| Enlarged cervix with gestational sac inside the cervical canal | Peritrophoblastic blood flow around the gestational sac detected with color Doppler |
| Absence of gestational sac inside the uterine cavity | |
| Enlarged uterus | |
| Diffuse irregular echoes within the uterus | |
| Absence of sliding sign | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, N.; Yordanov, A.; Popovski, N. Current Trends in the Treatment of Cervical Pregnancy: A Narrative Review. Medicina 2025, 61, 2072. https://doi.org/10.3390/medicina61112072
Stoyanova N, Yordanov A, Popovski N. Current Trends in the Treatment of Cervical Pregnancy: A Narrative Review. Medicina. 2025; 61(11):2072. https://doi.org/10.3390/medicina61112072
Chicago/Turabian StyleStoyanova, Nikoleta, Angel Yordanov, and Nikola Popovski. 2025. "Current Trends in the Treatment of Cervical Pregnancy: A Narrative Review" Medicina 61, no. 11: 2072. https://doi.org/10.3390/medicina61112072
APA StyleStoyanova, N., Yordanov, A., & Popovski, N. (2025). Current Trends in the Treatment of Cervical Pregnancy: A Narrative Review. Medicina, 61(11), 2072. https://doi.org/10.3390/medicina61112072







