Integrating Renal and Metabolic Parameters into a Derived Risk Score for Hyperuricemia in Uncontrolled Type 2 Diabetes: A Retrospective Cross-Sectional Study in Northwest Romania
Abstract
1. Introduction
Aims and Research Questions
- To evaluate the relative contribution of serum urea and the triglyceride-to-LDL cholesterol ratio to hyperuricemia risk.
- To examine the influence of gender, age, obesity, and medication use on hyperuricemia.
- To explore the discriminative ability of the derived score for identifying patients at risk.
- Which renal and metabolic parameters independently predict hyperuricemia in uncontrolled type 2 diabetes?
- Does the combination of serum urea and TG/LDL ratio improve risk assessment compared to single biomarkers?
- How does the derived risk score perform in distinguishing patients with and without hyperuricemia?
2. Materials and Methods
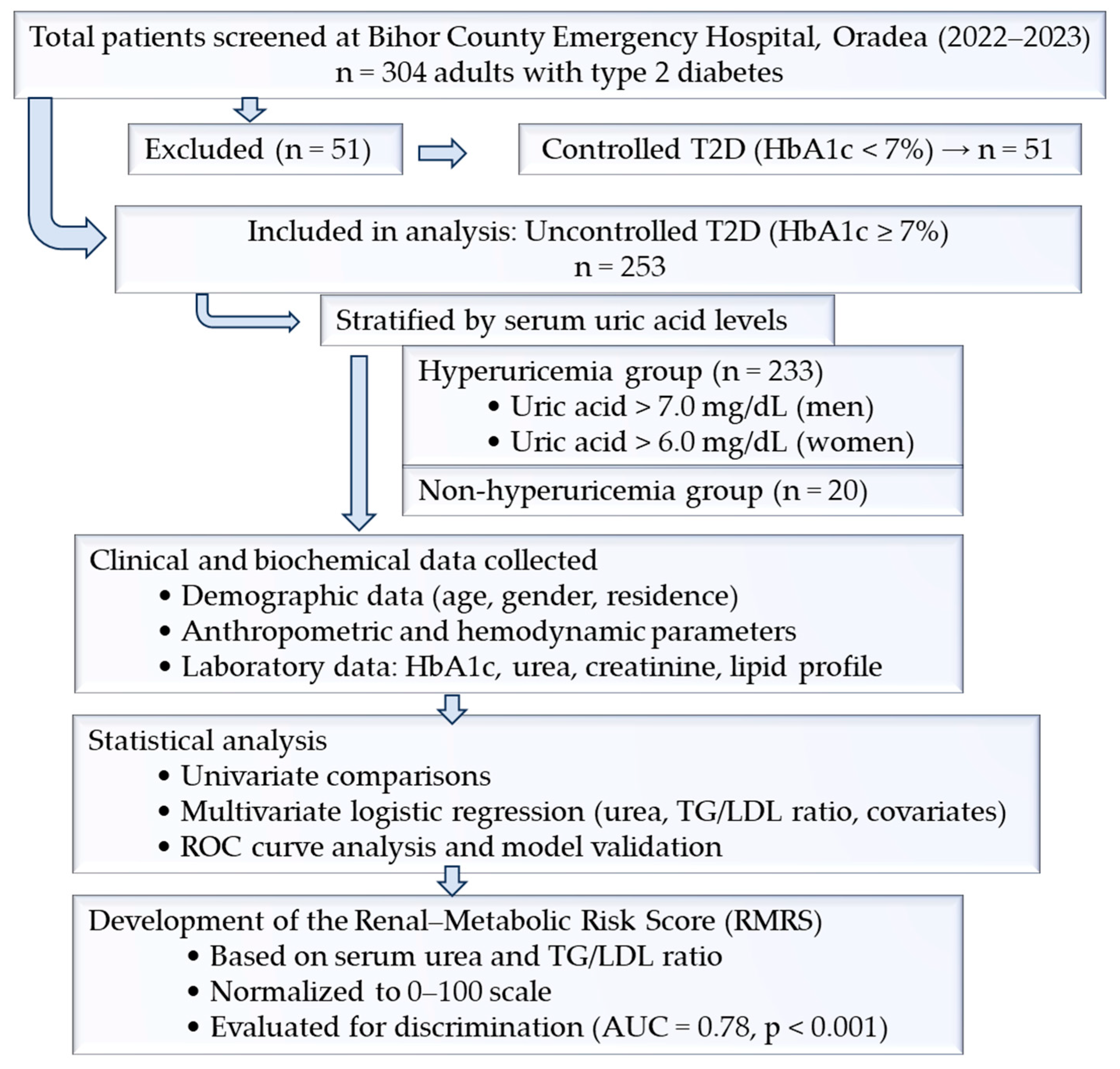
2.1. Study Design and Setting
2.2. Eligibility Criteria
2.3. Data Collection
- Blood pressure and heart rate (measured after 5–10 min rest, avoiding caffeine or strenuous activity for at least 30 min).
- Ankle–brachial index (ABI) measured with a Doppler device; ABI <0.9 indicated peripheral artery disease, and ABI >1.4 suggested arterial calcification.
- Anthropometric measurements: weight, height, BMI (kg/m2), and waist circumference (WC). Elevated WC was defined as >80 cm for females and >94 cm for males.
- Neurological assessment: peripheral sensitivity testing by a neurologist for diabetic polyneuropathy.
- Ophthalmologic assessment: fundoscopic examination by an ophthalmologist for diabetic retinopathy.
- Glycated hemoglobin (HbA1c) and fasting plasma glucose.
- Lipid profile: total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), triglycerides (TG), and calculated ratios (TG/HDL-C, TG/LDL, non-HDL cholesterol).
- Renal function: serum creatinine, urea, uric acid, albuminuria.
- Estimated glomerular filtration rate (eGFR (mL/min/1.73 m2)) calculated according to KDIGO guidelines.
2.4. Variable Definitions
- Diabetes: HbA1c ≥ 6.5%.
- Obesity was classified according to WHO BMI criteria; hypertension according to ESC/ESH 2018; dyslipidemia according to NCEP ATP-III; and chronic kidney disease was defined according to KDIGO guidelines, based on eGFR staging (<60 mL/min/1.73 m2) and/or albuminuria. Overweight/obesity: BMI 25.0–29.9 kg/m2 (overweight); BMI 30.0–34.9 kg/m2 (obesity class I); BMI 35.0–39.9 kg/m2 (obesity class II); BMI ≥40.0 kg/m2 (obesity class III).
- Hypertension: systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or ongoing antihypertensive therapy.
- Dyslipidemia cut-offs: TC ≥ 200 mg/dL, TG ≥ 150 mg/dL, LDL-C ≥ 100 mg/dL, HDL-C < 60 mg/dL (males) or <40 mg/dL (females).
- Renal function thresholds: creatinine > 1.1 mg/dL, urea > 20 mg/dL, uric acid > 6.0 mg/dL (females) or >7.0 mg/dL (males), albuminuria > 30 mg/dL. Definitions of hypertension, dyslipidemia, obesity, and CKD followed international guidelines (ESC/ESH, ATP III, KDIGO).
- RMRS = standardized serum urea + standardized TG/LDL ratio. HbA1c was examined in preliminary models but excluded from the final RMRS due to limited incremental contribution.
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
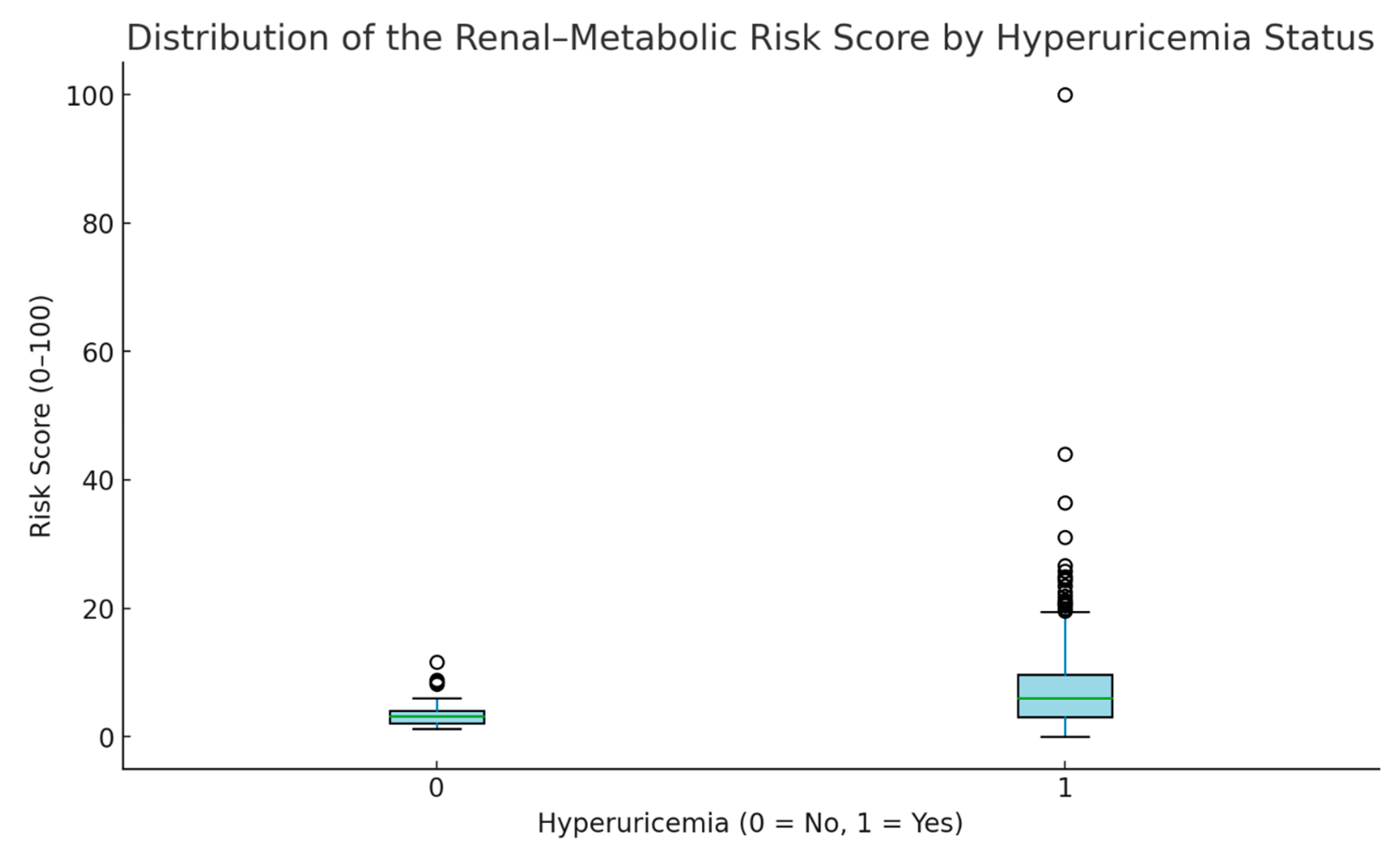
3.2. Renal–Metabolic Risk Score
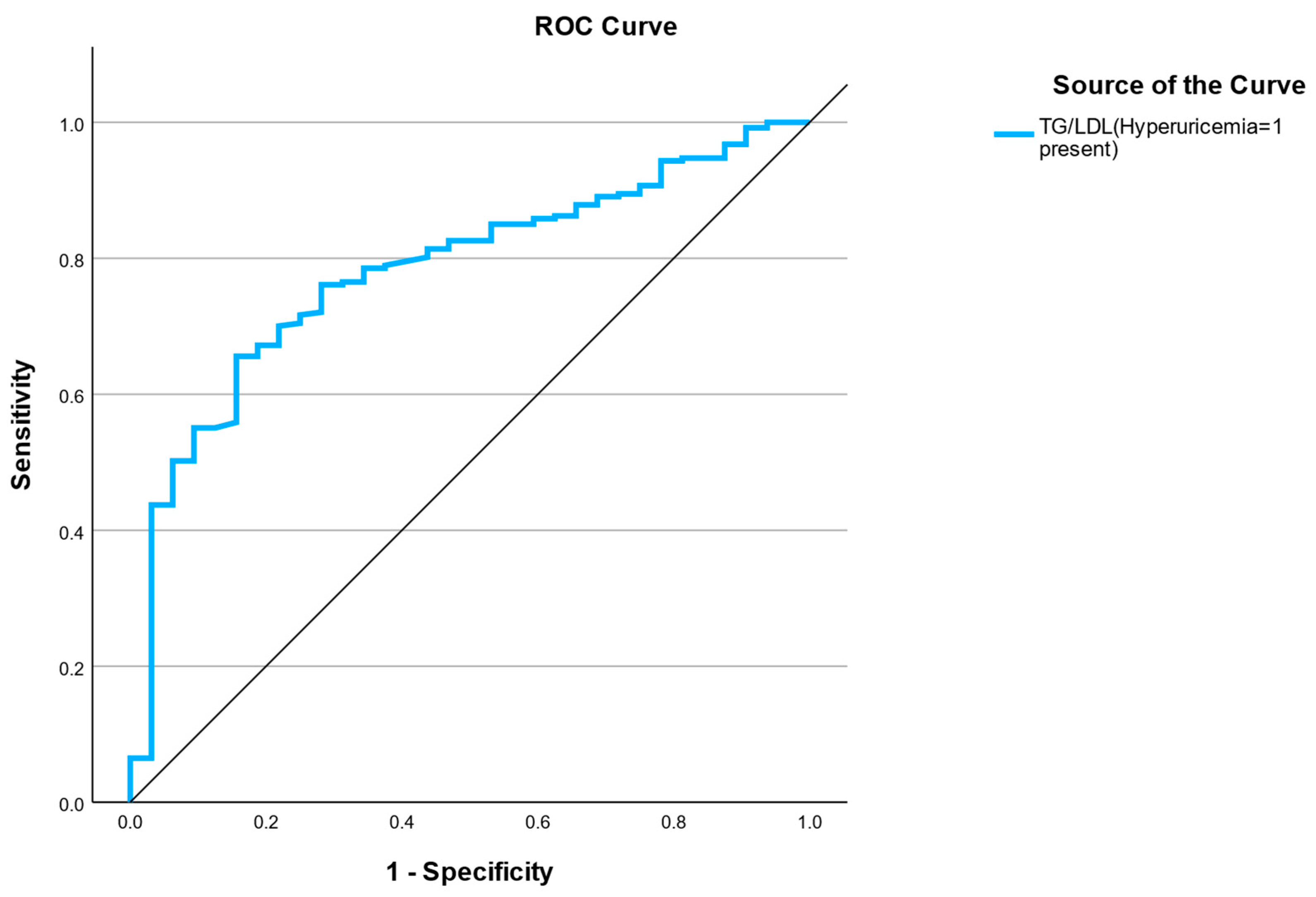
3.3. ROC—Renal–Metabolic Risk Score
3.4. Development of the Renal–Metabolic Risk Score
3.5. Bayesian Linear Regression Analysis
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Studies
4.3. Perspectives for Clinical and Assistive Practice
4.4. Clinical Implications
4.5. Strengths and Limitations
4.6. Future Directions
4.7. Emerging Directions and Future Biomarkers
5. Conclusions
Take-Home Message
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The insulin resistance atherosclerosis study. Kidney Int. 2000, 58, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, H.B.; Read, S.H.; Sattar, N.; Franzén, S.; Halbesma, N.; Dorresteijn, J.A.N.; Westerink, J.; Visseren, F.L.J.; Wild, S.H.; Eliasson, B.; et al. Development and validation of a lifetime risk model for kidney failure and treatment benefit in type 2 diabetes: 10-year and lifetime risk prediction models. Clin. J. Am. Soc. Nephrol. 2022, 17, 1783–1791. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Yadav, D.; Lee, E.S.; Kim, H.M.; Lee, E.Y.; Choi, E.; Chung, C.H. Hyperuricemia as a potential determinant of metabolic syndrome. J. Lifestyle Med. 2013, 3, 98–106. [Google Scholar] [PubMed]
- Freilich, M.; Arredondo, A.; Zonnoor, S.L.; McFarlane, I.M. Elevated serum uric acid and cardiovascular disease: A review and potential therapeutic interventions. Cureus 2022, 14, e23582. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Nakagawa, T.; Kanbay, M.; Kuwabara, M.; Kumar, A.; Arroyo, F.E.G.; Roncal-Jimenez, C.; Sasai, F.; Kang, D.H.; Jensen, T.; et al. Hyperuricemia in kidney disease: A major risk factor for cardiovascular events, vascular calcification, and renal damage. Semin. Nephrol. 2020, 40, 574–585. [Google Scholar] [CrossRef]
- Potra Cicalău, G.I.; Marcu, O.A.; Ghitea, T.C.; Ciavoi, G.; Iurcov, R.C.; Beiusanu, C.; Trifan, D.F.; Vicaș, L.G.; Ganea, M. Study of periodontal bacteria in diabetic wistar rats: Assessing the anti-inflammatory effects of carvacrol and magnolol hydrogels. Biomedicines 2024, 12, 1445. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapalowska-Chwyć, M.; Drozd, R. A low glycemic index decreases inflammation by increasing the concentration of uric acid and the activity of glutathione peroxidase (gpx3) in patients with polycystic ovary syndrome (pcos). Molecules 2019, 24, 1508. [Google Scholar] [CrossRef]
- Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo-Clemente, L.; Goya, L.; Sarriá, B. Colonic coffee phenols metabolites, dihydrocaffeic, dihydroferulic, and hydroxyhippuric acids protect hepatic cells from tnf-α-induced inflammation and oxidative stress. Int. J. Mol. Sci. 2023, 24, 1440. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Yuan, M.; Xu, Y.; Xu, H. Gout and diet: A comprehensive review of mechanisms and management. Nutrients 2022, 14, 3525. [Google Scholar] [CrossRef]
- Deji-Oloruntoba, O.O.; Balogun, J.O.; Elufioye, T.O.; Ajakwe, S.O. Hyperuricemia and insulin resistance: Interplay and potential for targeted therapies. Int. J. Transl. Med. 2025, 5, 30. [Google Scholar] [CrossRef]
- Timsans, J.; Palomäki, A.; Kauppi, M. Gout and hyperuricemia: A narrative review of their comorbidities and clinical implications. J. Clin. Med. 2024, 13, 7616. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah, M.; Siddiqui, K.; Al-Rubeaan, K. Association between serum uric acid levels and metabolic markers in patients with type 2 diabetes from a community with high diabetes prevalence. Int. J. Clin. Pract. 2020, 74, e13466. [Google Scholar] [CrossRef] [PubMed]
- Danciu, A.M.; Ghitea, T.C.; Bungau, A.F.; Vesa, C.M. The relationship between oxidative stress, selenium, and cumulative risk in metabolic syndrome. In Vivo 2023, 37, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. 2019. Available online: https://www.diabetesatlas.org/ (accessed on 29 September 2025).
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Aron, R.A.C.; Zaha, D.C. Current data regarding the relationship between type 2 diabetes mellitus and cardiovascular risk factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Kdigo Guidelines Focus on Topics Related to the Prevention or Management of Individuals with Kidney Diseases. 2016. Available online: https://kdigo.org/guidelines/ (accessed on 6 June 2021).
- Pană, N.; Ștefan, G.; Stancu, S.; Zugravu, A.; Ciurea, O.; Petre, N.; Mircescu, G.; Căpușă, C. Clinicopathological characteristics and disease chronicity in glomerular diseases: A decade-long study at Romania’s largest kidney biopsy reference center. Biomedicines 2024, 12, 1143. [Google Scholar] [CrossRef]
- Helou, N.; Talhouedec, D.; Shaha, M.; Zanchi, A. The impact of a multidisciplinary self-care management program on quality of life, self-care, adherence to anti-hypertensive therapy, glycemic control, and renal function in diabetic kidney disease: A cross-over study protocol. BMC Nephrol. 2016, 17, 88. [Google Scholar] [CrossRef]
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S. Us renal data system 2020 annual data report: Epidemiology of kidney disease in the united states. Am. J. Kidney Diseases 2021, 77, A7–A8. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Rayner, B.; Lakshmanan, M.C.; Kwan, A.Y.; Konig, M.; Shurzinske, L.; Botros, F.T. Clinical outcomes by albuminuria status with dulaglutide versus insulin glargine in participants with diabetes and ckd: Award-7 exploratory analysis. Kidney360 2021, 2, 254–262. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Li, L.; Li, Y.; Yang, P.; Duan, Y.; Wang, X.; Zhang, H.; Wang, J.; Zhang, H. Identification of age-specific risk factors for hyperuricemia: A machine learning-driven stratified analysis in health examination cohorts. BMC Med. Inform. Decis. Mak. 2025, 25, 280. [Google Scholar] [CrossRef]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Ma, L.; Wang, X.M. The link between hyperuricemia and diabetes: Insights from a quantitative analysis of scientific literature. Front. Endocrinol. 2024, 15, 1441503. [Google Scholar] [CrossRef]
- Paduraru, L.; CVesa, M.; Popoviciu, M.S.; Ghitea, T.C.; Zaha, D.C. Interrelationship between dyslipidemia and hyperuricemia in patients with uncontrolled type 2 diabetes: Clinical implications and a risk identification algorithm. Healthcare 2025, 13, 2605. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Y.; Cao, Y.; Gao, Y.; Pi, Y.; Tang, X.; Liu, Y.; Zhong, Y. The association between the cardiometabolic index and hyperuricemia: 2011–2016 nhanes. Front. Endocrinol. 2025, 16, 1545968. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Hillman, A.J.; Lohsoonthorn, V.; Hanvivatvong, O.; Jiamjarasrangsi, W.; Lertmaharit, S.; Williams, M.A. Association of high sensitivity c-reactive protein concentrations and metabolic syndrome among Thai adults. Asian Biomed. (Res. Rev. News) 2010, 4, 385–393. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Cibičková, Ľ.; Langová, K.; Vaverková, H.; Kubíčková, V.; Karásek, D. Correlation of uric acid levels and parameters of metabolic syndrome. Physiol. Res. 2017, 66, 481–487. [Google Scholar] [CrossRef]
- Soltani, Z.; Rasheed, K.; Kapusta, D.R.; Reisin, E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: Is it time for reappraisal? Curr. Hypertens. Rep. 2013, 15, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, L.; Pascalau, A.V.; Camarasan, A.; Muresan, M.M.; Pop, O.L.; Brata, R. The impact of dyslipidemia on the clinical profile of patients with uncontrolled diabetes. In Vivo 2025, 39, 2243–2258. [Google Scholar] [CrossRef]
- Alobaidi, S. Emerging biomarkers and advanced diagnostics in chronic kidney disease: Early detection through multi-omics and ai. Diagnostics 2025, 15, 1225. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.J.; Wu, C.Y.; Yang, C.W.; Yang, H.Y. Hyperuricemia and progression of chronic kidney disease: A review from physiology and pathogenesis to the role of urate-lowering therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef]
- Neagu, O.M.; Ghitea, T.; Marian, E.; Vlase, L.; Vlase, A.M.; Ciavoi, G.; Fehér, P.; Pallag, A.; Bácskay, I.; Nemes, D.; et al. Formulation and characterization of mucoadhesive polymeric films containing extracts of taraxaci folium and matricariae flos. Molecules 2023, 28, 4002. [Google Scholar] [CrossRef]
- Balakumar, P.; Alqahtani, A.; Khan, N.A.; Mahadevan, N.; Dhanaraj, S.A. Mechanistic insights into hyperuricemia-associated renal abnormalities with special emphasis on epithelial-to-mesenchymal transition: Pathologic implications and putative pharmacologic targets. Pharmacol. Res. 2020, 161, 105209. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. Micrornas in the diagnosis of digestive diseases: A comprehensive review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bellini, S.; Mastrocola, R.; Bruno, G.; Gruden, G. Microrna and microvascular complications of diabetes. Int. J. Endocrinol. 2018, 2018, 6890501. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Hyperuricemia | |
|---|---|---|
| No | Yes | |
| N | 25 | 279 |
| Age (years) | 65.12 ± 10.49 | 66.94 ± 11.13 |
| p-value | 0.415 | |
| Gender | Male 68.0%; Female 32.0% | Male 51.6%; Female 48.4% |
| p-value | 0.173 | |
| Provenance | Rural 52.0%; Urban 48.0% | Rural 56.3%; Urban 43.7% |
| p-value | 0.840 | |
| On uric acid–lowering therapy | Yes 0.0%; No 100.0% | Yes 15.4%; No 84.6% |
| p-value | 0.069 | |
| On lipid-lowering therapy | Yes 48.0%; No 52.0% | Yes 59.1%; No 40.9% |
| p-value | 0.384 | |
| On antihypertensive therapy | Yes 72.0%; No 28.0% | Yes 89.2%; No 10.8% |
| p-value | 0.027 * | |
| Variable | No Hyperuricemia (Mean ± SD) | Hyperuricemia (Mean ± SD) | p-Value |
|---|---|---|---|
| Urea (mg/dL) | 19.76 ± 10.02 | 32.15 ± 21.21 | <0.001 |
| TG/LDL ratio | 1.95 ± 1.28 | 2.94 ± 6.73 | 0.062 |
| Uric acid (mg/dL) | 6.77 ± 2.12 | 5.69 ± 1.87 | 0.038 |
| Variable | β Coefficient | Std. Error | z-Value | OR | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|---|---|---|
| Urea (mg/dL) | 0.066 | 0.024 | 2.75 | 1.068 | 1.016 | 1.123 | 0.010 |
| TG/LDL ratio | 0.077 | 0.098 | 0.79 | 1.080 | 0.827 | 1.410 | 0.574 |
| Age (years) | −0.018 | 0.023 | −0.75 | 0.982 | 0.938 | 1.029 | 0.452 |
| Gender | 0.551 | 0.495 | 1.11 | 1.736 | 0.651 | 4.626 | 0.270 |
| Uricosuric agents (use) | 0.000 | 13 554 | 0.00 | 5.3 × 108 | 0.000 | — | 0.999 |
| Diuretics (use) | 0.476 | 0.440 | 1.08 | 1.610 | 0.680 | — | 0.279 |
| SGLT2 inhibitors (use) | 0.176 | 0.591 | 0.30 | 1.193 | 0.375 | — | 0.765 |
| Hyperuricemia Status | Mean | SD | Minimum | Maximum | n | p |
|---|---|---|---|---|---|---|
| No (0) | 6.82 | 5.22 | 2.29 | 18.30 | 20 | 0.001 |
| Yes (1) | 13.44 | 12.39 | 0.00 | 100.00 | 233 |
| Aspect | Frequentist Result | Bayesian Confirmation | Meaning |
|---|---|---|---|
| Overall model significance | F(284,19) = 2.17, p = 0.025 | Posterior credible variance narrow, confirming model’s partial fit | Predictive relationship exists but modest |
| Predictor significance | TG/LDL weakly related to urea | Posterior mean near 0 but slightly positive | Possible small metabolic effect |
| eGFR (mL/min/1.73 m2) | Minimal effect | Posterior centered at 0 | eGFR adds limited incremental information |
| Residual variance | ~43,000–48,000 | Credible interval consistent | Substantial unexplained variance remains |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduraru, L.; Zaha, D.C.; Ghitea, T.C.; Fodor, R.; Vesa, C.M.; Popoviciu, M.S. Integrating Renal and Metabolic Parameters into a Derived Risk Score for Hyperuricemia in Uncontrolled Type 2 Diabetes: A Retrospective Cross-Sectional Study in Northwest Romania. Medicina 2025, 61, 2042. https://doi.org/10.3390/medicina61112042
Paduraru L, Zaha DC, Ghitea TC, Fodor R, Vesa CM, Popoviciu MS. Integrating Renal and Metabolic Parameters into a Derived Risk Score for Hyperuricemia in Uncontrolled Type 2 Diabetes: A Retrospective Cross-Sectional Study in Northwest Romania. Medicina. 2025; 61(11):2042. https://doi.org/10.3390/medicina61112042
Chicago/Turabian StylePaduraru, Lorena, Dana Carmen Zaha, Timea Claudia Ghitea, Radu Fodor, Cosmin Mihai Vesa, and Mihaela Simona Popoviciu. 2025. "Integrating Renal and Metabolic Parameters into a Derived Risk Score for Hyperuricemia in Uncontrolled Type 2 Diabetes: A Retrospective Cross-Sectional Study in Northwest Romania" Medicina 61, no. 11: 2042. https://doi.org/10.3390/medicina61112042
APA StylePaduraru, L., Zaha, D. C., Ghitea, T. C., Fodor, R., Vesa, C. M., & Popoviciu, M. S. (2025). Integrating Renal and Metabolic Parameters into a Derived Risk Score for Hyperuricemia in Uncontrolled Type 2 Diabetes: A Retrospective Cross-Sectional Study in Northwest Romania. Medicina, 61(11), 2042. https://doi.org/10.3390/medicina61112042









