Risk Factors for QRS-Fragmentation in Patients with STEMI Undergoing PCI
Abstract
1. Introduction
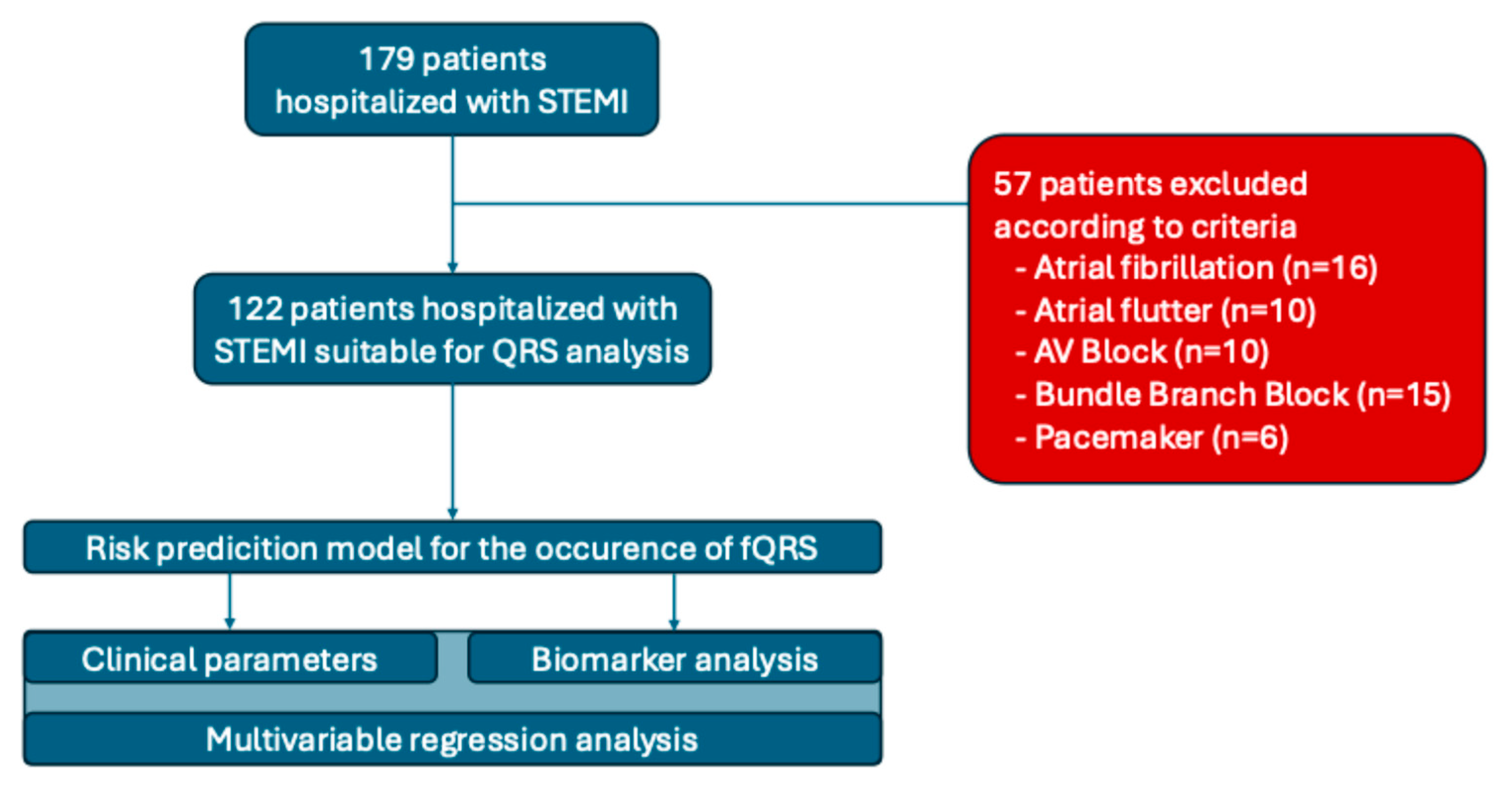
2. Materials and Methods
- -
- BIOMARKER ANALYSIS: Within the first hour of hospital admission and always before CA/PCI, venous blood samples were collected from all patients, centrifuged, and the resulting serum was aliquoted and stored at −80 °C for subsequent analysis. Serum concentrations of the biomarkers were measured using enzyme-linked immunosorbent assay (ELISA) kits, following the manufacturer’s instructions (Vektor-Best, Russia).
- -
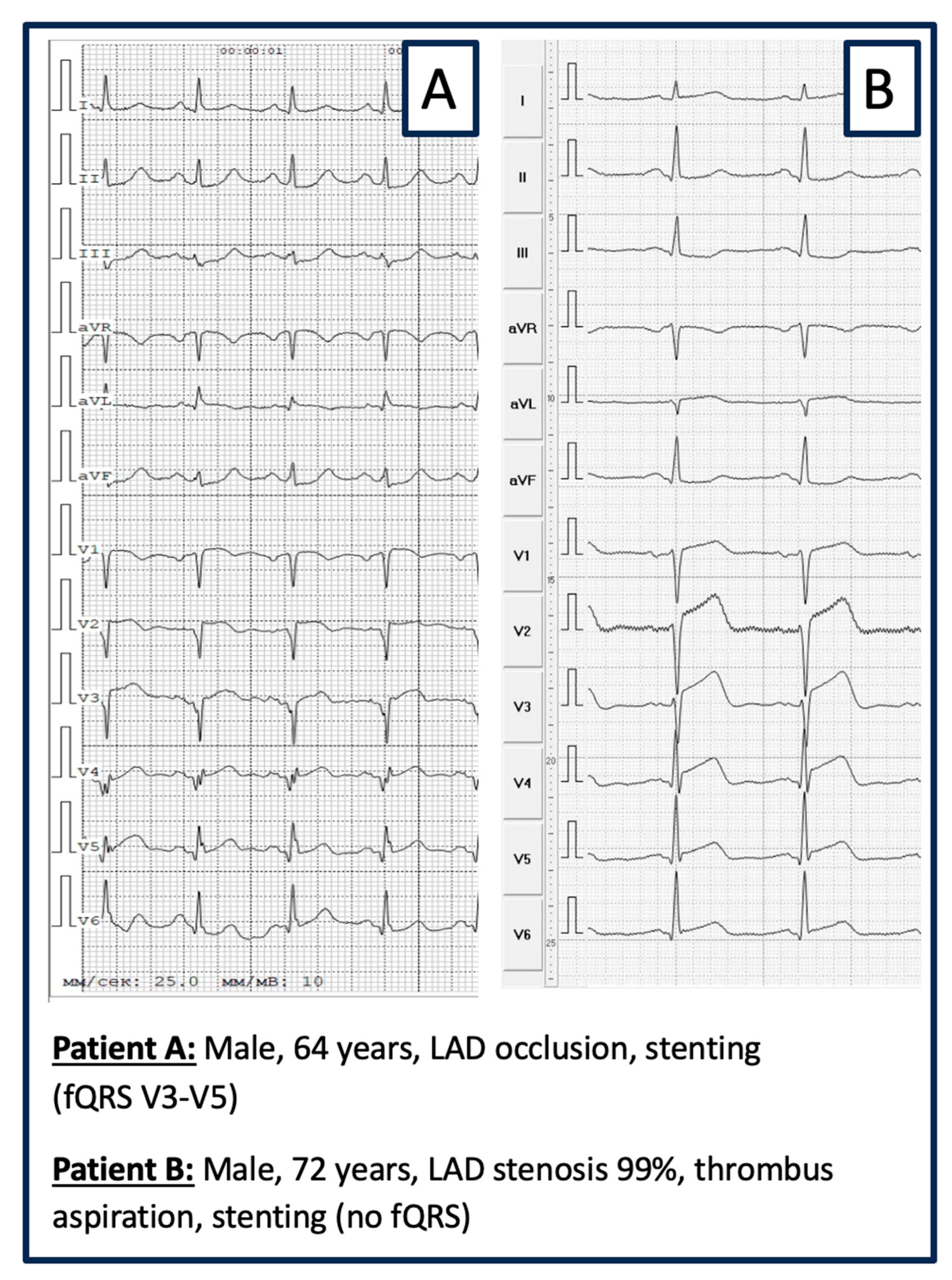
- ELECTROGRAM ANALYSIS: The ECG was recorded on ECG GE MAC 1200 ST, 12-channels: filters from 0.16 to 100 Hz; AC filter 60 Hz, ECG speed 25 mm/s, voltage 10 mm/mV. ECG evaluation regarding fQRS was performed visually by 2 experienced cardiologists blinded to the patient’s medical records and history. Inter-observer consensus regarding the presence of fQRS was mandatory. fQRS was defined as visible notching of the R- or S-wave in at least 2 contiguous leads (same coronary perfusion territory) in a routine 12-lead ECG (Figure 2) [20].
3. Results
3.1. Baseline and Procedural Characteristics of the Patient Cohort
3.2. Risk Factors for QRS Fragmentation
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| ACM | arrhythmogenic cardiomyopathy |
| ASAT | aspartate aminotransferase |
| CA | coronary angiography |
| CVD | cardiovascular disease |
| ELISA | enzyme-linked immunosorbent assay |
| ESC | European Society of Cardiology |
| fQRS | QRS fragmentation |
| GCP | Good Clinical Practice |
| GFR | glomerular filtration rate |
| HCM | hypertrophic cardiomyopathy |
| H-FABP | Heart-type Fatty Acid Binding Protein |
| MACE | major adverse cardiac events |
| MI | myocardial infarction |
| NSTEMI | non-ST elevation myocardial infarction |
| PCI | percutaneous coronary intervention |
| pPCI | primary percutaneous coronary intervention |
| sST2 | soluble ST2 |
| SLE | systemic lupus erythematosus |
| STEMI | ST-segment elevation myocardial infarction |
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021: Executive Summary. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 377–382. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Zagidullin, N.; Motloch, L.J.; Gareeva, D.; Hamitova, A.; Lakman, I.; Krioni, I.; Popov, D.; Zulkarneev, R.; Paar, V.; Kopp, K.; et al. Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients with ST-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 550. [Google Scholar] [CrossRef]
- Xia, W.; Feng, X.Y. Fragmented QRS (fQRS) Complex Predicts Adverse Cardiac Events of ST-Segment Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Intervention and Thrombolysis. Med. Sci. Monit. 2018, 24, 4634–4640. [Google Scholar] [CrossRef]
- Tanriverdi, Z.; Colluoglu, T.; Unal, B.; Dursun, H.; Kaya, D. The prognostic value of the combined use of QRS distortion and fragmented QRS in patients with acute STEMI undergoing primary percutaneous coronary intervention. J. Electrocardiol. 2018, 51, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Karacimen, D.; Ozcan, K.S.; Osmonov, D.; Turkkan, C.; Altay, S.; Ceylan, U.S.; Ugur, M.; Bozbay, M.; Erdinler, I. The relationship between fragmentation on electrocardiography and in-hospital prognosis of patients with acute myocardial infarction. Med. Sci. Monit. 2014, 20, 913–919. [Google Scholar]
- Ma, L.; Ma, S.; Lv, J.; Zhang, Y.; Liu, Z.; Bu, P.; Li, Z.; Wang, L. Fragmented QRS complex on ECG is associated with ventricular arrhythmias in patients with a prior myocardial infarction. Acta Cardiol. 2016, 71, 671–677. [Google Scholar] [CrossRef]
- Redfors, B.; Kosmidou, I.; Crowley, A.; Maehara, A.; Ben-Yehuda, O.; Arif, A.; Dizon, J.M.; Mintz, G.S.; Stone, G.W. Prognostic significance of QRS fragmentation and correlation with infarct size in patients with anterior ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: Insights from the INFUSE-AMI trial. Int. J. Cardiol. 2018, 253, 20–24. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Mekraksakit, P.; Chongsathidkiet, P.; Riangwiwat, T.; Kanjanahattakij, N.; Vutthikraivit, W.; Klomjit, S.; Thavaraputta, S. Baseline fragmented QRS is associated with increased all-cause mortality in heart failure with reduced ejection fraction: A systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2019, 24, e12597. [Google Scholar] [CrossRef]
- Tanriverdi, Z.; Dursun, H.; Colluoglu, T.; Kaya, D. Single Derivation Fragmented QRS Can Predict Poor Prognosis in Successfully Revascularized Acute STEMI Patients. Arq. Bras. Cardiol. 2017, 109, 213–221. [Google Scholar] [CrossRef]
- Umapathy, S.; Yadav, R.; Goswami, K.C.; Karthikeyan, G.; Parakh, N.; Bahl, V.K. Prognostic significance of fragmented QRS in patients with ST-elevation myocardial infarction undergoing revascularization. Indian Heart J. 2018, 70 (Suppl. S3), S126–S132. [Google Scholar] [CrossRef]
- Villa, A.; Vandenberk, B.; Kentta, T.; Ingelaere, S.; Huikuri, H.V.; Zabel, M.; Friede, T.; Sticherling, C.; Tuinenburg, A.; Malik, M.; et al. A machine learning algorithm for electrocardiographic fQRS quantification validated on multi-center data. Sci. Rep. 2022, 12, 6783. [Google Scholar] [CrossRef]
- Kurtul, A.; Duran, M. Fragmented QRS complex predicts contrast-induced nephropathy and in-hospital mortality after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin. Cardiol. 2017, 40, 235–242. [Google Scholar] [CrossRef]
- Kadi, H.; Inanir, A.; Habiboglu, A.; Ceyhan, K.; Koc, F.; Celik, A.; Onalan, O.; Arslan, S. Frequency of fragmented QRS on ECG is increased in patients with rheumatoid arthritis without cardiovascular disease: A pilot study. Mod. Rheumatol. 2012, 22, 238–242. [Google Scholar] [CrossRef]
- Erdem, F.H.; Tavil, Y.; Yazici, H.; Aygul, N.; Abaci, A.; Boyaci, B. Association of fragmented QRS complex with myocardial reperfusion in acute ST-elevated myocardial infarction. Ann. Noninvasive Electrocardiol. 2013, 18, 69–74. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on Behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Das, M.K.; Khan, B.; Jacob, S.; Kumar, A.; Mahenthiran, J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006, 113, 2495–2501. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Das, M.K.; Saha, C.; El Masry, H.; Peng, J.; Dandamudi, G.; Mahenthiran, J.; McHenry, P.; Zipes, D.P. Fragmented QRS on a 12-lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007, 4, 1385–1392. [Google Scholar] [CrossRef]
- Luo, G.; Li, Q.; Duan, J.; Peng, Y.; Zhang, Z. The Predictive Value of Fragmented QRS for Cardiovascular Events in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Kanjanahattakij, N.; Rattanawong, P.; Riangwiwat, T.; Prasitlumkum, N.; Limpruttidham, N.; Chongsathidkiet, P.; Vutthikraivit, W.; Crossey, E. Fragmented QRS and mortality in patients undergoing percutaneous intervention for ST-elevation myocardial infarction: Systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2018, 23, e12567. [Google Scholar] [CrossRef]
- Turkmen, S.; Bozkurt, M.; Hosoglu, Y.; Gol, M. Significance of fragmented QRS and predictors of outcome in ST-elevation myocardial infarction. J. Res. Med. Sci. 2024, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Haukilahti, M.A.; Eranti, A.; Kentta, T.; Huikuri, H.V. QRS Fragmentation Patterns Representing Myocardial Scar Need to Be Separated from Benign Normal Variants: Hypotheses and Proposal for Morphology Based Classification. Front. Physiol. 2016, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Kurdi, M.I.; Al Aseri, Z.; Suriya, M.O. CRP levels are higher in patients with ST elevation than non-ST elevation acute coronary syndrome. Arq. Bras. Cardiol. 2011, 96, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Milwidsky, A.; Ziv-Baran, T.; Letourneau-Shesaf, S.; Keren, G.; Taieb, P.; Berliner, S.; Shacham, Y. CRP velocity and short-term mortality in ST segment elevation myocardial infarction. Biomarkers 2017, 22, 383–386. [Google Scholar] [CrossRef]
- Stumpf, C.; Sheriff, A.; Zimmermann, S.; Schaefauer, L.; Schlundt, C.; Raaz, D.; Garlichs, C.D.; Achenbach, S. C-reactive protein levels predict systolic heart failure and outcome in patients with first ST-elevation myocardial infarction treated with coronary angioplasty. Arch. Med. Sci. 2017, 13, 1086–1093. [Google Scholar] [CrossRef]
- Tanveer, S.; Banu, S.; Jabir, N.R.; Khan, M.S.; Ashraf, G.M.; Manjunath, N.C.; Tabrez, S. Clinical and angiographic correlation of high-sensitivity C-reactive protein with acute ST elevation myocardial infarction. Exp. Ther. Med. 2016, 12, 4089–4098. [Google Scholar] [CrossRef]
- Karadeniz, M.; Duran, M.; Akyel, A.; Yarlioglues, M.; Ocek, A.H.; Celik, I.E.; Kilic, A.; Yalcin, A.A.; Ergun, G.; Murat, S.N. High Sensitive CRP Level Is Associated with Intermediate and High Syntax Score in Patients with Acute Coronary Syndrome. Int. Heart J. 2015, 56, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Wohlford, G.F.; Del Buono, M.G.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Kadariya, D.; Trankle, C.R.; Biondi-Zoccai, G.; Lipinski, M.J.; et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: Results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 503–510. [Google Scholar] [CrossRef] [PubMed]
| PARAMETER | All, M (Q1–Q3) | with fQRS, M (Q1–Q3) | Without fQRS, M (Q1–Q3) | p |
|---|---|---|---|---|
| n | 122 | 41 | 81 | |
| Men (%) | 83 (68.0) | 33 (80.5) | 50 (61.7) | 0.036 |
| Women (%) | 39 (32.0) | 8 (19.5) | 31 (38.3) | 0.036 |
| Age, years | 63 (56–70) | 62 (57–69) | 63 (56–71) | 0.952 |
| Height, cm | 170 (166–174) | 170 (168–176) | 170 (165–173) | 0.093 |
| Weight, kg | 78 (71.3–88.0) | 78 (72–86) | 79 (71–88) | 0.92 |
| BMI, kg/m2 | 26.9 (24.3–30.4) | 27.0 (24.0–29.1) | 26.8 (24.4–30.8) | 0.318 |
| Pulse, beat/min | 74 (65–85) | 74 (66–80) | 72 (65–86) | 0.899 |
| SBP, mm Hg | 130 (111–140) | 130 (100–140) | 130 (119–140) | 0.344 |
| DBP, mm Hg | 80 (70–90) | 80 (64–86) | 80 (70–90) | 0.259 |
| Glucose, mmol/L | 6 (4.6–7.7) | 5.1 (4.2–7.1) | 6.3 (4.9–9.2) | 0.894 |
| CRP, mg/L | 1 (0.8–1.2) | 0.9 (0.8–1.1) | 1.1 (1.0–1.3) | 0.024 |
| Creatinine, mg/dL | 87.5 (76.0–108.5) | 85.5 (75.8–98.5) | 89 (76–128) | 0.030 |
| GFR, mL/min/m2 | 60 (47.3–73.3) | 60.5 (50.8–71.8) | 60.0 (40.0–74.0) | 0.049 |
| Urea, mmol/L | 6.0 (4.6–7.7) | 5.1 (4.2–7.1) | 5.5 (4.9–9.2) | 0.020 |
| ASAT, mmol/L | 70.0 (44.0–120.0) | 68 (44–116) | 84 (56–144) | 0.281 |
| ALAT, mmol/L | 42 (28.0–61.0) | 44.5 (29.0–56.5) | 38 (28.0–85.3) | 0.243 |
| Troponin, ng/mL | 1200 (568–2657) | 1200 (478–2696) | 993 (699–2525) | 0.202 |
| LDH, mmol/L | 619.5 (538.3–1026.8) | 596.0 (518.5–916.3) | 769.0 (567.0–1200.0) | 0.249 |
| PARAMETER | All | with fQRS | Without fQRS | p |
|---|---|---|---|---|
| DM2, n (%) | 31 (25.4) | 10 (24.4) | 21 (25.92) | 0.854 |
| CKD, n (%) | 22 (18.0) | 9 (22.0) | 13 (16.05) | 0.424 |
| AH, n (%) | 119 (97.5) | 40 (97.6) | 78 (96.30) | 0.711 |
| Prior Stroke, n (%) | 10 (8.2) | 4 (9.8) | 6 (7.41) | 0.656 |
| Dyslipidaemia, n (%) | 122 (100) | 41 (100) | 80 (100) | 0.475 |
| AF, n (%) | 14 (11.5) | 4 (9.8) | 9 (11.1) | 0.819 |
| Prior MI, n (%) | 20 (16.4) | 7 (17.1) | 13 (16.1) | 0.886 |
| PARAMETER | All, M (Q1–Q3) | with fQRS, M (Q1–Q3) | Without fQRS, M (Q1–Q3) | p |
|---|---|---|---|---|
| LVEF, % | 54 (48.3–59.8) | 55 (48.0–58.0) | 54.5 (49.8–60.0) | 0.332 |
| FS EF % | 28 (25–32) | 29 (24–32) | 28 (25–32) | 0.478 |
| EDV, mm | 4.8 (4.6–5.1) | 5.1 (4.6–5.1) | 4.8 (4.6–5.0) | 0.369 |
| ESV, mm | 3.5 (3.2–3.7) | 3.5 (3.3–4.0) | 3.5 (3.2–3.7) | 0.138 |
| IVS, mm | 1.1 (1.0–1.2) | 1.1 (1.0–1. 2) | 1.2 (1.0–1.2) | 0.185 |
| Pulmonary pressure, m | 35 (28–45) | 32 (29–41) | 35 (28–47) | 0.645 |
| PARAMETER | Whole Cohort | with fQRS | Without fQRS | p |
|---|---|---|---|---|
| Thrombolysis, n (%) | 7 (5.7) | 2 (3.7) | 5 (6.2) | 0.772 |
| CAG | 120 (98.4) | 39 (95.1) | 80 (98.8) | 0.220 |
| LAD, n (%) | 79 (64.8) | 26 (63.4) | 52 (64.2) | 0.933 |
| RCA, n (%) | 47 (38.5) | 16 (39.0) | 31 (38.3) | 0.936 |
| CX, n (%) | 14 (11.5) | 3 (7.3) | 11 (13.6) | 0.306 |
| Stenting, n (%) | 83 (68.0) | 27 (65.9) | 55 (67.9) | 0.820 |
| Onset-to-door, min | 120 (60; 240) | 60 (60; 1080) | 0.082 | |
| Door-to-ballon, min | 35 (30; 50) | 30 (20; 40) | 0.069 | |
| Anterior wall MI | 41 (33.6%) | 16 (39.0%) | 25 (30.9%) | 0.368 |
| Posterior wall MI | 81 (66.4%) | 25 (61.0%) | 56 (69.1%) | 0.854 |
| TIMI, 0 | 84 (68.9%) | 31 (75.6%) | 53 (65.4%) | 0.654 |
| 1 | 35 (28.7%) | 9 (21.9%) | 26 (32.1%) | |
| 2 | 2 (1.6%) | 0 | 2 (2.5%) | |
| 3 | 1 (0.8%) | 1 (2.5%) | 0 | |
| Killip, I | 85 (69.7%) | 26 (63.4%) | 59 (72.8%) | 0.465 |
| III | 20 (16.4%) | 9 (22.0%) | 11 (13.6%) | |
| IV | 17(13.9%) | 6 (14.6%) | 11(13.6%) |
| Variable | Coefficient ± SE | p-Level |
|---|---|---|
| Glucose | −0.0015 ± 0.0033 | 0.655 |
| NT-proBNP | 0.0062 ± 0.0047 | 0.195 |
| CRP | 1.4190 ± 0.5794 | 0.014 * |
| Creatinine | 0.0088 ± 0.0047 | 0.058 |
| GFR | −0.0182 ± 0.0091 | 0.050 * |
| Urea | −0.0014 ± 0.0032 | 0.654 |
| ASAT | 0.0013 ± 0.0015 | 0.400 |
| Onset-to-door time (min) | 0.0031 ± 0.0015 | 0.035 * |
| Door-ballon time (min) | 0.0165 ± 0.0088 | 0.061 |
| MI location | 0.3602 ± 0.4004 | 0.368 |
| TIMI | 1 −0.6012 ± 0.4651 | 0.203 |
| 2 −15.02 ± 1455.40 | 0.99 | |
| 3 16.11 ± 1455.40 | 0.99 | |
| Killip | III 0.2007 ± 0.45 | 0.655 |
| III 0.6188 ± 0.50 | 0.223 | |
| IV 0.2133 ± 0.56 | 0.703 |
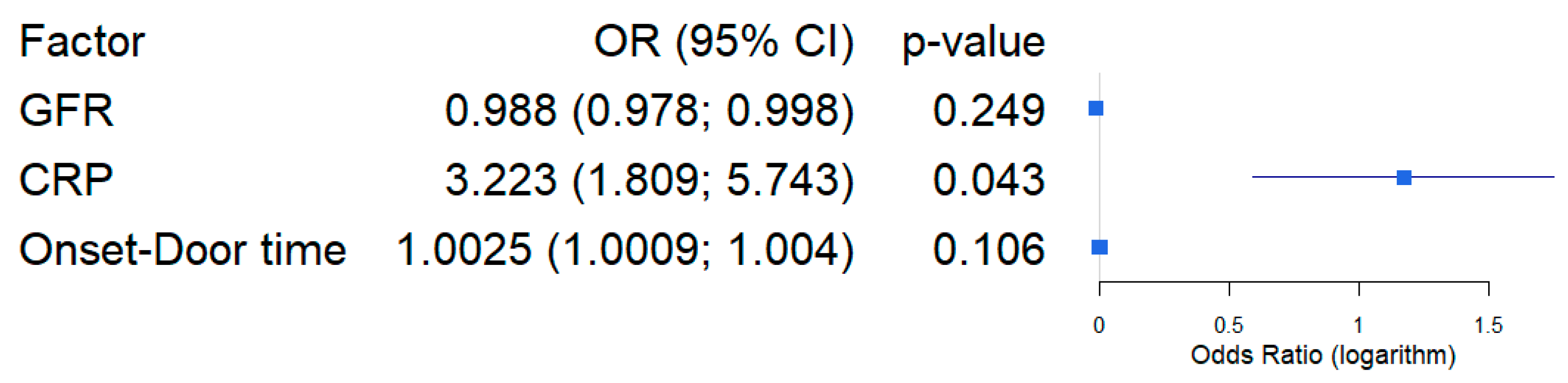
| Variable | Coefficient ± SE | OR, CI95% | p-Level |
|---|---|---|---|
| Intercept | −1.5744 ± 0.9773 | - | 0.107 |
| GFR | −0.0117 ± 0.0101 | 0.988 (0.978; 0.998) | 0.249 |
| CRP | 1.1704 ± 0.5777 | 3.223 (1.809; 5.743) | 0.043 * |
| Onset-Door time | 1.0025 ± 0.0015 | 1.0025 (1.0009; 1.004) | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinhofer, F.; Rakhimova, R.; Badykova, E.A.; Fiedler, L.; Semo, D.; Kaufmann, C.C.; Lakman, I.A.; Agletdinov, E.F.; Grishaev, D.M.; Cheremisina, K.A.; et al. Risk Factors for QRS-Fragmentation in Patients with STEMI Undergoing PCI. Medicina 2025, 61, 2023. https://doi.org/10.3390/medicina61112023
Tinhofer F, Rakhimova R, Badykova EA, Fiedler L, Semo D, Kaufmann CC, Lakman IA, Agletdinov EF, Grishaev DM, Cheremisina KA, et al. Risk Factors for QRS-Fragmentation in Patients with STEMI Undergoing PCI. Medicina. 2025; 61(11):2023. https://doi.org/10.3390/medicina61112023
Chicago/Turabian StyleTinhofer, Florian, Rosana Rakhimova, Elena A. Badykova, Lukas Fiedler, Dilvin Semo, Christoph C. Kaufmann, Irina A. Lakman, Eduard F. Agletdinov, Dimitry M. Grishaev, Ksenia A. Cheremisina, and et al. 2025. "Risk Factors for QRS-Fragmentation in Patients with STEMI Undergoing PCI" Medicina 61, no. 11: 2023. https://doi.org/10.3390/medicina61112023
APA StyleTinhofer, F., Rakhimova, R., Badykova, E. A., Fiedler, L., Semo, D., Kaufmann, C. C., Lakman, I. A., Agletdinov, E. F., Grishaev, D. M., Cheremisina, K. A., Baraboshkina, A. V., Motloch, L. J., Pistulli, R., & Zagidullin, N. S. (2025). Risk Factors for QRS-Fragmentation in Patients with STEMI Undergoing PCI. Medicina, 61(11), 2023. https://doi.org/10.3390/medicina61112023





