Coronary Intravascular Imaging: A Comprehensive Review of Techniques, Applications, and Future Directions
Abstract
1. Introduction
2. Intravascular Imaging Technology
2.1. IVUS Technology
2.2. OCT Technology
3. Intravascular Imaging Application in Different Clinical Scenarios
3.1. Chronic Coronary Syndrome
3.2. Left Main Coronary Artery Lesion Assessment and PCI Guidance
3.3. Acute Coronary Syndrome
3.4. Chronic Total Occlusion
4. Intravascular Imaging Used to Define Vulnerable Plaque
5. The Role of Intravascular Imaging in Percutaneous Coronary Intervention: Stent Sizing, Optimization, Complication, and Clinical Outcomes
5.1. Optimal Stent Landing Zones and Lesion Preparation
5.2. Stent Sizing and Optimal Expansion
5.3. Stent Malapposition and Edge Dissections: Clinical Implications
5.4. Intravascular Imaging in Stent Failure: In-Stent Restenosis and Stent Thrombosis
- Homogeneous: a uniform, high-signal-intensity pattern with minimal backscatter, typically reflecting neointimal tissue rich in smooth muscle cells;
- Heterogeneous: a mixed-signal-intensity appearance, suggestive of proteoglycan-rich neointima or early neoatherosclerotic changes;
- Attenuated: a superficial, high-intensity signal with marked backscatter, most consistent with lipid-laden neoatherosclerotic plaque;
- Layered: a configuration most often characterized by a superficial high-intensity band overlying a deeper low-intensity layer, frequently localized around stent struts.
6. Novel Technologies
6.1. NIRS
6.2. NIRF
6.3. NIRAF
6.4. IVPA
6.5. FLIm
6.6. Artificial Intelligence Integrated Software Analysis
7. Barriers and Facilitators to Clinical Adoption
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| ADE | Automated Differential Echogenicity |
| AHA | American Heart Association |
| ALA | Automated Lesion Assessment |
| AI | Artificial Intelligence |
| BMS | Bare-Metal Stent |
| CCS | Chronic Coronary Syndrome |
| CKD | Chronic Kidney Disease |
| CART | Controlled Antegrade and Retrograde subintimal Tracking; including “reverse-CART” |
| CTO | Chronic Total Occlusion |
| DES | Drug-Eluting Stent |
| DoCE | Device-Oriented Composite Endpoint |
| EAPCI | European Association of Percutaneous Cardiovascular Interventions |
| EEL | External Elastic Lamina |
| ESC | European Society of Cardiology |
| FCT | Fibrous Cap Thickness |
| FFR | Fractional Flow Reserve |
| FLIm | Fluorescence Lifetime Imaging |
| HD-IVUS | High-Definition IVUS |
| IB-IVUS | Integrated Backscatter IVUS |
| IEL | Internal Elastic Lamina |
| iMAP-IVUS | Intravascular Ultrasound with Integrated Mapping Analysis of Plaque |
| ISR | In-Stent Restenosis |
| IVI | Intravascular Imaging |
| IVL | Intravascular Lithotripsy |
| IVPA | Intravascular Photoacoustic |
| IVUS | Intravascular Ultrasound |
| LCBI | Lipid Core Burden Index |
| LCR | Lipid-to-Cap Ratio |
| LM | Left Main |
| LMCA | Left Main Coronary Artery |
| LPSM | Late Persistent Stent Malapposition |
| LASM | Late Acquired Stent Malapposition |
| LRP | Lipid-Rich Plaque |
| MACE | Major Adverse Cardiac Events |
| MI | Myocardial Infarction |
| MLA | Minimum Lumen Area |
| MLD | Minimum Lumen Diameter |
| MSA | Minimum Stent Area |
| MINOCA | Myocardial Infarction with Non-Obstructive Coronary Arteries |
| NIRAF | Near-Infrared Autofluorescence |
| NIRF | Near-Infrared Fluorescence |
| NIRS | Near-Infrared Spectroscopy |
| OCT | Optical Coherence Tomography |
| OFDI | Optical Frequency Domain Imaging |
| OFR | Optical Flow Ratio |
| PCI | Percutaneous Coronary Intervention |
| RF | Radio Frequency |
| SCAD | Spontaneous Coronary Artery Dissection |
| ST | Stent Thrombosis |
| SVG | Saphenous Vein Graft |
| TCFA | Thin-Cap Fibroatheroma |
| TLF | Target Lesion Failure |
| TLR | Target Lesion Revascularization |
| TMV | Total Malapposition Volume |
| TVF | Target Vessel Failure |
| VH-IVUS | Virtual Histology IVUS |
References
- Kheiri, B.; Simpson, T.F.; Osman, M.; German, D.M.; Fuss, C.S.; Ferencik, M. Computed Tomography vs Invasive Coronary Angiography in Patients With Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2022, 15, 2147–2149. [Google Scholar] [CrossRef]
- Hachinohe, D.; Mitomo, S.; Candilio, L.; Latib, A. A Practical Approach to Assessing Stent Results with IVUS or OCT. Methodist DeBakey Cardiovasc. J. 2018, 14, 32–41. [Google Scholar] [CrossRef]
- Hong, S.-J.; Zhang, J.-J.; Mintz, G.S.; Ahn, C.-M.; Kim, J.-S.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; Jang, Y.; Kan, J. Improved 3-Year Cardiac Survival After IVUS–Guided Long DES Implantation: A Patient-Level Analysis From 2 Randomized Trials. Cardiovasc. Interv. 2022, 15, 208–216. [Google Scholar]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M. Clinical outcomes following intravascular imaging-guided versus coronary angiography–guided percutaneous coronary intervention with stent implantation: A systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef]
- Jones, D.A.; Rathod, K.S.; Koganti, S.; Hamshere, S.; Astroulakis, Z.; Lim, P.; Sirker, A.; O’Mahony, C.; Jain, A.K.; Knight, C.J. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: Outcomes from the Pan-London PCI Cohort. JACC Cardiovasc. Interv. 2018, 11, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Mintz, G.S. Intravascular ultrasound-guidance is associated with lower cardiovascular mortality and myocardial infarction for drug-eluting stent implantation―insights from an updated meta-analysis of randomized trials. Circ. J. 2019, 83, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Nakamura, M.; Räber, L.; Colleran, R.; Kadota, K.; Capodanno, D.; Wijns, W.; Akasaka, T.; Valgimigli, M.; Guagliumi, G.; et al. Current use of intracoronary imaging in interventional practice-Results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, e475–e484. [Google Scholar] [CrossRef]
- Sung, J.-H.; Chang, J.-H. Mechanically Rotating Intravascular Ultrasound (IVUS) Transducer: A Review. Sensors 2021, 21, 3907. [Google Scholar] [CrossRef]
- Low, A.F.; Kawase, Y.; Chan, Y.-H.; Tearney, G.J.; Bouma, B.E.; Jang, I.-K. In vivo characterisation of coronary plaques with conventional grey-scale intravascular ultrasound: Correlation with optical coherence tomography. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2009, 4, 626. [Google Scholar] [CrossRef]
- van Veelen, A.; van der Sangen, N.M.; Henriques, J.P.; Claessen, B.E. Identification and treatment of the vulnerable coronary plaque. Rev. Cardiovasc. Med. 2022, 23, 39. [Google Scholar] [CrossRef]
- Xu, J.; Lo, S. Fundamentals and role of intravascular ultrasound in percutaneous coronary intervention. Cardiovasc. Diagn. Ther. 2020, 10, 1358. [Google Scholar] [CrossRef]
- Shimamura, K.; Kubo, T.; Akasaka, T. Evaluation of coronary plaques and atherosclerosis using optical coherence tomography. Expert Rev. Cardiovasc. Ther. 2021, 19, 379–386. [Google Scholar] [CrossRef]
- Roland, R.; Veselka, J. Optical Coherence Tomography of the Coronary Arteries. Int. J. Angiol. 2021, 30, 029–039. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; De Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Ozaki, Y.; Marco, V.; Boi, A.; Fineschi, M.; Fabbiocchi, F.; et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: The CLIMA study. Eur. Heart J. 2020, 41, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Biccirè, F.G.; Fabbiocchi, F.; Gatto, L.; La Manna, A.; Ozaki, Y.; Romagnoli, E.; Marco, V.; Boi, A.; Fineschi, M.; Piedimonte, G.; et al. Long-Term Prognostic Impact of OCT-Derived High-Risk Plaque Features. JACC Cardiovasc. Interv. 2025, 18, 1361–1372. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.-M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef]
- Vizzari, G.; Caminiti, R.; Ielasi, A.; Vetta, G.; Parlavecchio, A.; Mazzone, P.; Sacchetta, G.; Magnocavallo, M.; Della Rocca, D.G.; Siviglia, M.; et al. Contrast-enhanced excimer laser stepwise approach during PCI for resistant coronary lesions. Catheter. Cardiovasc. Interv. 2024, 104, 220–226. [Google Scholar] [CrossRef]
- Caminiti, R.; Vetta, G.; Parlavecchio, A.; Ielasi, A.; Magnocavallo, M.; Della Rocca, D.G.; Cerrato, E.; Carerj, S.; Di Bella, G.; Micari, A.; et al. A Systematic Review and Meta-Analysis Including 354 Patients from 13 Studies of Intravascular Lithotripsy for the Treatment of Underexpanded Coronary Stents. Am. J. Cardiol. 2023, 205, 223–230. [Google Scholar] [CrossRef]
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
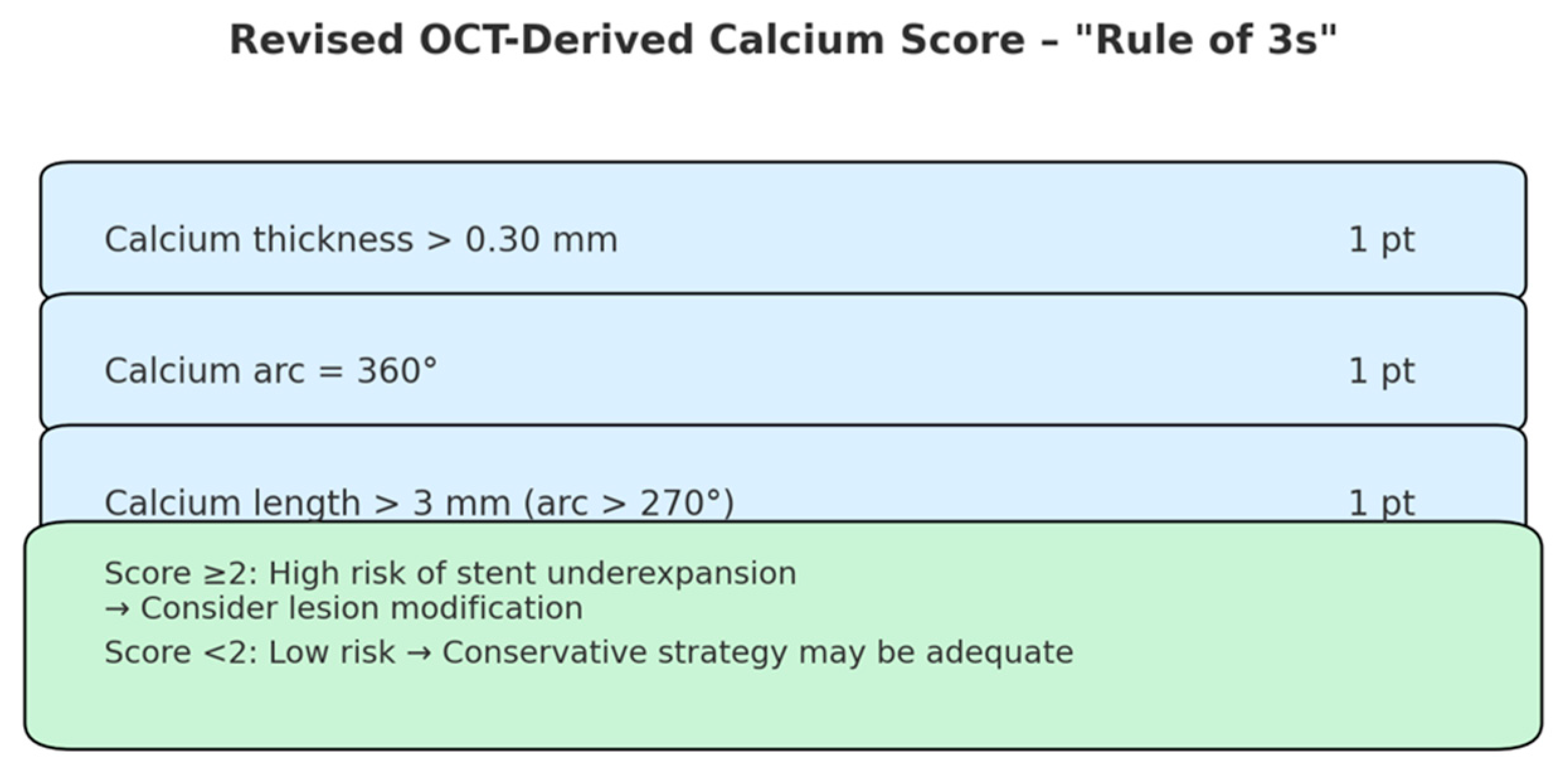
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.-Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018, 13, 2182–2189. [Google Scholar] [CrossRef]
- Sato, T.; Matsumura, M.; Yamamoto, K.; Sugizaki, Y.; Shlofmitz, E.; Moses, J.W.; Khalique, O.K.; Thomas, S.V.; Malik, S.; Dakroub, A.; et al. A Revised Optical Coherence Tomography–Derived Calcium Score to Predict Stent Underexpansion in Severely Calcified Lesions. JACC Cardiovasc. Interv. 2025, 18, 622–633. [Google Scholar] [CrossRef]
- Torres-Ruiz, G.; Mallofré-Vila, N.; Rojas-Flores, P.; Carrión-Montaner, P.; Bosch-Peligero, E.; Valcárcel-Paz, D.; Cardiel-Perez, A.; Guindo-Soldevila, J.; Martínez-Rubio, A. Evidence-based Management of Left Main Coronary Artery Disease. Eur. Cardiol. Rev. 2023, 18, e63. [Google Scholar] [CrossRef]
- Waksman, R.; Legutko, J.; Singh, J.; Orlando, Q.; Marso, S.; Schloss, T.; Tugaoen, J.; DeVries, J.; Palmer, N.; Haude, M.; et al. FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study. J. Am. Coll. Cardiol. 2013, 61, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, D.-W.; Kim, W.-J.; Lee, J.-Y.; Yun, S.-C.; Kang, S.-J.; Lee, S.-W.; Lee, C.W.; Hong, M.-K.; Park, S.-W.; et al. Impact of the Extent of Coronary Artery Disease on Outcomes After Revascularization for Unprotected Left Main Coronary Artery Stenosis. J. Am. Coll. Cardiol. 2010, 55, 2544–2552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Gao, X.; Kan, J.; Ge, Z.; Han, L.; Lu, S.; Tian, N.; Lin, S.; Lu, Q.; Wu, X.; et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. J. Am. Coll. Cardiol. 2018, 72, 3126–3137. [Google Scholar] [CrossRef]
- de la Torre Hernandez, J.M.; Hernández Hernandez, F.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Ruiz Nodar, J.M.; et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, J.M.; Kang, S.J.; Yoon, S.H.; Koo, B.K.; Lee, J.Y.; Kim, W.J.; Park, D.W.; Lee, S.W.; Kim, Y.H.; et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left main coronary artery stenosis. JACC Cardiovasc. Interv. 2014, 7, 868–874. [Google Scholar] [CrossRef]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of culprit lesion morphology in acute myocardial infarction: Ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef]
- Chamié, D.; Bezerra, H.G.; Attizzani, G.F.; Yamamoto, H.; Kanaya, T.; Stefano, G.T.; Fujino, Y.; Mehanna, E.; Wang, W.; Abdul-Aziz, A.; et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc. Interv. 2013, 6, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Groenland, F.T.W.; Mahmoud, K.D.; Neleman, T.; Plantes, A.C.Z.D.; Scoccia, A.; Ligthart, J.; Witberg, K.T.; Nuis, R.-J.; Dekker, W.K.D.; Wilschut, J.M.; et al. Tissue characterisation and primary percutaneous coronary intervention guidance using intravascular ultrasound: Rationale and design of the SPECTRUM study. Open Heart 2022, 9, e001955. [Google Scholar] [CrossRef]
- Mintz, G.S.; Nissen, S.E.; Anderson, W.D.; Bailey, S.R.; Erbel, R.; Fitzgerald, P.J.; Pinto, F.J.; Rosenfield, K.; Siegel, R.J.; Tuzcu, E.M.; et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001, 37, 1478–1492. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Mancini, G.B.J.; Humphries, K.; Fung, A.; Boone, R.; Starovoytov, A.; Aymong, E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2016, 87, E54–E61. [Google Scholar] [CrossRef]
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C. European Society of Cardiology, acute cardiovascular care association, SCAD study group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar] [CrossRef]
- Galassi, A.R.; Sumitsuji, S.; Boukhris, M.; Brilakis, E.S.; Di Mario, C.; Garbo, R.; Spratt, J.C.; Christiansen, E.H.; Gagnor, A.; Avran, A.; et al. Utility of Intravascular Ultrasound in Percutaneous Revascularization of Chronic Total Occlusions: An Overview. JACC Cardiovasc. Interv. 2016, 9, 1979–1991. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Tajti, P.; Karmpaliotis, D.; Garbo, R.; Gagnor, A.; Burke, M.N.; Brilakis, E.S. Intravascular Imaging for Chronic Total Occlusion Intervention. Curr. Cardiovasc. Imaging Rep. 2018, 11, 31. [Google Scholar] [CrossRef]
- Blessing, R.; Buono, A.; Ahoopai, M.; Geyer, M.; Knorr, M.; Brandt, M.; Steven, S.; Drosos, I.; Muenzel, T.; Wenzel, P.; et al. Use of intravascular ultrasound for optimal vessel sizing in chronic total occlusion percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 9, 922366. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Pavlidis, A.N.; Kaier, T.E.; Rigopoulos, A.G.; Karamasis, G.V.; Triantafyllis, A.S.; Vardas, P.; Brilakis, E.S.; Kalogeropoulos, A.S. The role of intravascular imaging in chronic total occlusion percutaneous coronary intervention. Front. Cardiovasc. Med. 2023, 10, 1199067. [Google Scholar] [CrossRef]
- Sherbet, D.P.; Christopoulos, G.; Karatasakis, A.; Danek, B.A.; Kotsia, A.; Navara, R.; Michael, T.T.; Roesle, M.; Rangan, B.V.; Haagen, D.; et al. Optical coherence tomography findings after chronic total occlusion interventions: Insights from the “AngiographiC evaluation of the everolimus-eluting stent in chronic Total occlusions” (ACE-CTO) study (NCT01012869). Cardiovasc. Revasc. Med. 2016, 17, 444–449. [Google Scholar] [CrossRef]
- Legutko, J.; Bryniarski, K.L.; Kaluza, G.L.; Roleder, T.; Pociask, E.; Kedhi, E.; Wojakowski, W.; Jang, I.-K.; Kleczynski, P. Intracoronary Imaging of Vulnerable Plaque—From Clinical Research to Everyday Practice. J. Clin. Med. 2022, 11, 6639. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.M.; Davies, M.J. Vulnerable Plaque: Relation of Characteristics to Degree of Stenosis in Human Coronary Arteries. Circulation 1996, 94, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. The Vulnerable Plaque: The Real Villain in Acute Coronary Syndromes. Open Cardiovasc. Med. J. 2011, 5, 123–129. [Google Scholar] [CrossRef]
- Apostolos, A.; Karanasos, A.; Ktenopoulos, N.; Tsalamandris, S.; Vlachakis, P.K.; Kachrimanidis, I.; Skalidis, I.; Sagris, M.; Koliastasis, L.; Drakopoulou, M.; et al. Unlocking the Secrets of Acute Coronary Syndromes Using Intravascular Imaging: From Pathophysiology to Improving Outcomes. J. Clin. Med. 2024, 13, 7087. [Google Scholar] [CrossRef]
- Rai, H.; Cassese, S.; Joner, M. Optical coherence tomography revisited: Imaging and imagination. EuroIntervention 2018, 14, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.-K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; Ijsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT–FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef]
- Vergallo, R.; Park, S.-J.; Stone, G.W.; Erlinge, D.; Porto, I.; Waksman, R.; Mintz, G.S.; D’aScenzo, F.; Seitun, S.; Saba, L.; et al. Vulnerable or High-Risk Plaque. A JACC: Cardiovascular Imaging Position Statement. JACC Cardiovasc. Imaging 2025, 18, 709–740. [Google Scholar] [CrossRef]
- Vergallo, R.; Ren, X.; Yonetsu, T.; Kato, K.; Uemura, S.; Yu, B.; Jia, H.; Abtahian, F.; Aguirre, A.D.; Tian, J.; et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: A 3-vessel optical coherence tomography study. Am. Heart J. 2014, 167, 59–67. [Google Scholar] [CrossRef]
- Biccirè, F.G.; Musto, C.; Limbruno, U.; Fabbiocchi, F.; Turturo, M.; Boi, A.; Cassano, F.; Calligaris, G.; Benenati, S.; Budassi, S.; et al. Rates of Percutaneous Coronary Revascularization in Morphological- vs. Functional-Guided Arms of the INTERCLIMA (Interventional Strategy for Non-Culprit Lesions With Major Vulnerability Criteria Identified by OCT in Patients With ACS) Randomized Controlled Trial: Preliminary Data. J. Am. Coll. Cardiol. 2022, 80, B115. [Google Scholar] [CrossRef]
- Hong, H.; Jia, H.; Zeng, M.; Gutiérrez-Chico, J.L.; Wang, Y.; Zeng, X.; Qin, Y.; Zhao, C.; Chu, M.; Huang, J.; et al. Risk Stratification in Acute Coronary Syndrome by Comprehensive Morphofunctional Assessment With Optical Coherence Tomography. JACC Asia 2022, 2, 460–472. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, Y.-H.; Park, D.-W.; Lee, S.-W.; Kim, W.-J.; Suh, J.; Yun, S.-C.; Lee, C.W.; Hong, M.-K.; Lee, J.-H.; et al. Impact of Intravascular Ultrasound Guidance on Long-Term Mortality in Stenting for Unprotected Left Main Coronary Artery Stenosis. Circ. Cardiovasc. Interv. 2009, 2, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-F.; Ge, Z.; Kong, X.-Q.; Kan, J.; Han, L.; Lu, S.; Tian, N.-L.; Lin, S.; Lu, Q.-H.; Wang, X.-Y.; et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Chamié, D.; Costa, J.R.; Damiani, L.P.; Siqueira, D.; Braga, S.; Costa, R.; Seligman, H.; Brito, F.; Barreto, G.; Staico, R.; et al. Optical Coherence Tomography Versus Intravascular Ultrasound and Angiography to Guide Percutaneous Coronary Interventions: The iSIGHT Randomized Trial. Circ. Cardiovasc. Interv. 2021, 14, e009452. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; La Manna, A.; Burzotta, F.; Gatto, L.; Marco, V.; Fineschi, M.; Fabbiocchi, F.; Versaci, F.; Trani, C.; et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention: The Centro Per La Lotta Contro L’infarto–Optimization Of Percutaneous Coronary Intervention (CLI-OPCI) LATE study. EuroIntervention 2018, 14, e443–e451. [Google Scholar] [CrossRef]
- Prati, F.; Romagnoli, E.; Burzotta, F.; Limbruno, U.; Gatto, L.; La Manna, A.; Versaci, F.; Marco, V.; Di Vito, L.; Imola, F. Clinical impact of OCT findings during PCI: The CLI-OPCI II study. Cardiovasc. Imaging 2015, 8, 1297–1305. [Google Scholar]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Shin, D.; Sakai, K.; Matsumura, M.; Shlofmitz, R.A.; Leistner, D.; Canova, P.; Alfonso, F.; et al. OCT-Guided vs Angiography-Guided Coronary Stent Implantation in Complex Lesions. J. Am. Coll. Cardiol. 2024, 84, 368–378. [Google Scholar] [CrossRef]
- Holm, N.R.; Andreasen, L.N.; Neghabat, O.; Laanmets, P.; Kumsars, I.; Bennett, J.; Olsen, N.T.; Odenstedt, J.; Hoffmann, P.; Dens, J.; et al. OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N. Engl. J. Med. 2023, 389, 1477–1487. [Google Scholar] [CrossRef]
- Cortese, B.; Hernandez, J.M.d.l.T.; Lanocha, M.; Ielasi, A.; Giannini, F.; Campo, G.; D’AScenzo, F.; Latini, R.A.; Krestianinov, O.; Alfonso, F.; et al. Optical coherence tomography, intravascular ultrasound or angiography guidance for distal left main coronary stenting: The ROCK cohort II study. Catheter. Cardiovasc. Interv. 2022, 99, 664–673. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Maehara, A.; Cristea, E.; Witzenbichler, B.; Guagliumi, G.; Brodie, B.; Kellett, M.A.; Dressler, O.; Lansky, A.J.; Parise, H.; et al. Usefulness of minimum stent cross sectional area as a predictor of angiographic restenosis after primary percutaneous coronary intervention in acute myocardial infarction (from the HORIZONS-AMI Trial IVUS substudy). Am. J. Cardiol. 2012, 109, 455–460. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Hill, J.M.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results From the Disrupt CAD III Study. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Calvert, P.A.; Brown, A.J.; Hoole, S.P.; Obaid, D.R.; West, N.E.; Bennett, M.R. Geographical miss is associated with vulnerable plaque and increased major adverse cardiovascular events in patients with myocardial infarction. Catheter. Cardiovasc. Interv. 2016, 88, 340–347. [Google Scholar] [CrossRef]
- Mintz, G.S.; Guagliumi, G. Intravascular imaging in coronary artery disease. Lancet 2017, 390, 793–809. [Google Scholar] [CrossRef]
- Mintz, G.S. Intravascular ultrasound and outcomes after drug-eluting stent implantation. Coron. Artery Dis. 2017, 28, 346–352. [Google Scholar] [CrossRef]
- Imola, F.; Occhipinti, M.; Biondi-Zoccai, G.; Di Vito, L.; Ramazzotti, V.; Manzoli, A.; Pappalardo, A.; Cremonesi, A.; Albertucci, M.; Prati, F. Association between proximal stent edge positioning on atherosclerotic plaques containing lipid pools and postprocedural myocardial infarction (from the CLI-POOL Study). Am. J. Cardiol. 2013, 111, 526–531. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef]
- Kang, S.-J.; Ahn, J.-M.; Song, H.; Kim, W.-J.; Lee, J.-Y.; Park, D.-W.; Yun, S.-C.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ. Cardiovasc. Interv. 2011, 4, 562–569. [Google Scholar] [CrossRef]
- Sonoda, S.; Morino, Y.; Ako, J. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: Serial intravascular ultrasound analysis from the sirius trial. J. Am. Coll. Cardiol. 2004, 43, 1959–1963. [Google Scholar] [CrossRef]
- Song, H.; Kang, S.; Ahn, J.; Kim, W.; Lee, J.; Park, D.; Lee, S.; Kim, Y.; Lee, C.W.; Park, S.; et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter. Cardiovasc. Interv. 2014, 83, 873–878. [Google Scholar] [CrossRef]
- Kandzari, D.; Smits, P.; Ali, Z. Influence of procedural imaging and optimal stent expansion on clinical outcomes: Insights from the ADAPT-DES study. J. Am. Coll. Cardiol. 2020, 75, 1439–1442. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Bavry, A.A. Outcomes with intravascular ultrasound-guided stent implantation: A meta-analysis of randomized trials in the era of drug-eluting stents. Circ. Cardiovasc. Interv. 2016, 9, e004251. [Google Scholar] [CrossRef]
- Jang, J.-S.; Song, Y.-J.; Kang, W.; Jin, H.-Y.; Seo, J.-S.; Yang, T.-H.; Kim, D.-K.; Cho, K.-I.; Kim, B.-H.; Park, Y.H.; et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: A meta-analysis. JACC Cardiovasc. Interv. 2014, 7, 233–243. [Google Scholar] [CrossRef]
- Ahn, J.-M.; Kang, S.-J.; Yoon, S.-H.; Park, H.W.; Kang, S.M.; Lee, J.-Y.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; et al. Meta-analysis of outcomes after intravascular ultrasound–guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am. J. Cardiol. 2014, 113, 1338–1347. [Google Scholar] [CrossRef]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef]
- Doi, H.; Maehara, A.; Mintz, G.S.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.J.; Grube, E.; Dawkins, K.D.; et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: An integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc. Interv. 2009, 2, 1269–1275. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Maehara, A.; Lansky, A.J.; Guagliumi, G.; Brodie, B.; Kellett, M.A.; Dressler, O.; Parise, H.; Mehran, R.; Dangas, G.D.; et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: A Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ. Cardiovasc. Interv. 2011, 4, 239–247. [Google Scholar] [CrossRef]
- Fujii, K.; Carlier, S.; Mintz, G. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: An intravascular ultrasound study. J. Am. Coll. Cardiol. 2005, 45, 995–998. [Google Scholar] [CrossRef]
- Liu, X.; Doi, H.; Maehara, A.; Mintz, G.S.; Costa, J.d.R.; Sano, K.; Weisz, G.; Dangas, G.D.; Lansky, A.J.; Kreps, E.M.; et al. A volumetric intravascular ultrasound comparison of early drug-eluting stent thrombosis versus restenosis. JACC Cardiovasc. Interv. 2009, 2, 428–434. [Google Scholar] [CrossRef]
- Soeda, T.; Uemura, S.; Park, S.J.; Jang, Y.; Lee, S.; Cho, J.M.; Kim, S.J.; Vergallo, R.; Minami, Y.; Ong, D.S.; et al. Incidence and clinical significance of poststent optical coherence tomography findings: One-year follow-up study from a multicenter registry. Circulation 2015, 132, 1020–1029. [Google Scholar] [CrossRef]
- Hong, M.-K.; Mintz, G.S.; Lee, C.W.; Park, D.-W.; Choi, B.-R.; Park, K.-H.; Kim, Y.-H.; Cheong, S.-S.; Song, J.-K.; Kim, J.-J.; et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur. Heart J. 2006, 27, 1305–1310. [Google Scholar] [CrossRef]
- Di Vito, L.; Yoon, J.H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Costa, M.; Bezerra, H.G.; Arbustini, E.; Narula, J.; Crea, F.; et al. Comprehensive overview of definitions for optical coherence tomography-based plaque and stent analyses. Coron. Artery Dis. 2014, 25, 172–185. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, B.K.; Shin, D.H.; Nam, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kang, T.S.; Kang, W.C.; Her, A.Y.; et al. Effect of intravascular ultrasound–guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. J. Am. Med. Assoc. 2015, 314, 2155–2163. [Google Scholar] [CrossRef]
- Im, E.; Kim, B.-K.; Ko, Y.-G.; Shin, D.-H.; Kim, J.-S.; Choi, D.; Jang, Y.; Hong, M.-K. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ. Cardiovasc. Interv. 2014, 7, 88–96. [Google Scholar] [CrossRef]
- Romagnoli, E.; Gatto, L.; La Manna, A.; Burzotta, F.; Taglieri, N.; Saia, F.; Amico, F.; Marco, V.; Ramazzotti, V.; Di Giorgio, A.; et al. Role of residual acute stent malapposition in percutaneous coronary interventions. Catheter. Cardiovasc. Interv. 2017, 90, 566–575. [Google Scholar] [CrossRef]
- Prati, F.; Kodama, T.; Romagnoli, E.; Gatto, L.; Di Vito, L.; Ramazzotti, V.; Chisari, A.; Marco, V.; Cremonesi, A.; Parodi, G.; et al. Suboptimal stent deployment is associated with subacute stent thrombosis: Optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: The CLI-THRO study. Am. Heart J. 2015, 169, 249–256. [Google Scholar] [CrossRef]
- Wang, B.; Mintz, G.S.; Witzenbichler, B.; Souza, C.F.; Metzger, D.C.; Rinaldi, M.J.; Duffy, P.L.; Weisz, G.; Stuckey, T.D.; Brodie, B.R.; et al. Predictors and long-term clinical impact of acute stent malapposition: An assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) intravascular ultrasound substudy. J. Am. Heart Assoc. 2016, 5, e004438. [Google Scholar] [CrossRef]
- Kubo, T.; Akasaka, T.; Shite, J.; Suzuki, T.; Uemura, S.; Yu, B.; Kozuma, K.; Kitabata, H.; Shinke, T.; Habara, M.; et al. OCT compared with IVUS in a coronary lesion assessment: The OPUS-CLASS study. JACC Cardiovasc. Imaging 2013, 6, 1095–1104. [Google Scholar] [CrossRef]
- Guo, N.; Maehara, A.; Mintz, G.S.; He, Y.; Xu, K.; Wu, X.; Lansky, A.J.; Witzenbichler, B.; Guagliumi, G.; Brodie, B.; et al. Incidence, mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: An intravascular ultrasound substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Circulation 2010, 122, 1077–1084. [Google Scholar]
- Steinberg, D.H.; Mintz, G.S.; Mandinov, L.; Yu, A.; Ellis, S.G.; Grube, E.; Dawkins, K.D.; Ormiston, J.; Turco, M.A.; Stone, G.W.; et al. Long-term impact of routinely detected early and late incomplete stent apposition: An integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. JACC Cardiovasc. Interv. 2010, 3, 486–494. [Google Scholar] [CrossRef]
- Karalis, I.; Ahmed, T.A.; Jukema, J.W. Late acquired stent malapposition: Why, when and how to handle? Heart 2012, 98, 1529–1536. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Mintz, G.S.; Kim, J.-S.; Kim, B.-K.; Jang, Y.; Hong, M.-K. Long-term clinical outcomes of drug-eluting stent malapposition. Korean Circ. J. 2020, 50, 880–889. [Google Scholar] [CrossRef]
- Kim, B.G.; Kachel, M.; Kim, J.-S.; Guagliumi, G.; Kim, C.; Kim, I.-S.; Lee, Y.-J.; Lee, O.-H.; Byun, Y.S.; Milewski, K.; et al. Clinical Implications of Poststent Optical Coherence Tomographic Findings. JACC Cardiovasc. Imaging 2022, 15, 126–137. [Google Scholar] [CrossRef]
- Taniwaki, M.; Radu, M.D.; Zaugg, S.; Amabile, N.; Garcia-Garcia, H.M.; Yamaji, K.; Jørgensen, E.; Kelbæk, H.; Pilgrim, T.; Caussin, C.; et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 2016, 133, 650–660. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Joner, M.; Godschalk, T.C.; Malik, N.; Alfonso, F.; Xhepa, E.; Cock, D.; Komukai, K.; Tada, T.; Cuesta, J.; et al. Optical coherence tomography findings in patients with coronary stent thrombosis: A report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation 2017, 136, 1007–1021. [Google Scholar] [CrossRef]
- van Zandvoort, L.J.; Tomaniak, M.; Tovar Forero, M.N.; Masdjedi, K.; Visseren, L.; Witberg, K.; Ligthart, J.; Kardys, I.; Lemmert, M.E.; Diletti, R.; et al. Predictors for clinical outcome of untreated stent edge dissections as detected by optical coherence tomography. Circ. Cardiovasc. Interv. 2020, 13, e008685. [Google Scholar] [CrossRef]
- Prati, F.; Gatto, L.; La Manna, A.; Burzotta, F.; Limbruno, U.; Versaci, F.; Fabbiocchi, F.; Giorgio, A.D.; Marco, V.; Ramazzotti, V.; et al. Clinical impact of suboptimal stenting and residual intrastent plaque/thrombus protrusion in patients with acute coronary syndrome: The CLI-OPCI ACS Substudy (Centro per la Lotta Contro L’Infarto-Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ. Cardiovasc. Interv. 2016, 9, e003726. [Google Scholar]
- Ugo, F.; Franzino, M.; Massaro, G.; Maltese, L.; Cavallino, C.; Abdirashid, M.; Benedetto, D.; Costa, F.; Rametta, F.; Sangiorgi, G.M. The Role of IVUS in Coronary Complications. Catheter. Cardiovasc. Interv. 2025, 105, 1171–1182. [Google Scholar] [CrossRef]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Stefanini, G.G.; Mavridis, D.; Siontis, K.C.; Alfonso, F.; Pérez-Vizcayno, M.J.; Byrne, R.A.; Kastrati, A.; Meier, B.; Salanti, G.; et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: A network meta-analysis. Lancet 2015, 386, 655–664. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of drug-eluting stents: A new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef]
- Goto, K.; Zhao, Z.; Matsumura, M.; Dohi, T.; Kobayashi, N.; Kirtane, A.J.; Rabbani, L.E.; Collins, M.B.; Parikh, M.A.; Kodali, S.K.; et al. Mechanisms and patterns of intravascular ultrasound in-stent restenosis among bare metal stents and first-and second-generation drug-eluting stents. Am. J. Cardiol. 2015, 116, 1351–1357. [Google Scholar] [CrossRef]
- Song, L.; Mintz, G.; Yin, D.; Yamamoto, M.H.; Chin, C.Y.; Matsumura, M.; Kirtane, A.; Parikh, M.; Moses, J.; Ali, Z.; et al. Characteristics of early versus late in-stent restenosis in second-generation drug-eluting stents: An optical coherence tomography study. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 294–302. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Kang, S.-J.; Mintz, G.S.; Akasaka, T.; Park, D.-W.; Lee, J.-Y.; Kim, W.-J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug–eluting stent implantation. Circulation 2011, 123, 2954–2963. [Google Scholar] [CrossRef]
- Ali, Z.A.; Roleder, T.; Narula, J.; Mohanty, B.D.; Baber, U.; Kovacic, J.C.; Mintz, G.S.; Otsuka, F.; Pan, S.; Virmani, R.; et al. Increased thin-cap neoatheroma and periprocedural myocardial infarction in drug-eluting stent restenosis: Multimodality intravascular imaging of drug-eluting and bare-metal stents. Circ. Cardiovasc. Interv. 2013, 6, 507–517. [Google Scholar] [CrossRef]
- Gonzalo, N.; Serruys, P.W.; Okamura, T.; van Beusekom, H.M.; Garcia-Garcia, H.M.; van Soest, G.; van der Giessen, W.; Regar, E. Optical coherence tomography patterns of stent restenosis. Am. Heart J. 2009, 158, 284–293. [Google Scholar] [CrossRef]
- Falk, E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Heart 1983, 50, 127–134. [Google Scholar] [CrossRef]
- Constantinides, P. Plaque fissures in human coronary thrombosis. J. Atheroscler. Res. 1966, 6, 1–17. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Maini, B.; Dixon, S.R.; Brilakis, E.S.; Grines, C.L.; Rizik, D.G.; Powers, E.R.; Steinberg, D.H.; Shunk, K.A.; Weisz, G.; et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ. Cardiovasc. Interv. 2011, 4, 429–437. [Google Scholar] [CrossRef]
- Kang, S.-J.; Mintz, G.S.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Xu, K.; Goldstein, J.A.; Stone, G.W.; Muller, J.E. Combined IVUS and NIRS detection of fibroatheromas: Histopathological validation in human coronary arteries. JACC Cardiovasc. Imaging 2015, 8, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Mintz, G.S.; Brilakis, E.S.; Banerjee, S.; Abdel-Karim, A.-R.R.; Maini, B.; Biro, S.; Lee, J.-B.; Stone, G.W.; Weisz, G.; et al. In vivo characterization of coronary plaques: Novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur. Heart J. 2012, 33, 372–383. [Google Scholar] [CrossRef]
- Waksman, R.; Torguson, R.; Spad, M.-A.; Garcia-Garcia, H.; Ware, J.; Wang, R.; Madden, S.; Shah, P.; Muller, J. The Lipid-Rich Plaque Study of vulnerable plaques and vulnerable patients: Study design and rationale. Am. Heart J. 2017, 192, 98–104. [Google Scholar] [CrossRef]
- Danek, B.A.; Karatasakis, A.; Karacsonyi, J.; Alame, A.; Resendes, E.; Kalsaria, P.; Nguyen-Trong, P.-K.J.; Rangan, B.V.; Roesle, M.; Abdullah, S.; et al. Long-term follow-up after near-infrared spectroscopy coronary imaging: Insights from the lipid cORe plaque association with CLinical events (ORACLE-NIRS) registry. Cardiovasc. Revasc. Med. 2017, 18, 177–181. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; van Geuns, R.-J.; de Boer, S.P.M.; Simsek, C.; Kardys, I.; Lenzen, M.J.; van Domburg, R.T.; Regar, E.; et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Anesäter, E.; Fransson, K.; Andell, P.; Persson, J.; Erlinge, D. Intracoronary near-infrared spectroscopy and the risk of future cardiovascular events. Open Heart 2019, 6, e000917. [Google Scholar] [CrossRef]
- Madder, R.D.; Husaini, M.; Davis, A.T.; VanOosterhout, S.; Khan, M.; Wohns, D.; McNamara, R.F.; Wolschleger, K.; Gribar, J.; Collins, J.S.; et al. Large lipid-rich coronary plaques detected by near-infrared spectroscopy at non-stented sites in the target artery identify patients likely to experience future major adverse cardiovascular events. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Montarello, N.J.; Nelson, A.J.; Verjans, J.; Nicholls, S.J.; Psaltis, P.J. The role of intracoronary imaging in translational research. Cardiovasc. Diagn. Ther. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Khraishah, H.; Jaffer, F.A. Intravascular molecular imaging: Near-infrared fluorescence as a new frontier. Front. Cardiovasc. Med. 2020, 7, 587100. [Google Scholar] [CrossRef] [PubMed]
- Ughi, G.J.; Wang, H.; Gerbaud, E.; Gardecki, J.A.; Fard, A.M.; Hamidi, E.; Vacas-Jacques, P.; Rosenberg, M.; Jaffer, F.A.; Tearney, G.J. Clinical characterization of coronary atherosclerosis with dual-modality OCT and near-infrared autofluorescence imaging. JACC Cardiovasc. Imaging 2016, 9, 1304–1314. [Google Scholar] [CrossRef]
- Bozhko, D.; Osborn, E.A.; Rosenthal, A.; Verjans, J.W.; Hara, T.; Kellnberger, S.; Wissmeyer, G.; Ovsepian, S.V.; McCarthy, J.R.; Mauskapf, A.; et al. Quantitative intravascular biological fluorescence-ultrasound imaging of coronary and peripheral arteries in vivo. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1253–1261. [Google Scholar] [CrossRef]
- Vinegoni, C.; Botnaru, I.; Aikawa, E.; Calfon, M.A.; Iwamoto, Y.; Folco, E.J.; Ntziachristos, V.; Weissleder, R.; Libby, P.; Jaffer, F.A. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 2011, 3, 84ra45. [Google Scholar] [CrossRef]
- de Vries, B.M.W.; Hillebrands, J.-L.; van Dam, G.M.; Tio, R.A.; de Jong, J.S.; Slart, R.H.; Zeebregts, C.J. Images in cardiovascular medicine. Multispectral near-infrared fluorescence molecular imaging of matrix metalloproteinases in a human carotid plaque using a matrix-degrading metalloproteinase-sensitive activatable fluorescent probe. Circulation 2009, 119, e534–e536. [Google Scholar]
- Jaffer, F.A.; Calfon, M.A.; Rosenthal, A.; Mallas, G.; Razansky, R.N.; Mauskapf, A.; Weissleder, R.; Libby, P.; Ntziachristos, V. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J. Am. Coll. Cardiol. 2011, 57, 2516–2526. [Google Scholar] [CrossRef]
- Zaheer, A.; Lenkinski, R.E.; Mahmood, A.; Jones, A.G.; Cantley, L.C.; Frangioni, J.V. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat. Biotechnol. 2001, 19, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Jaffer, F.A.; Ntziachristos, V. Intravascular multispectral optoacoustic tomography of atherosclerosis: Prospects and challenges. Imaging Med. 2012, 4, 299. [Google Scholar] [CrossRef]
- Jansen, K.; van Soest, G.; van der Steen, A.F. Intravascular photoacoustic imaging: A new tool for vulnerable plaque identification. Ultrasound Med. Biol. 2014, 40, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Karpiouk, A.; Yeager, D.; Amirian, J.; Litovsky, S.; Smalling, R.; Emelianov, S. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis. Ultrasound Med. Biol. 2012, 38, 2098–2103. [Google Scholar] [CrossRef]
- Bec, J.; Vela, D.; Phipps, J.E.; Agung, M.; Unger, J.; Margulies, K.B.; Buja, M.; Marcu, L. Visualization and quantification of biochemical markers of atherosclerotic plaque progression using intravascular fluorescence lifetime (Conference Presentation). In Proceedings of the Diagnostic and Therapeutic Applications of Light in Cardiology 2020, San Francisco, CA, USA, 1–2 February 2020; SPIE: Bellingham, WA, USA, 2020; Volume 11215, p. 112150E. [Google Scholar]
- Lee, M.W.; Song, J.W.; Kang, W.J.; Nam, H.S.; Kim, T.S.; Kim, S.; Oh, W.-Y.; Kim, J.W.; Yoo, H. Comprehensive intravascular imaging of atherosclerotic plaque in vivo using optical coherence tomography and fluorescence lifetime imaging. Sci Rep 2018, 8, 14561. [Google Scholar] [CrossRef]
- Kim, J.W. First-In-Human Intracoronary Dual-Modal Optical Coherence Tomography and Fluorescence Lifetime Imaging (OCT-FLIM) In Patients Undergoing Percutaneous Coronary Intervention. Clinicaltrials.gov, Clinical Trial Registration NCT04835467, Ago. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04835467 (accessed on 2 December 2022).
- Matsumura, M.; Mintz, G.S.; Dohi, T.; Li, W.; Shang, A.; Fall, K.; Sato, T.; Sugizaki, Y.; Chatzizisis, Y.S.; Moses, J.W.; et al. Accuracy of IVUS-Based Machine Learning Segmentation Assessment of Coronary Artery Dimensions and Balloon Sizing. JACC Adv. 2023, 2, 100564. [Google Scholar] [CrossRef]
- Cioffi, G.M.; Pinilla-Echeverri, N.; Sheth, T.; Sibbald, M.G. Does artificial intelligence enhance physician interpretation of optical coherence tomography: Insights from eye tracking. Front. Cardiovasc. Med. 2023, 10, 1283338. [Google Scholar] [CrossRef]
- Bezerra, H.G.; Quimby, D.L.; Matar, F.; Mohanty, B.D.; Bassily, E.; Ughi, G.J. High-Frequency Optical Coherence Tomography (HF-OCT) for Preintervention Coronary Imaging. JACC Cardiovasc. Imaging 2023, 16, 982–984. [Google Scholar] [CrossRef]
- Ali, Z.A.; Dager, A.; Zúñiga, M.; Fonseca, J.; Arana, C.; Chamié, D.; Hill, J.M.; Madder, R.D.; Muller, J.E.; Simonton, C.A.; et al. First-in-Human Experience With a Novel Multimodality DeepOCT-NIRS Intracoronary Imaging System. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101344. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ahn, J.-M.; Kang, D.-Y.; Yun, S.-C.; Ahn, Y.-K.; Kim, W.-J.; Nam, C.-W.; Jeong, J.-O.; Chae, I.-H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Bakhos, J.J.; Wu, W.; Zhao, S.; Kassab, G.S.; Khan, B.; Panagopoulos, A.; Makadia, J.; Oguz, U.M.; Banga, A.; et al. Artificial Intelligence, Computational Simulations, and Extended Reality in Cardiovascular Interventions. JACC Cardiovasc. Interv. 2023, 16, 2479–2497. [Google Scholar] [CrossRef] [PubMed]
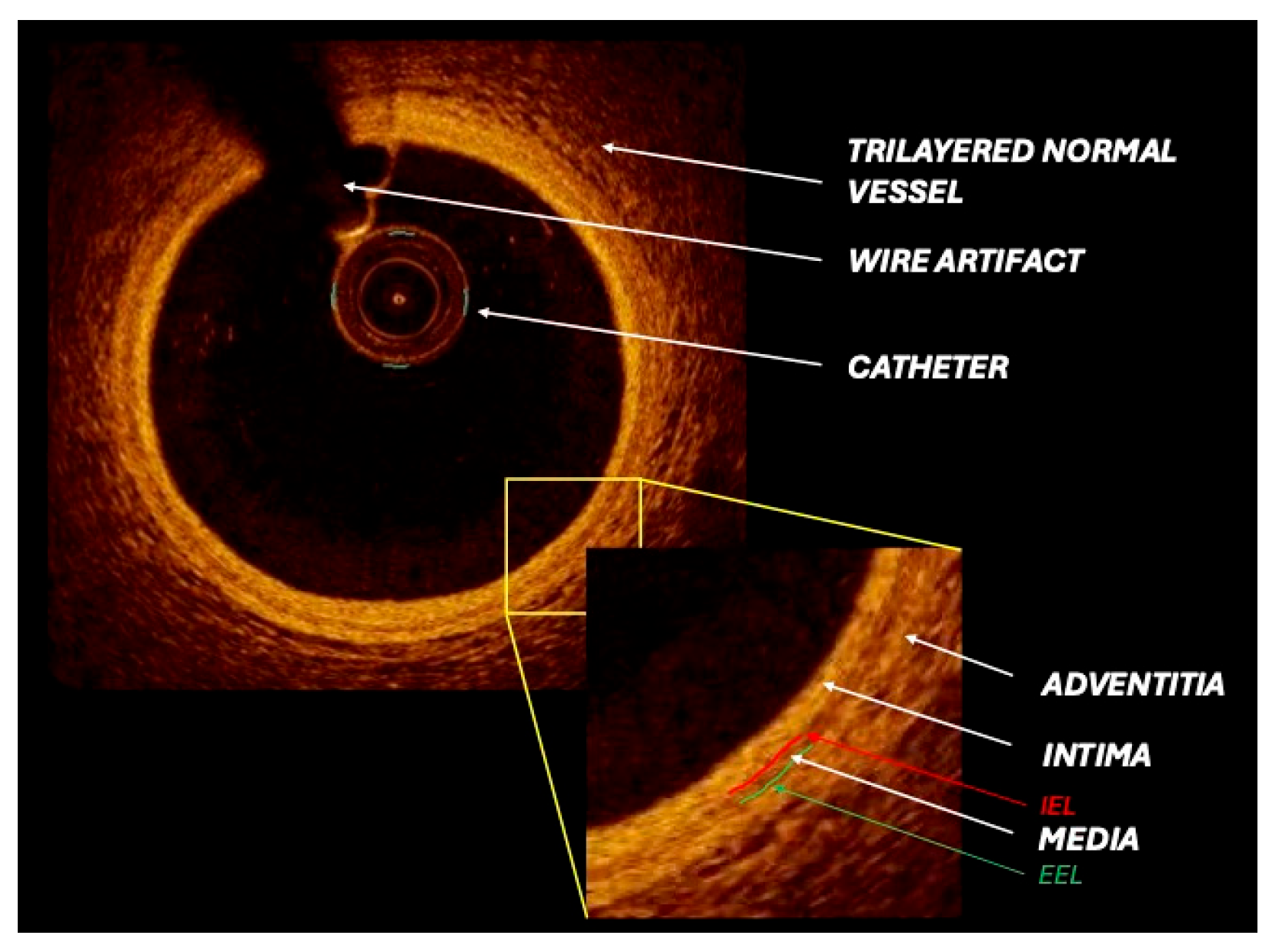
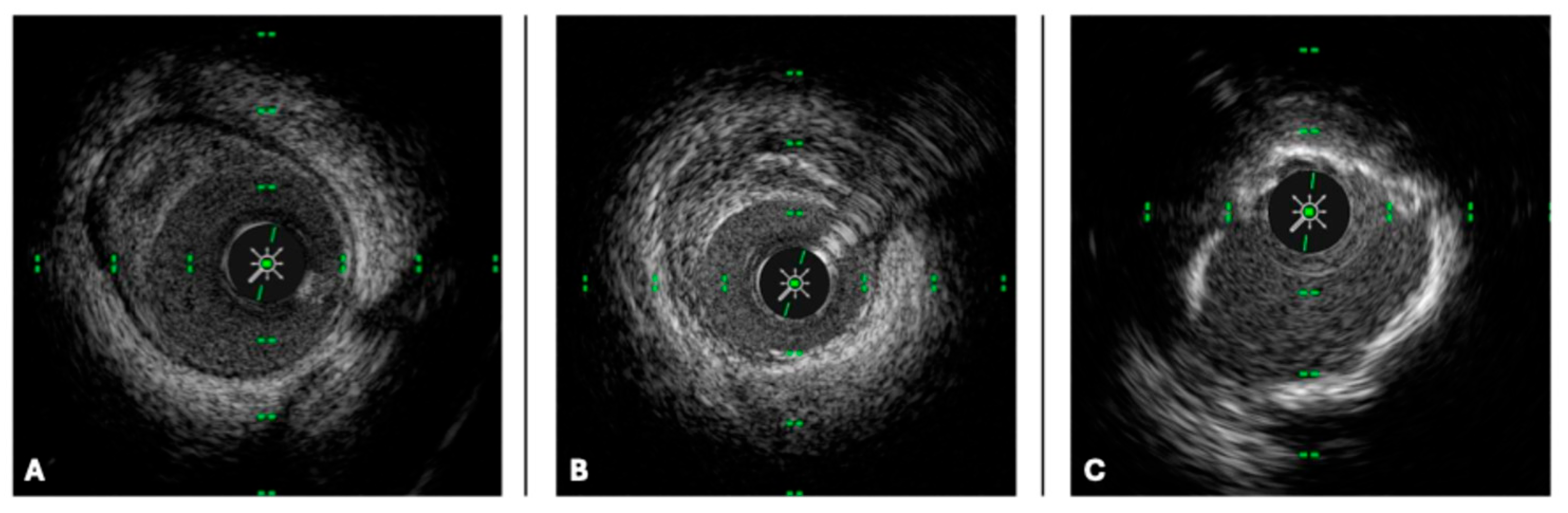
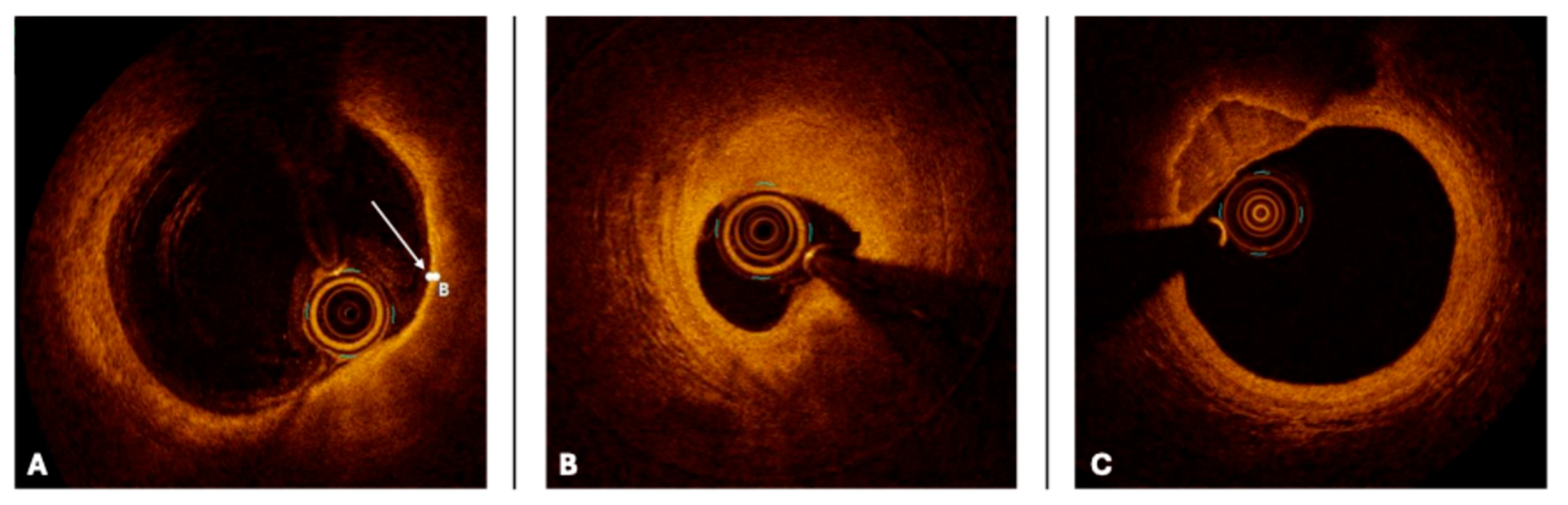

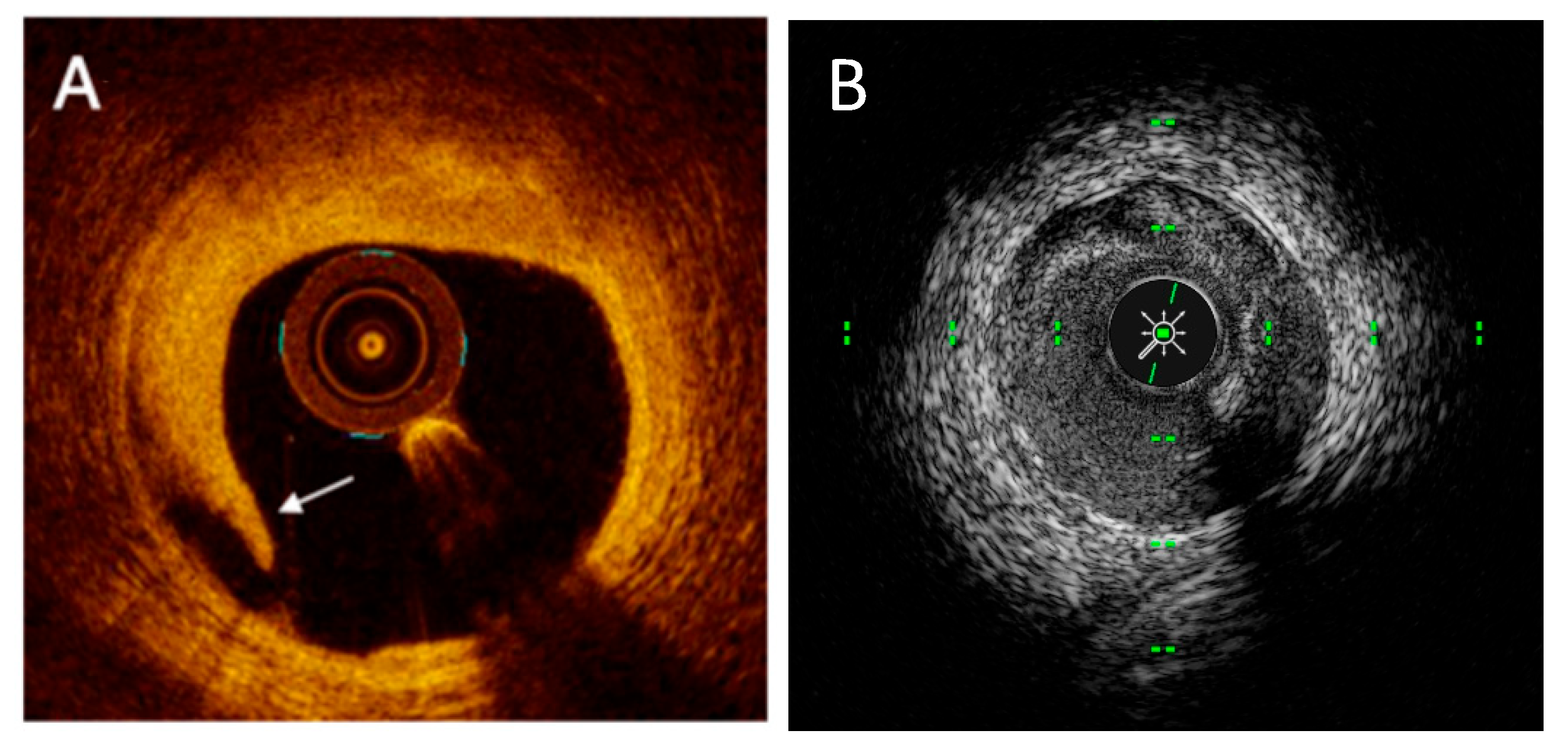
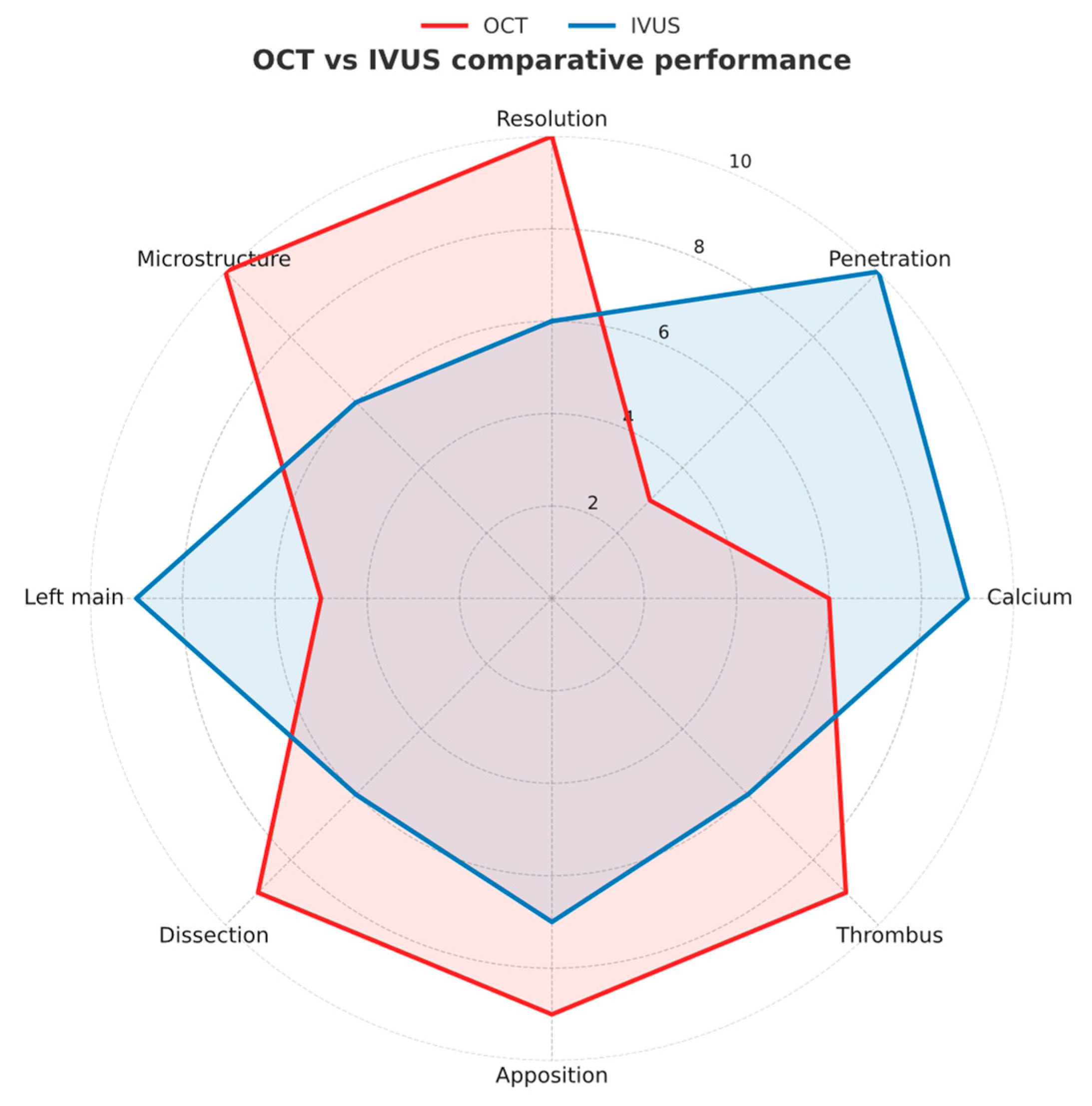
| IVUS (40–45 MHz) | HD IVUS (50–60 MHz) | OCT Frequency Domain | |
|---|---|---|---|
| Wave source | Ultrasound | Ultrasound | Near-infrared light |
| Axial resolution (μm) | 38–46 | 20–40 | 15–20 |
| Penetration depth in soft tissue (mm) | >5 | 3–8 | 1–2 |
| Distance between adjacent frames (mm) | 0.02–0.03 | 0.02–0.17 | 0.1–0.25 |
| Study | Year of Publication | Study Design | Patients (n.) | ACS (%) | Stent Type | Follow-Up (Months) | Primary Endpoint |
|---|---|---|---|---|---|---|---|
| CLI-OPCI | 2012 | Retrospective (OCT vs. angiography) | 670 | 60 | DES or BMS | 12 | OCT-guidance PCI is associated with significantly lower rate of the composite of MACEs at 1 year |
| OCTACS | 2015 | Randomized (OCT vs. angiography) | 100 | 100 | DES | 6 | OCT-guided optimization improves stent strut coverage at 6-month follow-up |
| IVUS-XPL | 2015 | Randomized (IVUS vs. angiography) | 1400 | 49 | DES | 12 | IVUS-guided PCI is associated with significantly lower rate of the composite of MACEs at 1 year |
| ILUMIEN III | 2016 | Randomized (IVUS vs. OCT vs. angiography) | 450 | 36 | DES | 1 | OCT in MSA was non inferior vs. IVUS and not superior vs. angiography |
| DOCTORS | 2016 | Randomized (OCT vs. angiography) | 240 | 100 | DES vs. BMS | 6 | OCT-guidance PCI is associated with significantly greater post procedural FFR |
| OPINION | 2017 | Randomized (OCT vs. IVUS) | 829 | 12 | DES | 12 | TVF by OCT at 1 year—non-inferiority compared to IVUS guidance |
| Choi et al. | 2019 | Prospective registry (IVUS vs. angiography) | 6005 | 37 | DES | 64 | IVUS-guided PCI is associated with significantly lower risk of cardiac death |
| ROCK II | 2021 | Retrospective (OCT/IVUS vs. angiography) | 730 | 60 | DES | 12 | Intravascular imaging was superior to angiography for distal LM stenting, with no difference between OCT and IVUS. |
| OCTOBER | 2023 | Randomized (OCT vs. angiography) | 1201 | 45 | DES | 24 | OCT-guided PCI was associated with a lower incidence of MACEs in patients with complex coronary-artery bifurcation lesions |
| Clinical Factors | Anatomical Factors | Stent/Procedural Factors |
|---|---|---|
| Chronic kidney disease (CKD) | Calcified lesion | Bare metal stent |
| Diabetes mellitus | Ostial lesion | Stent underexpansion |
| Older age | Bifurcation lesion | Multiple stent layers |
| Obesity | Long lesion | Stent fracture |
| Antiproliferative drug resistance | Small vessel | Geographical miss |
| Untreated cardiovascular risk factors | Previous ISR | Stent gap |
| CTO revascularization | Small post-PCI MLA | |
| SVG revascularization | Smaller stent diameter | |
| Longer stent |
| IVUS | |
|---|---|
| Focal ISR | Defined as a lumen area < 4.0 mm2 and a lesion length < 10 mm, further classified according to its location within the stented segment:
|
| Multifocal ISR | Characterized by multiple discrete restenotic segments, with the following subtypes:
|
| Diffuse ISR | Defined as a minimum lumen area < 4.0 mm2 with a lesion length > 10 mm, and categorized as follows:
|
| OCT | |
| Type I | Thin-cap neoatheroma: presence of one or more thin fibrous caps with neoatherosclerotic tissue located between the lumen and the stent struts |
| Type II | Thick-cap neoatheroma: neoatherosclerosis situated between the lumen and the stent struts without thin-cap features |
| Type III | Peri-strut neoatheroma: neoatherosclerotic tissue predominantly distributed around the stent struts |
| Type IV | Pre-existing fibroatheroma: native atherosclerotic plaque appearing as a signal-poor region with blurred borders between the stent struts and the adventitia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuvara, G.; Franzino, M.; Carciotto, G.; De Ferrari, T.; Lo Giudice, S.; Pallante, F.; Giannino, F.; Ajello, M.; Tomasi, S.; Sciortino, L.; et al. Coronary Intravascular Imaging: A Comprehensive Review of Techniques, Applications, and Future Directions. Medicina 2025, 61, 2019. https://doi.org/10.3390/medicina61112019
Iuvara G, Franzino M, Carciotto G, De Ferrari T, Lo Giudice S, Pallante F, Giannino F, Ajello M, Tomasi S, Sciortino L, et al. Coronary Intravascular Imaging: A Comprehensive Review of Techniques, Applications, and Future Directions. Medicina. 2025; 61(11):2019. https://doi.org/10.3390/medicina61112019
Chicago/Turabian StyleIuvara, Giustina, Marco Franzino, Gabriele Carciotto, Tommaso De Ferrari, Stefania Lo Giudice, Francesco Pallante, Federico Giannino, Manuela Ajello, Sofia Tomasi, Luigi Sciortino, and et al. 2025. "Coronary Intravascular Imaging: A Comprehensive Review of Techniques, Applications, and Future Directions" Medicina 61, no. 11: 2019. https://doi.org/10.3390/medicina61112019
APA StyleIuvara, G., Franzino, M., Carciotto, G., De Ferrari, T., Lo Giudice, S., Pallante, F., Giannino, F., Ajello, M., Tomasi, S., Sciortino, L., Monciino, G., Licandri, W., Caminiti, R., Virga, V., Costa, F., Micari, A., & Vizzari, G. (2025). Coronary Intravascular Imaging: A Comprehensive Review of Techniques, Applications, and Future Directions. Medicina, 61(11), 2019. https://doi.org/10.3390/medicina61112019







