Sodium Phenylbutyrate Ameliorates Ovariectomy-Induced Bone Loss in Rats
Abstract
1. Introduction
2. Material and Method
2.1. Animals
Drug
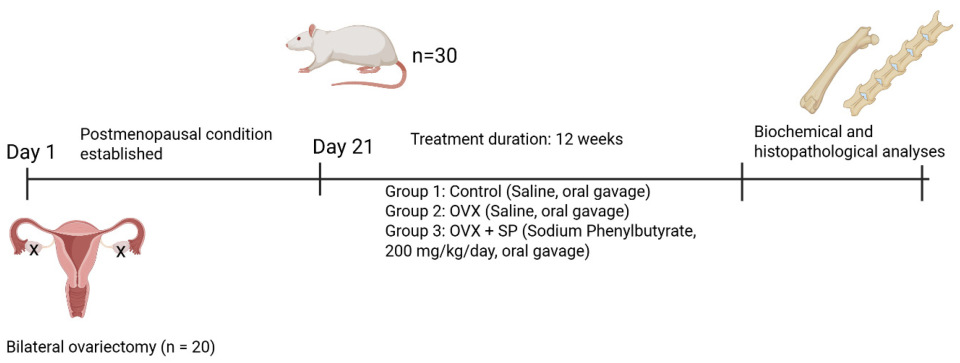
2.2. Experimental Protocol
2.2.1. Ovariectomy Procedure
2.2.2. Experimental Groups and Treatments
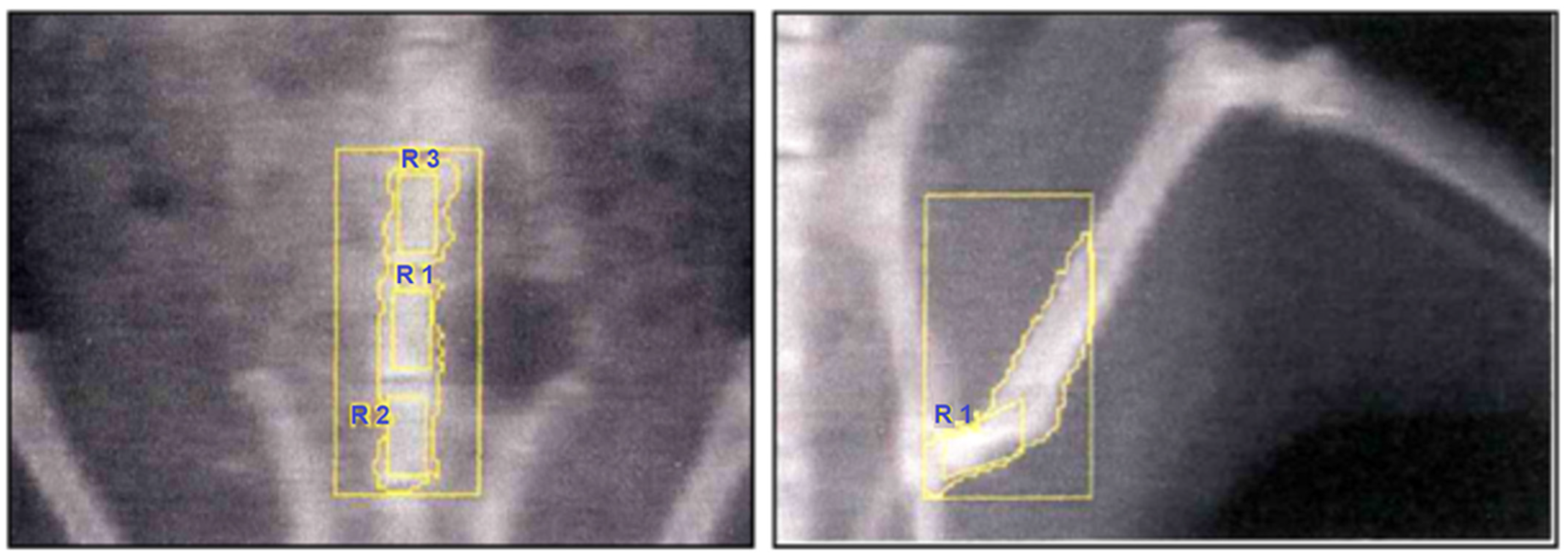
2.2.3. Bone Mineral Density (BMD) Measurements
Histomorphometric Evaluations
2.2.4. Sample Collection and Tissue Processing
2.3. Histopathological Examination of Bone Tissue
2.3.1. Tissue Preparation
2.3.2. Morphometric Analysis
Evaluation of Histopathological Findings
2.3.3. Measurement Criteria
2.4. Inflammatory-Related Cytokine Analysis (IL-6, TNF-α, RANKL)
2.4.1. Sample Preparation
2.4.2. ELISA Quantification
2.5. Measurement of Plasma Cathepsin K Levels
2.6. Measurement of Malondialdehyde (MDA)
2.7. Statistical Analysis
Sample Size Determination and Power Analysis
3. Results
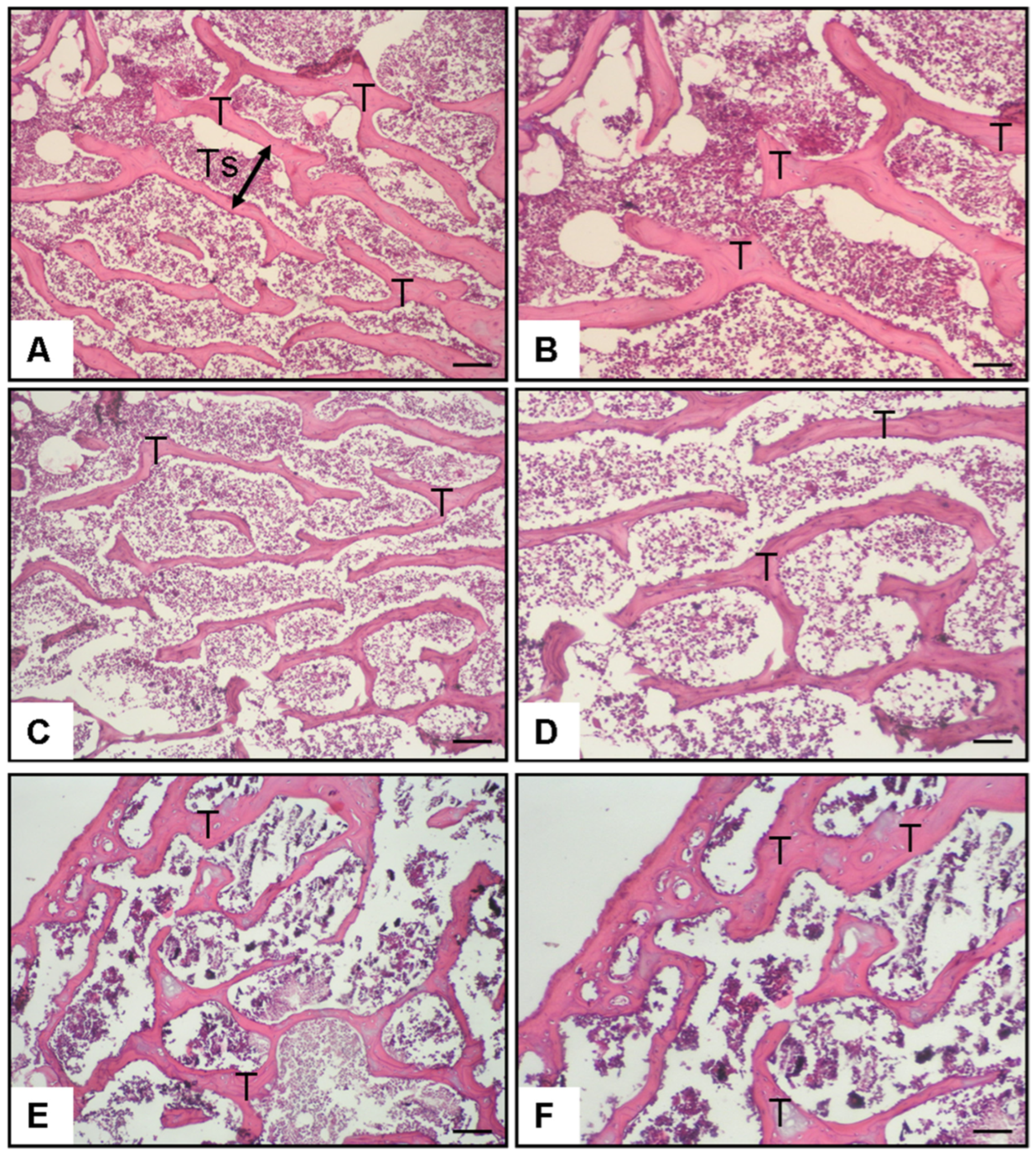
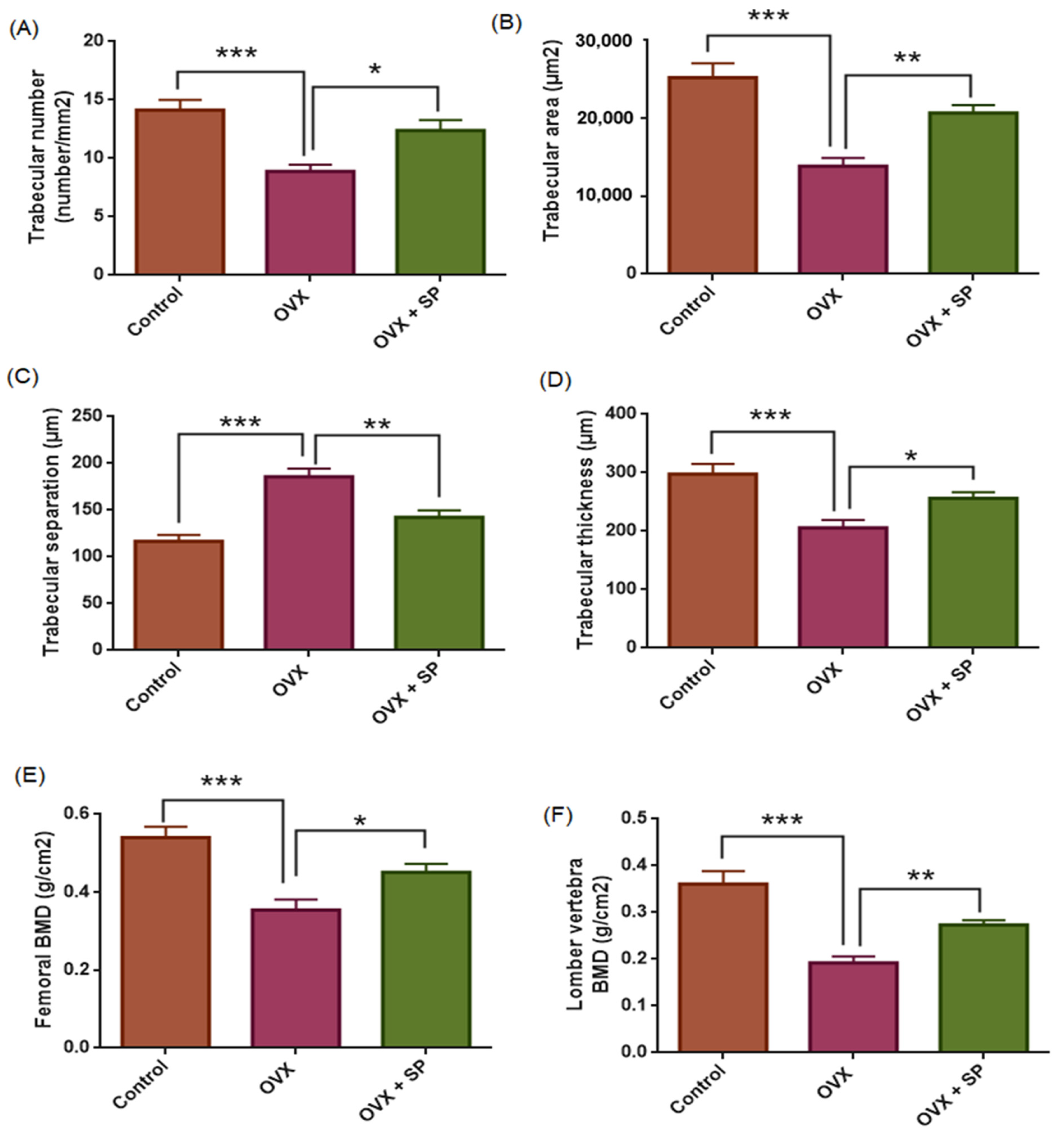
3.1. Bone Histomorphometry and Bone Mineral Density
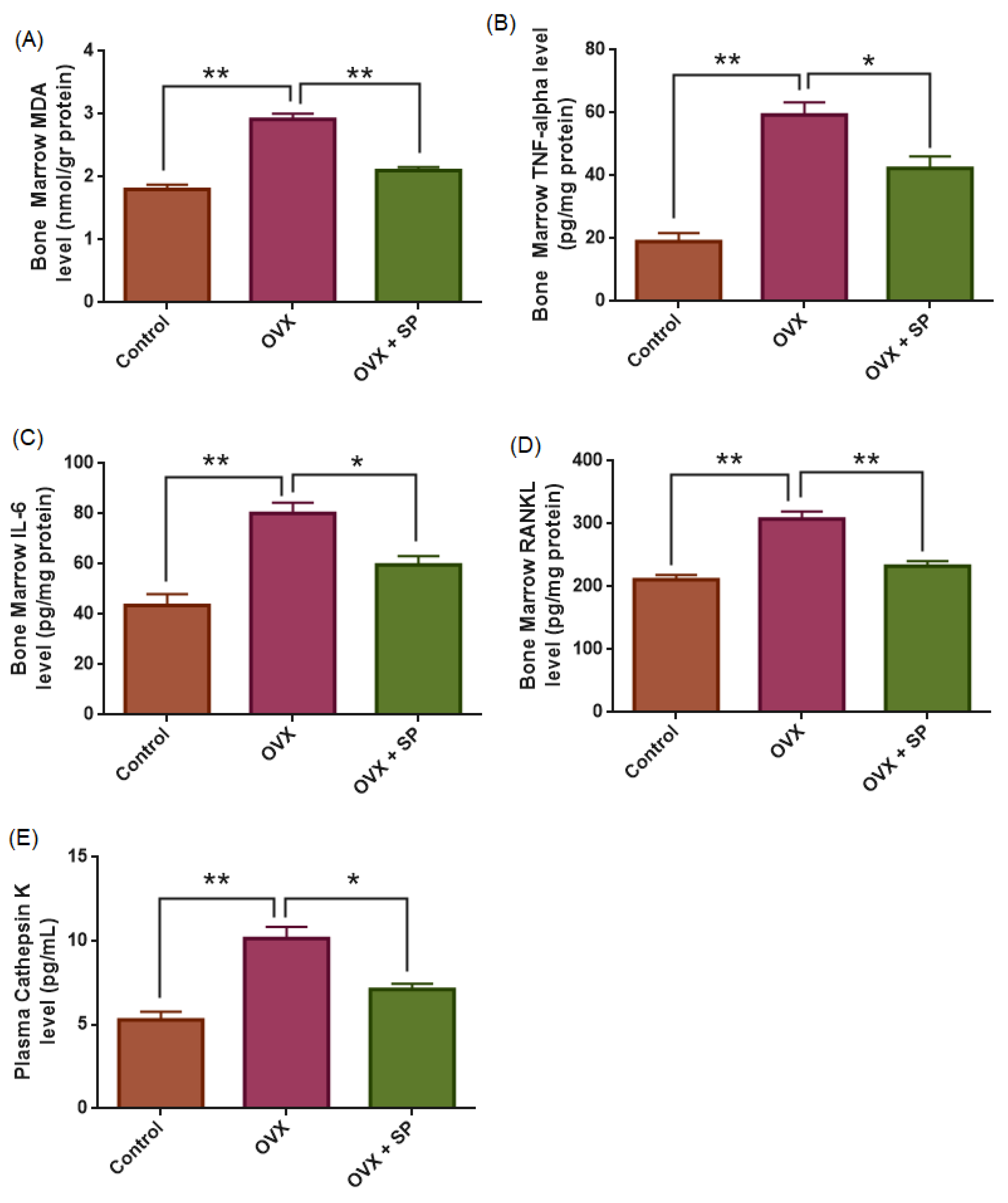
3.2. Oxidative Stress Marker (MDA)
3.3. Inflammatory Cytokines (TNF-α and IL-6)
3.4. RANKL
3.5. Plasma Cathepsin K
3.6. General Observations
4. Discussion
Future Directions
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Shan, P.F. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology, and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Dennison, E.M.; Leufkens, H.A.; Cooper, C. Epidemiology of fractures in england and wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mason, B.; Horne, A.; Ames, R.; Clearwater, J.; Liu, M.; Evans, M.C.; Gamble, G.D.; Reid, I.R. Fractures Between the Ages of 20 and 50 Years Increase Women’s Risk of Subsequent Fractures. Arch. Intern. Med. 2002, 162, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A., 3rd; Berger, M. Patients With Prior Fractures Have an Increased Risk of Future Fractures: A Summary of the Literature and Statistical Synthesis. J. Bone Miner. 2000, 15, 721–739. [Google Scholar] [CrossRef]
- Walker, J. Osteoporosis and Fragility Fractures: Risk Assessment, Management and Prevention. Nurs. Older People 2020, 32, 34–41. [Google Scholar] [CrossRef]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D.; Hanley, D.A.; Sellmeyer, D.; Cheung, A.M.; Shane, E.; Kearns, A.; Thomas, T.; Boyd, S.K.; Boutroy, S.; et al. Microarchitectural deterioration of cortical and trabecular bone: Differing effects of denosumab and alendronate. J. Bone Miner. Res. 2010, 25, 1886–1894. [Google Scholar] [CrossRef]
- Hussain, M.A.; Joseph, A.; Cherian, V.M.; Srivastava, A.; Cherian, K.E.; Kapoor, N.; Paul, T.V. Bisphosphonate-induced atypical femoral fracture in tandem: Long-term follow-up is warranted. Endocrinol. Diabetes Metab. Case Rep. 2022; Online ahead of print. [Google Scholar]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Reid, I.R. Short-term and long-term effects of osteoporosis therapies. Nat. Rev. Endocrinol. 2015, 11, 418–428. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89. [Google Scholar]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Liu, T.; Ding, S.; Yin, D.; Cuan, X.; Xie, C.; Xu, H.; Sheng, J. Pu-erh tea extract ameliorates ovariectomy-induced osteoporosis in rats and suppresses osteoclastogenesis in vitro. Front. Pharmacol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Rey, J.R.C.; Cervino, E.V.; Rentero, M.L.; Crespo, E.C.; Álvaro, A.O.; Casillas, M. Raloxifene: Mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop. J. 2009, 3, 14. [Google Scholar] [CrossRef]
- Falsetti, I.; Palmini, G.; Aurilia, C.; Donati, S.; Iantomasi, T.; Brandi, M.L. Selective estrogen receptor modulators in post-menopausal osteoporosis. Int. J. Bone Fragility 2022, 2, 93–96. [Google Scholar] [CrossRef]
- Black, L.J.; Sato, M.; Rowley, E.R.; Magee, D.E.; Bekele, A.; Williams, D.C.; Bensch, W.R. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J. Clin. Investig. 1994, 93, 63–69. [Google Scholar] [CrossRef]
- Turner, C.H.; Sato, M.; Bryant, H.U. Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology 1994, 135, 2001–2005. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are oxidative stress and inflammation mediators of bone loss due to estrogen deficiency? A review of current evidence. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Brandi, M.L. Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Zha, L.; He, L.; Liang, Y.; Qin, H.; Yu, B.; Chang, L.; Xue, L. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018, 102, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Li, H.; Avraham, S.; Jiang, S.; Avraham, H.K. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor α. Int. J. Cancer 2003, 107, 353–358. [Google Scholar] [CrossRef]
- deFazio, A.; Chiew, Y.E.; Donoghue, C.; Lee, C.S.; Sutherland, R.L. Effect of sodium butyrate on estrogen receptor and epidermal growth factor receptor gene expression in human breast cancer cell lines. J. Biol. Chem. 1992, 267, 18008–18012. [Google Scholar] [CrossRef]
- Alao, J.P.; Lam, E.W.; Ali, S.; Buluwela, L.; Bordogna, W.; Lockey, P.; Vigushin, D.M. Histone deacetylase inhibitor trichostatin A represses estrogen receptor α-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin. Cancer Res. 2004, 10, 8094–8104. [Google Scholar] [CrossRef]
- Oie, S.; Matsuzaki, K.; Yokoyama, W.; Murayama, A.; Yanagisawa, J. HDAC3 regulates stability of estrogen receptor α mRNA. Biochem. Biophys. Res. Commun. 2013, 432, 236–241. [Google Scholar] [CrossRef]
- Schoeller, A.; Karki, K.; Jayaraman, A.; Chapkin, R.S.; Safe, S. Short chain fatty acids exhibit selective estrogen receptor downregulator (SERD) activity in breast cancer. Am. J. Cancer Res. 2022, 12, 3422. [Google Scholar] [PubMed]
- Zhou, Q.; Atadja, P.; Davidson, N.E. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol. Ther. 2007, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Munster, P.N. Histone deacetylase inhibitor induced modulation of anti-estrogen therapy. Cancer Lett. 2009, 280, 184–191. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Hu, X.; Li, Q.; Sun, T.; Li, W.; Shang, H. Entinostat, a class I selective histone deacetylase inhibitor, plus exemestane for Chinese patients with hormone receptor-positive advanced breast cancer: A multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Acta Pharm. Sin. B 2023, 13, 2250–2258. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, W.; Jiao, G.; Li, C.; Liu, H. MiR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects osteoblasts from oxidative stress. Int. J. Biol. Macromol. 2018, 107, 2094–2101. [Google Scholar] [CrossRef]
- Dudakovic, A.; Evans, J.M.; Li, Y.; Middha, S.; McGee-Lawrence, M.E.; Van Wijnen, A.J.; Westendorf, J.J. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J. Biol. Chem. 2013, 288, 28783–28791. [Google Scholar] [CrossRef]
- Peng, X.; Wang, T.; Wang, Q.; Zhao, Y.; Xu, H.; Yang, H.; Geng, D. Pan-histone deacetylase inhibitor vorinostat suppresses osteoclastic bone resorption through modulation of RANKL-evoked signaling and ameliorates ovariectomy-induced bone loss. Cell Commun. Signal. 2024, 22, 160. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Jana, A.; Liu, X.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PLoS ONE 2012, 7, e38113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Jiang, N.; Zhang, A. Sodium Phenylbutyrate ameliorates inflammatory response induced by Staphylococcus aureus lipoteichoic acid via suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules 2018, 23, 3056. [Google Scholar] [CrossRef]
- Park, H.-J.; Son, H.-J.; Sul, O.-J.; Suh, J.-H.; Choi, H.-S. 4-Phenylbutyric acid protects against lipopolysaccharide-induced bone loss by modulating autophagy in osteoclasts. Biochem. Pharmacol. 2018, 151, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Q.; Lv, J.; Nie, H. 4-Phenyl butyric acid prevents glucocorticoid-induced osteoblast apoptosis by attenuating endoplasmic reticulum stress. J. Bone Miner. Metab. 2017, 35, 366–374. [Google Scholar] [CrossRef]
- Cantley, M.D.; Bartold, P.M.; Marino, V.; Fairlie, D.P.; Le, G.T.; Lucke, A.J.; Haynes, D.R. Histone deacetylase inhibitors and periodontal bone loss. J. Periodontal Res. 2011, 46, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ma, S.; Li, Y.; Sun, Y.; Zhang, K.; Zhou, Q.; Yu, R. Evaluating the Activity of Sodium Butyrate to Prevent Osteoporosis in Rats by Promoting Osteal GSK-3β/Nrf2 Signaling and Mitochondrial Function. J. Agric. Food Chem. 2020, 68, 6588–6603. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Pérez-Mediavilla, A.; Frechilla, D.; Del Río, J.; García-Osta, A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2009, 34, 1721–1732. [Google Scholar] [CrossRef]
- Barış Güven, B.; Tanoglu, A.; Ozcelik, F.; Guzel Tanoglu, E.; Kaya Terzi, N. 4-phenyl butyric acid improves hepatic ischemia/reperfusion and affects gene expression of ABC transporter Abcc5 in rats. Croat. Med. J. 2023, 64, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gong, H.; Bai, T.; Fu, Y.; Li, X.; Lu, J.; Chen, J. Pharmacological Intervention with 4-Phenylbutyrate Ameliorates TiAl6V4 Nanoparticles-Induced Inflammatory Osteolysis by Promoting Macrophage Apoptosis. Bioengineering 2025, 12, 701. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units: Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.A.D.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Zamai, R.S.; Corrêa, M.G.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z.; Messora, M.R.; Pimentel, S.P. Does resveratrol favor peri-implant bone repair in rats with ovariectomy-induced osteoporosis? Gene expression, counter-torque and micro-CT analysis. Braz. Oral Res. 2023, 37, e003. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, N.; Zhang, Q.; Liu, Y.; Wu, Q.; He, Y.; Zhang, Q. Jin-Tian-Ge ameliorates ovariectomy-induced bone loss in rats and modulates osteoblastogenesis and osteoclastogenesis in vitro. Chin. Med. 2022, 17, 78. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Frigério, P.B.; Duarte, N.D.; Moura, J.D.; Monteiro, N.G.; Fabris, A.L.D.S.; Okamoto, R. Evaluation of Peri-Implant Bone Repair in Ovariectomized Rats Submitted to the Implant Placement Functionalized with Anti-Sclerostin. Bioengineering 2025, 12, 358. [Google Scholar] [CrossRef]
- Omi, N.; Ezawa, I. The effect of ovariectomy on bone metabolism in rats. Bone 1995, 17, S163–S168. [Google Scholar] [CrossRef]
- Teramura, K.; Fukushima, S.; Nozaki, K.; Kokubo, S.; Takahashi, K. Comparison of incadronate and alfacalcidol on increased bone turnover caused by ovariectomy in rats. Eur. J. Pharmacol. 2002, 449, 191–196. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Yao, Q.; Qin, J. Early effects of ovariectomy on bone microstructure, bone turnover markers and mechanical properties in rats. BMC Musculoskelet. Disord. 2022, 23, 316. [Google Scholar] [CrossRef]
- Ammann, P.; Rizzoli, R. Bone strength and its determinants. Osteoporos. Int. 2003, 14 (Suppl. 3), S13–S18. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Chen, Y.-J.; Ho, J.-T.; Huang, H.-L.; Wang, S.-P.; Cheng, F.-C.; Wu, J.; Tsai, M.-T. A comparison of micro-CT and dental CT in assessing cortical bone morphology and trabecular bone microarchitecture. PLoS ONE 2014, 9, 107545. [Google Scholar] [CrossRef]
- Dempster, D.W. The contribution of trabecular architecture to cancellous bone quality. J. Bone Miner. Res. 2000, 15, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.; Moreira-Gonçalves, D.; Coriolano, H.-J.A.; Duarte, J.A. Bone quality: The determinants of bone strength and fragility. Sports Med. 2014, 44, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.; Chines, A.; Clark, P.; Ebeling, P.R.; McClung, M.; Rhee, Y.; Stad, R.K. Bone mineral density after transitioning from denosumab to alendronate. J. Clin. Endocrinol. Metab. 2020, 105, e255–e264. [Google Scholar] [CrossRef] [PubMed]
- Hagino, H.; Sugimoto, T.; Tanaka, S.; Sasaki, K.; Sone, T.; Nakamura, T.; Mori, S. A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: Primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos. Int. 2021, 32, 2301–2311. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Genovese, T.; Di Paola, R.; Ruggeri, Z.; Vegeto, E.; Caputi, A.P.; Van de Loo, F.A.J.; Puzzolo, D.; et al. Inducible nitric oxide synthase mediates bone loss in ovariectomized mice. Endocrinology 2003, 144, 1098–1107. [Google Scholar] [CrossRef]
- Muthusami, S.; Ramachandran, I.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 2005, 360, 81–86. [Google Scholar] [CrossRef]
- Oktem, G.; Uslu, S.; Vatansever, S.; Aktug, H.; Yurtseven, M.; Uysal, A. Evaluation of the relationship between inducible nitric oxide synthase (iNOS) activity and effects of melatonin in experimental osteoporosis in the rat. Surg. Radiol. Anat. SRA 2006, 28, 157–162. [Google Scholar] [CrossRef]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.M.; Kim, H.H.; Lee, Z.H. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Feng, Y.L.; Jiang, X.T.; Ma, F.F.; Han, J.; Tang, X.L. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef]
- Ai, Z.; Wu, Y.O.; Yu, M.; Li, J.; Li, S. Theaflavin-3, 3′-digallate suppresses RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss in mice. Front. Pharmacol. 2020, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Orkun, S.; Aydin, G.; Keles, I.; Tosun, A.; Arslan, M.; Caglayan, O. Effects of ovariectomy and ascorbic acid supplement on oxidative stress parameters and bone mineral density in rats. Libyan J. Med. 2011, 6, 5965. [Google Scholar] [CrossRef]
- Yalin, S.; Sagir, O.; Comelekoglu, U.; Berköz, M.; Eroglu, P. Strontium ranelate treatment improves oxidative damage in osteoporotic rat model. Pharmacol. Rep. 2012, 64, 396–402. [Google Scholar] [CrossRef]
- Albayrak, A.; Uyanik, M.H.; Odabasoglu, F.; Halici, Z.; Uyanik, A.; Bayir, Y.; Albayrak, F.; Albayrak, Y.; Polat, B.; Suleyman, H. The effects of diabetes and/or polymicrobial sepsis on the status of antioxidant enzymes and pro-inflammatory cytokines on heart, liver, and lung of ovariectomized rats. J. Surg. Res. 2011, 169, 67–75. [Google Scholar] [CrossRef]
- Varga, C.; Veszelka, M.; Kupai, K.; Börzsei, D.; Deim, Z.; Szabó, R.; Török, S.; Priksz, D.; Gesztelyi, R.; Juhász, B.; et al. The Effects of Exercise Training and High Triglyceride Diet in an Estrogen Depleted Rat Model: The Role of the Heme Oxygenase System and Inflammatory Processes in Cardiovascular Risk. J. Sports Sci. Med. 2018, 17, 580–588. [Google Scholar] [PubMed]
- Delgobo, M.; Agnes, J.P.; Goncalves, R.M.; Dos Santos, V.W.; Parisotto, E.B.; Zamoner, A.; Zanotto-Filho, A. N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized and inflammatory processes in cardiovascular risk. J. Sports Sci. Med. 2018, 17, 580–588. [Google Scholar]
- Moon, N.; Effiong, L.; Song, L.; Gardner, T.R.; Soung, D.Y. Tart cherry prevents bone loss through inhibition of RANKL in TNFoverexpressing mice. Nutrients 2018, 11, 63. [Google Scholar] [CrossRef]
- Theill, L.E.; Boyle, W.J.; Penninger, J.M. RANK-L AND RANK: T Cells, Bone Loss, and Mammalian Evolution. Annu. Rev. Immunol. 2002, 20, 795–823. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Takayanagi, H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011, 1240, E13–E18. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of osteoporosis—Role of T cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Cui, Z.; Gu, G.; Chen, F.; Li, J.; Du, X.; Chen, S.; Xi, Y. Targeting Irgm1 to combat osteoporosis: Suppressing ROS and restoring bone remodeling. Cell Death Dis. 2025, 16, 651. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D. Biochemical Markers of Bone Turnover. Clin. Biochem. 1993, 26, 431–432. [Google Scholar] [CrossRef]
- Shi, X.L.; Li, C.W.; Wan, Q.Z.; Li, A.Q.; Wang, H.; Liu, K. Drynaria total flavonoids decrease cathepsin K expression in ovariectomized rats. Genet. Mol. Res. 2014, 13, 4311–4319. [Google Scholar] [CrossRef]
- Yang, L.; Ding, S.; Zhang, B.; Liu, J.; Dong, Y.; Tang, Q.; Ma, X. Beneficial effects of total phenylethanoid glycoside fraction isolated from Cistanche deserticola on bone microstructure in ovariectomized rats. Oxidative Med. Cell. Longev. 2019, 2019, 2370862. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, H.D.; Park, J.H.; Lim, J.I.; Yang, J.S.; Kwak, W.Y.; Son, M.H. An orally active cathepsin K inhibitor, furan-2-carboxylic acid, 1-{1-[4-fluoro-2-(2-oxo-pyrrolidin-1-yl)-phenyl]-3-oxo-piperidin-4-ylcarbamoyl}-cyclohexyl)-amide (OST-4077), inhibits osteoclast activity in vitro and bone loss in ovariectomized rats. J. Pharmacol. Exp. Ther. 2006, 318, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, B.L.; Duong, L.T.; Cusick, T.E.; Masarachia, P.J.; Gentile, M.A.; Gauthier, J.Y.; Kimmel, D.B. Cathepsin K inhibitors prevent bone loss in estrogen-deficient rabbits. J. Bone Miner. Res. 2011, 26, 252–262. [Google Scholar] [CrossRef]
- Ochi, Y.; Yamada, H.; Mori, H.; Nakanishi, Y.; Nishikawa, S.; Kayasuga, R.; Kawabata, K. Effects of eight-month treatment with ONO-5334, a cathepsin K inhibitor, on bone metabolism, strength and microstructure in ovariectomized cynomolgus monkeys. Bone 2014, 65, 1–8. [Google Scholar] [CrossRef]
- Panwar, P.; Xue, L.; Søe, K.; Srivastava, K.; Law, S.; Delaisse, J.M.; Brömme, D. An ectosteric inhibitor of cathepsin K inhibits bone resorption in ovariectomized mice. J. Bone Miner. Res. 2017, 32, 2415–2430. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Kim, Y.; Li, J.; Huang, S.; Saleem, S.; Cao, W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic. Biol. Med. 2012, 52, 928–936. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Sutar, Y.; Mallick, S.; Date, A.; Shukla, D. Topical phenylbutyrate antagonizes NF-κB signaling and resolves corneal inflammation. iScience 2022, 25, 105682. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.L.; Daniels, E.G.; Molenaars, M.; Houtkooper, R.H.; Janssens, G.E. From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs. EMBO Mol. Med. 2019, 11, e9854. [Google Scholar] [CrossRef]
- Yadav, A.; Huang, T.C.; Chen, S.H.; Ramasamy, T.S.; Hsueh, Y.Y.; Lin, S.P.; Wu, C.C. Sodium phenylbutyrate inhibits Schwann cell inflammation via HDAC and NFκB to promote axonal regeneration and remyelination. J. Neuroinflamm. 2021, 18, 238. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R&D 2011, 11, 227–249. [Google Scholar] [CrossRef]
- Kennedy, C.; Byth, K.; Clarke, C.; DeFazio, A. Cell proliferation in the normal mouse mammary gland and inhibition by phenylbutyrate. Mol. Cancer Ther. 2002, 1, 1025–1033. [Google Scholar] [PubMed]
- Fu, Q.; Li, T.; Zhang, C.; Ma, X.; Meng, L.; Liu, L.; Zhao, X. Butyrate mitigates metabolic dysfunctions via the ERα-AMPK pathway in muscle in OVX mice with diet-induced obesity. Cell Commun. Signal. 2023, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Munster, P.N.; Marchion, D.; Thomas, S.; Egorin, M.; Minton, S.; Springett, G.; Lee, J.-H.; Simon, G.; Chiappori, A.; Sullivan, D.; et al. Phase I trial of vorinostat and doxorubicin in solid tumours: Histone deacetylase 2 expression as a predictive marker. Br. J. Cancer 2009, 101, 1044–1050. [Google Scholar] [CrossRef]
- Ma, J.; Luo, T.; Zeng, Z.; Fu, H.; Asano, Y.; Liao, Y.; Kitakaze, M. Histone deacetylase inhibitor phenylbutyrate exaggerates heart failure in pressure overloaded mice independently of HDAC inhibition. Sci. Rep. 2016, 6, 34036. [Google Scholar] [CrossRef]
- Harris, S.T.; Watts, N.B.; Genant, H.K.; McKeever, C.D.; Hangartner, T.; Keller, M.; Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. JAMA 1999, 282, 1344–1352. [Google Scholar] [CrossRef]
- Nayak, S.; Greenspan, S.L. A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos. Int. 2019, 30, 705–720. [Google Scholar] [CrossRef]
- Zhu, J.; March, L. Treating osteoporosis: Risks and management. Aust. Prescr. 2022, 45, 150. [Google Scholar] [CrossRef]
- Aapro, M.; Hadji, P.; Santini, D.; Schmidmaier, R.; Eastell, R. Biosimilars in osteoporosis treatment: Focus on denosumab. Expert. Opin. Biol. Ther. 2025, 25, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Del Signore, S.J.; Amante, D.J.; Kim, J.; Stack, E.C.; Goodrich, S.; Cormier, K.; Smith, K.; Cudkowicz, M.E.; Ferrante, R.J. Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph. Lateral Scler. 2009, 10, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Batshaw, M.L.; MacArthur, R.B.; Tuchman, M. Alternative pathway therapy for urea cycle disorders: Twenty years later. J. Pediatr. 2001, 138, S46–S54, discussion S54–S55. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.H.; Olson, J.; Tong, W.P.; Young, C.W.; Spriggs, D.R.; Malkin, M.G. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Investig. New Drugs 2007, 25, 131–138. [Google Scholar] [CrossRef]
- Peña-Quintana, L.; Llarena, M.; Reyes-Suárez, D.; Aldamiz-Echevarría, L. Profile of sodium phenylbutyrate granules for the treatment of urea-cycle disorders: Patient perspectives. Patient Prefer. Adherence 2017, 11, 1489–1496. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). PHEBURANE® (Sodium Phenylbutyrate) Oral Pellets: Prescribing Information; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216513s000lbl.pdf (accessed on 15 October 2025).
- Farias, V.; Jerkovich, F.; Barragán, A.M.; Pereyra, A.; Pernas, M.G.; Abdala, R.; Zanchetta, M.B. Three-year effect of bisphosphonates on bone mineral density after denosumab withdrawal: Observations from a real-world study. JBMR Plus 2024, 8, ziae044. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Colarossi, G.; Eschweiler, J.; Tingart, M.; Betsch, M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: A Bayesian network meta-analysis. J. Orthop. Surg. Res. 2021, 16, 533. [Google Scholar] [CrossRef]
- Leder, B.Z.; Tsai, J.N.; Uihlein, A.V.; Wallace, P.M.; Lee, H.; Neer, R.M.; Burnett-Bowie, S.-A.M. Denosumab and Teriparatide Transitions in Postmenopausal Osteoporosis (The DATA-Switch Study): Extension of a Randomised Controlled Trial. Lancet 2015, 386, 1147–1155. [Google Scholar] [CrossRef]
- Costa, A.G.; Cusano, N.E.; Silva, B.C.; Cremers, S.; Bilezikian, J.P. Cathepsin K: Its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 2011, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, T.; Kołt, S.; Ciastoń, I.; Vizovisek, M.; Poręba, M.; Turk, B.; Kasperkiewicz, P. Investigation of osteoclast cathepsin K activity in osteoclastogenesis and bone loss using a set of chemical reagents. Cell Chem. Biol. 2023, 30, 159–174. [Google Scholar] [CrossRef]
- Abe, K.; Aoki, Y. Sex differences in bone resorption in the mouse femur: A light and scanning electron-microscopic study. Cell Tissue Res. 1989, 255, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xie, N.; Sun, X.D.; Nice, E.C.; Liou, Y.C.; Huang, C.; Shen, Z. Insights and implications of sexual dimorphism in osteoporosis. Bone Res. 2024, 12, 8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbaş, B.; Dinç, G.; Akbaş, A.; Hacım, N.A.; Ercan, G.; Aygün, H.; Erbaş, O. Sodium Phenylbutyrate Ameliorates Ovariectomy-Induced Bone Loss in Rats. Medicina 2025, 61, 2016. https://doi.org/10.3390/medicina61112016
Akbaş B, Dinç G, Akbaş A, Hacım NA, Ercan G, Aygün H, Erbaş O. Sodium Phenylbutyrate Ameliorates Ovariectomy-Induced Bone Loss in Rats. Medicina. 2025; 61(11):2016. https://doi.org/10.3390/medicina61112016
Chicago/Turabian StyleAkbaş, Bakiye, Gülseren Dinç, Ahmet Akbaş, Nadir Adnan Hacım, Gülçin Ercan, Hatice Aygün, and Oytun Erbaş. 2025. "Sodium Phenylbutyrate Ameliorates Ovariectomy-Induced Bone Loss in Rats" Medicina 61, no. 11: 2016. https://doi.org/10.3390/medicina61112016
APA StyleAkbaş, B., Dinç, G., Akbaş, A., Hacım, N. A., Ercan, G., Aygün, H., & Erbaş, O. (2025). Sodium Phenylbutyrate Ameliorates Ovariectomy-Induced Bone Loss in Rats. Medicina, 61(11), 2016. https://doi.org/10.3390/medicina61112016




