Hepatitis E Virus (HEV) Seroprevalence in Cryptogenic Cirrhosis: From Evidence of High Frequency to the Impact on Disease Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.1.1. Cryptogenic Cirrhosis Patients
- Age > 18 years at the time of HEV serology testing.
- Confirmed diagnosis of liver cirrhosis (clinical, biochemical, and/or imaging/pathology).
- CC diagnosis established through definitive exclusion of all known etiologies prior to June 2020.
- Anti-HEV IgG serology test subsequently performed for any clinical reason.
- Viral Etiologies: Chronic Hepatitis B (HBV) (HBsAg negative), Chronic Hepatitis C (HCV) (Anti-HCV negative), and Hepatitis D (HDV).
- Autoimmune etiologies: Autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) (negative/low-titer autoantibodies, including ANA, ASMA, LKM-1, and AMA; and exclusion by imaging/pathology).
- Alcohol-related liver disease (ALD): History of consumption exceeding >20 g/day for women or >30 g/day for men for >5 years.
- Metabolic/genetic etiologies: Evidence of hemochromatosis (HFE), Wilson’s disease (ATP7B), or alpha-1 antitrypsin deficiency (A1AT).
- Metabolic dysfunction-associated steatotic liver disease (MASLD/MASH): Definitive exclusion of MASLD/MASH based on imaging, elastography, or pathology showing absence of significant steatosis or MASLD-associated metabolic factors at the time of diagnosis.
- Other rare causes: Drug-induced liver injury (DILI) and vascular causes (e.g., Budd-Chiari Syndrome).
2.1.2. Healthy Control Group
2.2. Data and Laboratory Analyses
2.3. Liver Fibrosis Scoring
2.4. Statistical Analysis and Ethical Approval
- Determination of factors associated with Anti-HEV IgG seropositivity: Multi-variable logistic regression analysis was employed to independently assess the association between CC status and Anti-HEV IgG seropositivity, adjusting for confounding factors such as age and sex.
- Evaluation of independent predictors for cirrhosis-related death: To evaluate the independent factors predicting the risk of cirrhosis-related death, a multivariable Cox regression analysis was performed, including Anti-HEV IgG seropositivity, age, sex, and the MELD-Na core.
3. Results
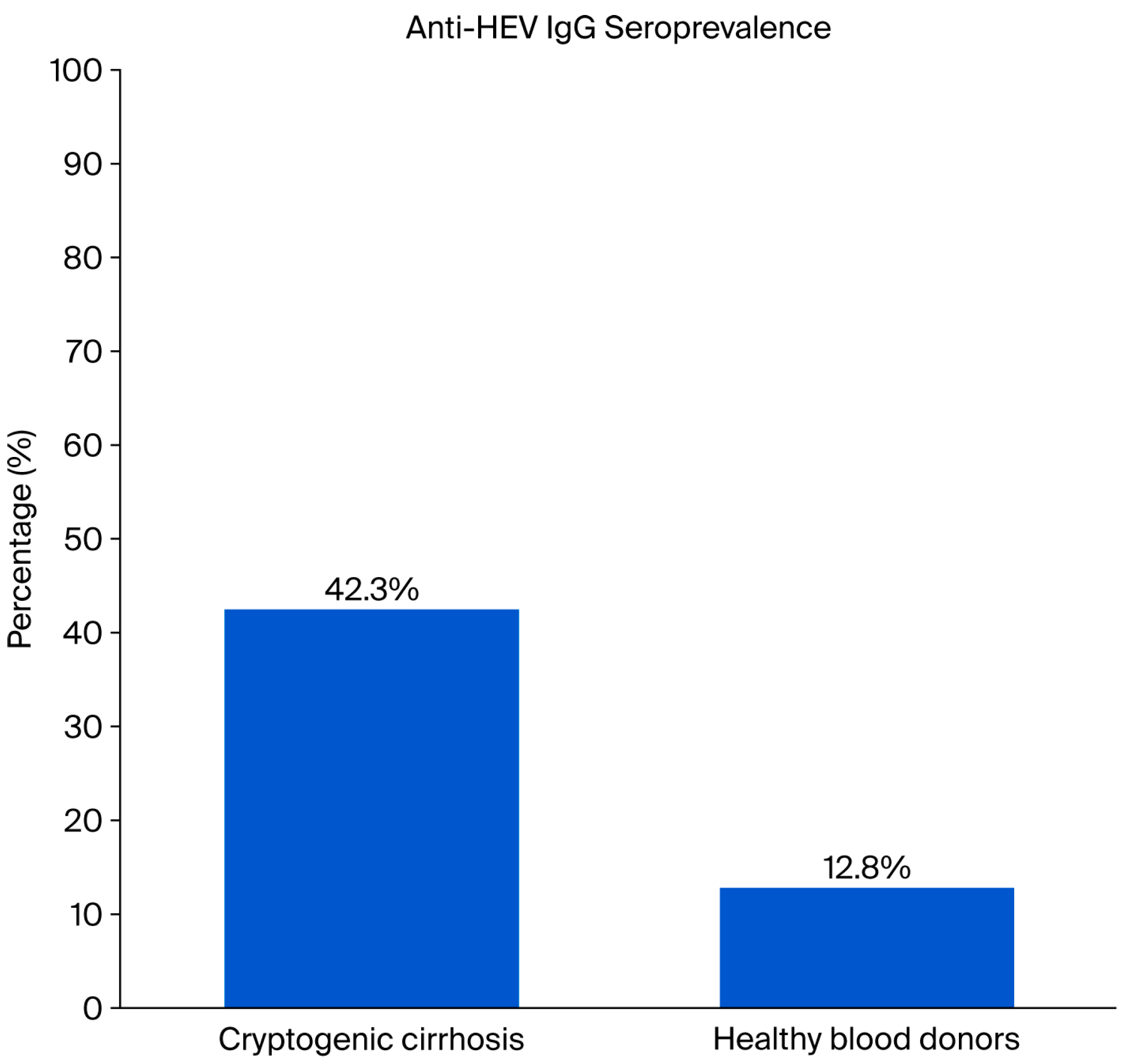
3.1. HEV Seroprevalence in Cryptogenic Cirrhosis Patients and Healthy Controls
3.2. Comparison of Baseline Characteristics Between Anti-HEV IgG Seropositive and Seronegative Cryptogenic Cirrhosis Patients
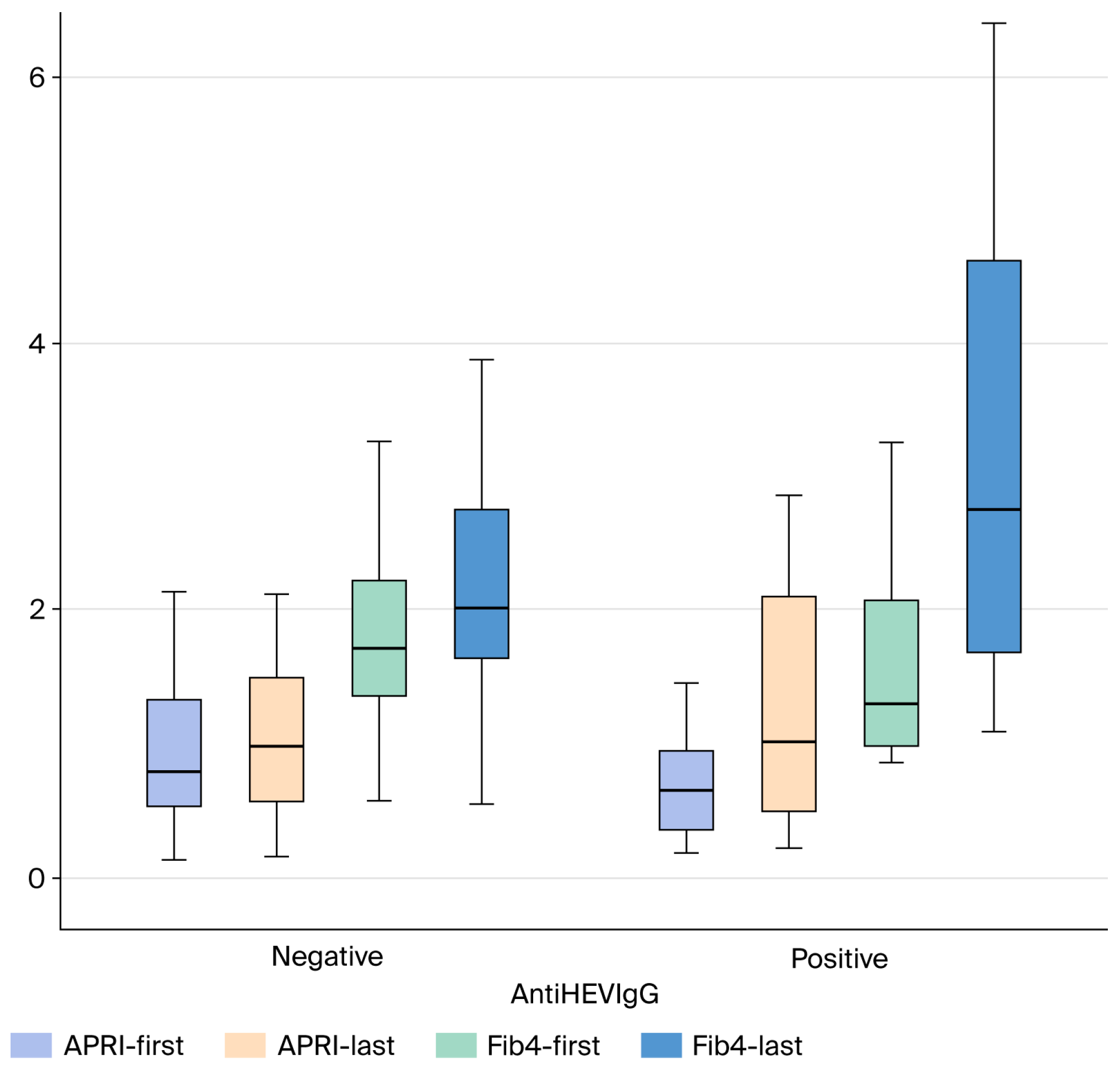
3.3. Long-Term Data of Seropositive and Seronegative Cryptogenic Cirrhosis Patients
4. Discussion
4.1. Increased HEV Seroprevalence in Cryptogenic Cirrhosis
4.2. Age, Immunosuppression, and Mechanisms of Chronicity
4.3. Comparison of Demographic Features and Liver Severity Scores by Anti-HEV Serology
4.4. Impact of HEV Positivity on Disease Severity and Prognosis
4.5. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APRI | AST-to-Platelet ratio |
| HEV | Hepatitis E virus |
| FIB-4 | Fibrosis-4 score |
| INR | International normalized ratio |
| MELD | Model for end-stage liver disease |
References
- Hoofnagle, J.H.; Nelson, K.E.; Purcell, R.H. Hepatitis E. N. Engl. J. Med. 2012, 367, 1237–1244. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitajima, N.; Abe, N.; Mishiro, S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology 2004, 330, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007, 127, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, M.; Kumar, A.; Kar, P.; Jilani, N.; Sharma, J.B. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: A prospective study from north India. Indian. J. Med. Microbiol. 2003, 21, 184–185. [Google Scholar] [CrossRef]
- Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. [Google Scholar] [CrossRef]
- Aslan, A.T.; Balaban, H.Y. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J. Gastroenterol. 2020, 26, 5543–5560. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef]
- Gérolami, R.; Moal, V.; Colson, P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860. [Google Scholar] [CrossRef]
- Jagjit Singh, G.K.; Ijaz, S.; Rockwood, N.; Farnworth, S.P.; Devitt, E.; Atkins, M.; Tedder, R.; Nelson, M. Chronic Hepatitis E as a cause for cryptogenic cirrhosis in HIV. J. Infect. 2013, 66, 103–106. [Google Scholar] [CrossRef]
- Tavitian, S.; Peron, J.M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M.; et al. Ribavirin for Chronic Hepatitis Prevention among Patients with Hematologic Malignancies. Emerg. Infect. Dis. 2015, 21, 1466–1469. [Google Scholar] [CrossRef]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.W.; Dalton, H.R. Hepatitis E: An underestimated emerging threat. Ther. Adv. Infect. Dis. 2019, 6, 2049936119837162. [Google Scholar] [CrossRef]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022, 77, 1109–1123. [Google Scholar] [CrossRef]
- Meng, X.J.; Dea, S.; Engle, R.E.; Friendship, R.; Lyoo, Y.S.; Sirinarumitr, T.; Toth, T.E.; Harris, K.E. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J. Med. Virol. 1999, 59, 297–302. [Google Scholar] [CrossRef]
- Waar, K.; Herremans, M.M.; Vennema, H.; Koopmans, M.P.; Benne, C.A. Hepatitis E is a cause of unexplained hepatitis in The Netherlands. J. Clin. Virol. 2005, 33, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Hercun, J.; Heller, T.; Vilarinho, S. Undiagnosed liver diseases. Transl. Gastroenterol. Hepatol. 2021, 6, 28. [Google Scholar] [CrossRef]
- Thuluvath, P.J.; Kantsevoy, S.; Thuluvath, A.J.; Savva, Y. Is cryptogenic cirrhosis different from NASH cirrhosis? J. Hepatol. 2018, 68, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Beha, D.; Plehm, K.; Zöllner, C.; Hofmann, J.; Schott, E. High prevalence of anti-hepatitis E virus antibodies in outpatients with chronic liver disease in a university medical center in Germany. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1431–1436. [Google Scholar] [CrossRef]
- Sezgin, O.; Yaraş, S.; Tezcan Ülger, S.; Aslan, G.; Tiftik, E.N. The Prevalence of Hepatitis E Virus Infection in the Adult Turkish Population: A Systematic Review of the Literature and Prevalence Study in Blood Donors in Mersin Province. Turk. J. Gastroenterol. 2021, 32, 782–789. [Google Scholar] [CrossRef]
- Dalton, H.R.; Bendall, R.; Ijaz, S.; Banks, M. Hepatitis E: An emerging infection in developed countries. Lancet Infect. Dis. 2008, 8, 698–709. [Google Scholar] [CrossRef]
- Mellgren, Å.; Karlsson, M.; Karlsson, M.; Lagging, M.; Wejstål, R.; Norder, H. High seroprevalence against hepatitis E virus in patients with chronic hepatitis C virus infection. J. Clin. Virol. 2017, 88, 39–45. [Google Scholar] [CrossRef]
- Kondili, L.A.; Chionne, P.; Porcaro, A.; Madonna, E.; Taffon, S.; Resuli, B.; Taliani, G.; Rapicetta, M. Seroprevalence of hepatitis E virus (HEV) antibody and the possible association with chronic liver disease: A case-control study in Albania. Epidemiol. Infect. 2006, 134, 95–101. [Google Scholar] [CrossRef]
- Fantilli, A.C.; Trinks, J.; Marciano, S.; Zárate, R.F.; Balderramo, D.C.; Martínez Wassaf, M.G.; Haddad, L.; Gadano, A.; Debes, J.D.; Pisano, M.B.; et al. Unexpected high seroprevalence of hepatitis E virus in patients with alcohol-related cirrhosis. PLoS ONE 2019, 14, e0224404. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, F.; Çavuş, B.; Pınarbaşı, B.; Bozacı, M.; Baran, B.; Akyüz, U.; Özdemir, H.H.; Yılmaz, U.S.; Türkay, N. Cryptogenic liver cirrhosis and hepatitis E virus (HEV): Are they related? Ann. Hepatol. 2019, 18, 585–589. [Google Scholar] [CrossRef]
- Faber, M.S.; Wenzel, J.J.; Jilg, W.; Thamm, M.; Höhle, M.; Stark, K. Hepatitis E virus seroprevalence among adults, Germany. Emerg. Infect. Dis. 2012, 18, 1654–1657. [Google Scholar] [CrossRef]
- Krumbholz, A.; Neubert, A.; Joel, S.; Girschick, H.; Huppertz, H.I.; Kaiser, P.; Liese, J.; Streng, A.; Niehues, T.; Peters, J.; et al. Prevalence of hepatitis E virus antibodies in children in Germany. Pediatr. Infect. Dis. J. 2014, 33, 258–262. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Lhomme, S.; Rostaing, L.; Izopet, J. Hepatitis E virus: Chronic infection, extra-hepatic manifestations, and treatment. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, P.V.; Pischke, S.; Schlaphoff, V.; Grabowski, J.; Fytili, P.; Gronert, A.; Bremer, B.; Markova, A.A.; Jaroszewicz, J.; Bara, C.; et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology 2012, 55, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Van der Eijk, A.A.; Pas, S.D.; de Man, R.A. Hepatitis E virus: A potential threat for patients with liver disease and liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 143–150. [Google Scholar] [CrossRef]
- Barragué, H.; Condat, B.; Petitdidier, N.; Champagne, E.; Renou, C.; Izopet, J.; Abravanel, F. Chronic hepatitis E virus infection in a cirrhotic patient: A case report. Medicine 2017, 96, e7915. [Google Scholar] [CrossRef]
- Blasco-Perrin, H.; Abravanel, F.; Blasco-Baque, V.; Péron, J.M. Hepatitis E, the neglected one. Liver Int. 2016, 36, 130–134. [Google Scholar] [CrossRef]
- Kumar Acharya, S.; Kumar Sharma, P.; Singh, R.; Das, K.; Sakhuja, P.; Gondal, R.; Jha, N.A.; Sarin, S.K. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J. Hepatol. 2007, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Marion-Audibert, A.M.; Tesse, S.; Graillot, E.; Phelip, G.; Radenne, S.; Duperret, S.; Durieux, M.; Rode, A.; Mabrut, J.Y.; Souquet, J.C.; et al. Lethal acute HEV superinfection on hepatitis B cirrhosis. Gastroenterol. Clin. Biol. 2010, 34, 334–336. [Google Scholar] [CrossRef]
- Bayram, A.; Eksi, F.; Mehli, M.; Sözen, E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology 2007, 50, 281–286. [Google Scholar] [CrossRef]
- Hamid, S.S.; Atiq, M.; Shehzad, F.; Yasmeen, A.; Nissa, T.; Salam, A.; Siddiqui, A.; Jafri, W. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology 2002, 36, 474–478. [Google Scholar] [CrossRef]
- Radha Krishna, Y.; Saraswat, V.A.; Das, K.; Himanshu, G.; Yachha, S.K.; Aggarwal, R.; Choudhuri, G. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009, 29, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]

| Variable (Predictor) | Odds Ratio (Exp(B)) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Age (years) | 1.005 | 0.984–1.025 | 0.661 |
| Gender (male/female) | 0.595 | 0.223–1.589 | 0.300 |
| Cirrhosis state (present/absent) | 6.142 | 2.221–16.984 | <0.001 |
| Anti-HEV IgG Negative (n = 30) | Anti-HEV IgG Positive (n = 22) | p Value | |
|---|---|---|---|
| Age (years) (mean ± SD) | 61.7 ± 12.1 | 69.7 ± 7.2 | 0.011 |
| BMI (kg/m2)(mean ± SD) | 24.9 ± 3.0 | 25.0 ± 2.4 | 0.911 |
| AST (U/L) (mean ± SD) | 45.4 ± 39.5 | 46.7 ± 27.5 | 0.894 |
| ALT (U/L) (mean ± SD) | 34.0 ± 35.5 | 31.1 ± 21.4 | 0.733 |
| Total Bilirubin (mg/dL) | 1.4 ± 0.9 | 1.3 ± 0.5 | 0.098 |
| Sodium (mEq/L) (mean ± SD) | 138.3 ± 3.5 | 135.6 ± 4.5 | 0.032 |
| Albumin (g/dL) (mean ± SD) | 3.6 ± 0.6 | 3.5 ± 0.6 | 0.409 |
| Creatinine (mg/dL) (mean ± SD) | 0.8 ± 0.2 | 1.0 ± 0.2 | 0.004 |
| Hemoglobin (gr/dL) (mean ± SD) | 11.8 ± 2.1 | 11.6 ± 1.9 | 0.803 |
| WBC (/mm3)(mean ± SD) | 5255.3 ± 2767.9 | 6398.8 ± 2266.2 | 0.169 |
| Platelet (×1000/mm3) | 124.5 ± 76.4 | 153.8 ± 64.4 | 0.175 |
| INR(mean ± SD) | 1.3 ± 0.4 | 1.4 ± 0.2 | 0.641 |
| AFP ng/mL (mean ± SD) | 3.1 ± 1.5 | 2.7 ± 2.0 | 0.551 |
| CTP (A/B/C) (n/n/n) | 18/11/1 | 10/12/0 | 0.341 |
| CTP (mean ± SD) | 6.71 ± 1.58 | 7.00 ± 1.66 | 0.541 |
| MELD-Na (mean ± SD) | 13.4 ± 2.33 | 14.8 ± 2.54 | 0.086 |
| FIB-4 score (mean ± SD) | 4.39 ± 2.71 | 5.02 ± 5.05 | 0.570 |
| APRI score (mean ± SD) | 1.11 ± 1.06 | 1.07 ± 1.11 | 0.894 |
| Anti-HEV IgG Negative (n = 30) | Anti-HEV IgG Positive (n = 22) | p Value | |
|---|---|---|---|
| HCC n (%) | 0/30 (0%) | 1/22 (4.6%) | 0.423 |
| Death due to cirrhosis n (%) | 4/30 (13.4%) | 8/22 (36.4%) | 0.047 |
| Death due to other causes n (%) | 1/30 (3.3%) | 1/22 (4.6%) | 0.820 |
| AST (U/L) (mean ± SD) | 47.7 ± 42.3 | 49.6 ± 30.8 | 0.884 |
| ALT (U/L) (mean ± SD) | 35.4 ± 37.4 | 25.8 ± 14.8 | 0.355 |
| Total Bilirubin (mg/dL) | 2.14 ± 1.89 | 2.65 ± 3.18 | 0.552 |
| Sodium (mEq/L) (mean ± SD) | 136.2 ± 7.6 | 137.8 ± 6.2 | 0.500 |
| Albumin (g/dL) (mean ± SD) | 2.87 ± 0.74 | 2.88 ± 0.70 | 0.981 |
| Creatinine (mg/dL) (mean ± SD) | 1.38 ± 1.51 | 1.24 ± 0.72 | 0.744 |
| Hemoglobin (gr/dL) (mean ± SD) | 10.2 ± 2.1 | 9.4 ± 4.9 | 0.243 |
| WBC (/mm3)(mean ± SD) | 6768 ± 5680 | 6256 ± 5043 | 0.169 |
| Platelet (×1000/mm3) | 124.1 ± 65.9 | 103.3 ± 64.0 | 0.175 |
| INR(mean ± SD) | 1.75 ± 0.86 | 1.77 ± 0.52 | 0.641 |
| AFP ng/mL (mean ± SD) | 4.2 ± 4.5 | 3.1 ± 2.4 | 0.551 |
| CTP (A/B/C) (n/n/n) | 6/12/12 | 4/7/11 | 0.763 |
| CTP (mean ± SD) | 8.63 ± 2.38 | 9.5 ± 2.44 | 0.879 |
| MELD-Na (mean ± SD) | 19.6 ± 9.28 | 25.1 ± 7.74 | 0.029 |
| FIB-4 score (mean ± SD) | 4.39 ± 2.71 | 5.02 ± 5.05 | 0.570 |
| APRI score (mean ± SD) | 1.51 ± 1.12 | 1.79 ± 2.37 | 0.579 |
| Variable | Hazard Ratio | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Age (years) | 1.073 | 0.563–6.695 | 0.055 |
| Gender (male/female) | 1.207 | 0.394–3.694 | 0.742 |
| AntiHEV IgG (negative/positive) | 1.941 | 0.563–6.695 | 0.294 |
| MELD-Na | 0.971 | 0.905–1.041 | 0.400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaraş, S.; Özdoğan, O.; Tezcan Ülger, S.; Aslan, G.; Tiftik, E.N.; Sezgin, O. Hepatitis E Virus (HEV) Seroprevalence in Cryptogenic Cirrhosis: From Evidence of High Frequency to the Impact on Disease Progression. Medicina 2025, 61, 2014. https://doi.org/10.3390/medicina61112014
Yaraş S, Özdoğan O, Tezcan Ülger S, Aslan G, Tiftik EN, Sezgin O. Hepatitis E Virus (HEV) Seroprevalence in Cryptogenic Cirrhosis: From Evidence of High Frequency to the Impact on Disease Progression. Medicina. 2025; 61(11):2014. https://doi.org/10.3390/medicina61112014
Chicago/Turabian StyleYaraş, Serkan, Osman Özdoğan, Seda Tezcan Ülger, Gönül Aslan, Eyüp Naci Tiftik, and Orhan Sezgin. 2025. "Hepatitis E Virus (HEV) Seroprevalence in Cryptogenic Cirrhosis: From Evidence of High Frequency to the Impact on Disease Progression" Medicina 61, no. 11: 2014. https://doi.org/10.3390/medicina61112014
APA StyleYaraş, S., Özdoğan, O., Tezcan Ülger, S., Aslan, G., Tiftik, E. N., & Sezgin, O. (2025). Hepatitis E Virus (HEV) Seroprevalence in Cryptogenic Cirrhosis: From Evidence of High Frequency to the Impact on Disease Progression. Medicina, 61(11), 2014. https://doi.org/10.3390/medicina61112014






