Efficacy of Insulin Eye Drops in the Treatment of Corneal Ulcers in Patients with Facial Nerve Palsy and Lagophthalmos: A Retrospective Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMT | Amniotic Membrane Transplantation |
| AS-OCT | Anterior Segment Optical Coherence Tomography |
| CECs | Corneal Epithelial Cells |
| OR | Odds Ratio |
| CI | Confidence Interval |
| IQR | Interquartile range |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| PED | Persistent Epithelial Defect |
| PPG | Polypropylene Glycol |
| PEG | Polyethylene Glycol |
| SED | Serum Eye Drops |
| SOC | Standard of Care |
| TBUT | Tear Break-Up Time |
| OSDI | Ocular Surface Disease Index |
| KT | Corneal Transplantation |
References
- Ahmed, F.; House, R.J.; Feldman, B.H. Corneal Abrasions and Corneal Foreign Bodies. Prim. Care 2015, 42, 363–375. [Google Scholar] [CrossRef]
- Musch, D.C.; Sugar, A.; Meyer, R.F. Demographic and Predisposing Factors in Corneal Ulceration. Arch. Ophthalmol. 1983, 101, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Werli-Alvarenga, A.; Ercole, F.F.; Botoni, F.A.; Oliveira, J.A.; Chianca, T.C. Corneal Injuries: Incidence and Risk Factors in the Intensive Care Unit. Rev. Lat. Am. Enferm. 2011, 19, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Epidemiological Characteristics, Predisposing Factors and Microbiological Profiles of Infectious Corneal Ulcers: The Portsmouth Corneal Ulcer Study. Br. J. Ophthalmol. 2009, 93, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Byanju, R.; Kandel, R.P.; Poudyal, B.; Bhandari, S.; Ligal, A.; Pradhan, S.; Gautam, M.; Shrestha, P.; Sah, R.K.; A Gonzales, J.; et al. Risk Factors for Corneal Ulcers: A Population-Based Matched Case-Control Study in Nepal. Br. J. Ophthalmol. 2023, 107, 1771–1775. [Google Scholar] [CrossRef]
- Pereira, M.V.; Glória, A.L. Lagophthalmos. Semin. Ophthalmol. 2010, 25, 72–78. [Google Scholar] [CrossRef]
- George, E.; Richie, M.B.; Glastonbury, C.M. Facial Nerve Palsy: Clinical Practice and Cognitive Errors. Am. J. Med. 2020, 133, 1039–1044. [Google Scholar] [CrossRef]
- Singh, S.; Das, A.V.; Ali, M.H. Ocular involvement in facial nerve paralysis: Risk factors for severe visual impairment and ocular surface exposure in 1870 patients. Orbit 2023, 42, 256–261. [Google Scholar] [CrossRef]
- Pepose, J.S.; Wilhelmus, K.R. Divergent Approaches to the Management of Corneal Ulcers. Am. J. Ophthalmol. 1992, 114, 630–632. [Google Scholar] [CrossRef]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous Serum Eye Drops in Dry Eye Disease: Preferred Practice Pattern Guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic Membrane Extract and Eye Drops: A Review of Literature and Clinical Application. Clin. Ophthalmol. 2018, 12, 1105–1112. [Google Scholar] [CrossRef]
- Lamas-Francis, D.; Navarro, D.; Moreno, C.; De-Rojas, V.; Mansilla, R.; Dios, E.; Rigueiro, J.; Álvarez, D.; Crego, P.; Rodríguez-Ares, T.; et al. Amniotic Membrane Transplantation in the Management of Corneal Ulceration Following Infectious Keratitis. Ocul. Immunol. Inflamm. 2024, 32, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.J.; McCaig, C.D.; Forrester, J.V.; Zhao, M. Insulin, Not Leptin, Promotes In Vitro Cell Migration to Heal Monolayer Wounds in Human Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, M.; Lorenc, A.; Leszczyński, R.; Mrukwa-Kominek, E. Topical Insulin in Neurotrophic Keratopathy: A Review of Current Understanding of the Mechanism of Action and Therapeutic Approach. Pharmaceutics 2023, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdi, P.; Ghaffari, R.; Azad, N.; Alshaheeb, A.; Latifi, G.; Soltani Shahgoli, S.; Fakhredin, H. Topical Insulin for Refractory Persistent Corneal Epithelial Defects. Sci. Rep. 2024, 14, 12459. [Google Scholar] [CrossRef]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Use of Topical Insulin to Normalize Corneal Epithelial Healing in Diabetes Mellitus. Arch. Ophthalmol. 2007, 125, 1082–1088. [Google Scholar] [CrossRef]
- Wang, A.L.; Weinlander, E.; Metcalf, B.M.; Barney, N.P.; Gamm, D.M.; Nehls, S.M.; Struck, M.C. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 2017, 36, 1426–1428. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Desai, D.T.; Kalaiselvan, P.; Dumpati, S.; Kuppusamy, R.; Masoudi, S.; Shah, D.O.; Willcox, M.D.P. Lipid-based eye drop formulations for the management of evaporative dry eyes. Contact Lens Anterior Eye 2024, 47, 102154. [Google Scholar] [CrossRef]
- Alcon Laboratories Inc. SYSTANE® COMPLETE Lubricant Eye Drops. Available online: https://systane.myalcon.com/products/systane-complete/ (accessed on 27 October 2025).
- Bušić, M.; Kuzmanović Elabjer, B.; Bosnar, D. Seminaria Ophthalmologica; Cerovski: Osijek, Croatia, 2014. [Google Scholar]
- Mandić, Z. Oftalmologija; Medicinska naklada: Zagreb, Croatia, 2014. [Google Scholar]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wouters, C.; Saelens, I.; Delbeke, H. Topical Insulin for Neurotrophic-Related Epithelial Defects: Where Do We Stand? A Systematic Review. J. Curr. Ophthalmol. 2024, 36, 9–22. [Google Scholar] [CrossRef]
- Soares, R.J.D.S.M.; Arêde, C.; Sousa Neves, F.; da Silva Fernandes, J.; Cunha Ferreira, C.; Sequeira, J. Topical Insulin—Utility and Results in Refractory Neurotrophic Keratopathy in Stages 2 and 3. Cornea 2022, 41, 990–994. [Google Scholar] [CrossRef]
- Dasrilsyah, A.M.; Wan Abdul Halim, W.H.; Mustapha, M.; Tang, S.F.; Kaur, B.; Ong, E.Y.; Bastion, M.L.C. Randomized Clinical Trial of Topical Insulin Versus Artificial Tears for Healing Rates of Iatrogenic Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Cornea 2023, 42, 1395–1403. [Google Scholar] [CrossRef]
- Krolo, I.; Behaegel, J.; Termote, K.; de Bruyn, B.; De Schepper, M.; Oellerich, S.; Ní Dhubhghaill, S. The Role of Topical Insulin in Ocular Surface Restoration: A Review. Surv. Ophthalmol. 2024, 69, 805–817. [Google Scholar] [CrossRef]
- Bastion, M.L.; Ling, K.P. Topical Insulin for Healing of Diabetic Epithelial Defects? A Retrospective Review of Corneal Debridement during Vitreoretinal Surgery in Malaysian Patients. Med. J. Malays. 2013, 68, 208–216. [Google Scholar]
- Quiroz-Mendoza, J.L.; García-Roa, M.; Romero-Morales, V.; Valera-Cornejo, D.; Vázquez-Membrillo, M.; Ramírez-Neria, P.; Villalpando-Gómez, Y.; García-Franco, R. Clinical Trial of Topical Insulin and Sodium Hyaluronate in the Treatment of Epithelial Defects Produced by Intraoperative Corneal Epithelial Debridement during Pars Plana Vitrectomy in Diabetics. Rev. Mex. Oftalmol. 2021, 95, 63–70. [Google Scholar] [CrossRef]
- NaPier, E.; Camacho, M.; McDevitt, T.F.; Sweeney, A.R. Neurotrophic Keratopathy: Current Challenges and Future Prospects. Ann. Med. 2022, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Ghimire, D.; Basu, S.; Agrawal, V.; Jacobs, D.S.; Shanbhag, S.S. Contact Lenses in Dry Eye Disease and Associated Ocular Surface Disorders. Indian J. Ophthalmol. 2023, 71, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Wirta, D.; Lipsky, W.; Toyos, M.; Martel, J.; Goosey, J.; Verachtert, A.; El-Harazi, S.; Karpecki, P.; Allegretti, M.; Goisis, G.; et al. Recombinant Human Nerve Growth Factor (Cenegermin) for Moderate-to-Severe Dry Eye: Phase II, Randomized, Vehicle-Controlled, Dose-Ranging Trial. BMC Ophthalmol. 2024, 24, 290. [Google Scholar] [CrossRef]
- Liu, S.H.; Saldanha, I.J.; Abraham, A.G.; Rittiphairoj, T.; Hauswirth, S.; Gregory, D.; Ifantides, C.; Li, T. Topical Corticosteroids for Dry Eye. Cochrane Database Syst. Rev. 2022, 10, CD015070. [Google Scholar] [CrossRef] [PubMed]
| Insulin Group (n = 20) | Control Group (n = 12) | p-Value | Test | |
|---|---|---|---|---|
| Female gender (n [%]) | 11 (55%) | 7 (58%) | 1.00 | Continuity corrected chi-square test |
| Male gender (n [%]) | 9 (45%) | 5 (42%) | ||
| Age (years, median [IQR]) | 58 (52–65) | 60 (50–64) | 0.58 | t-test |
| Oxford staining score | 2 (2–3) | 2 (2–3) | 0.47 | t-test |
| Schirmer I test (millimeters, median [IQR]) | 7 (5–9) | 8 (5–10) | 0.55 | t-test |
| TBUT (median [IQR]) | 6 (5–7) | 6 (5–8) | 0.73 | t-test |
| visual acuity (logMAR, median [IQR]) | 0.4 (0.3–0.5) | 0.4 (0.3–0.6) | 0.64 | t-test |
| OSDI score | 45 (40–55) | 46 (42–56) | 0.59 | t-test |
| Ulcer characteristics | ||||
| Ulcer size (mm2), n (%) | Small (<2): 7 (35%) Medium (2–5): 9 (45%) Large (>5): 4 (20%) | Small (<2): 4 (33%) Medium (2–5): 6 (50%) Large (>5): 2 (17%) | 1.00 | Fisher–Freeman–Halton exact test |
| Defect depth, n (%) | Superficial: 13 (65%) Stromal: 7 (35%) | Superficial: 7 (58%) Stromal: 5 (42%) | 0.72 | Fisher’s exact test |
| Defect duration before therapy (days), median [IQR] | 14 [10,11,12,13,14,15,16,17,18,19,20] | 15 [11,12,13,14,15,16,17,18,19,20,21,22] | 0.73 | Mann–Whitney test |
| Outcome | Insulin Group (n = 20) | Control Group (n = 12) | Test | p-Value | 95% CI for Difference in Proportions |
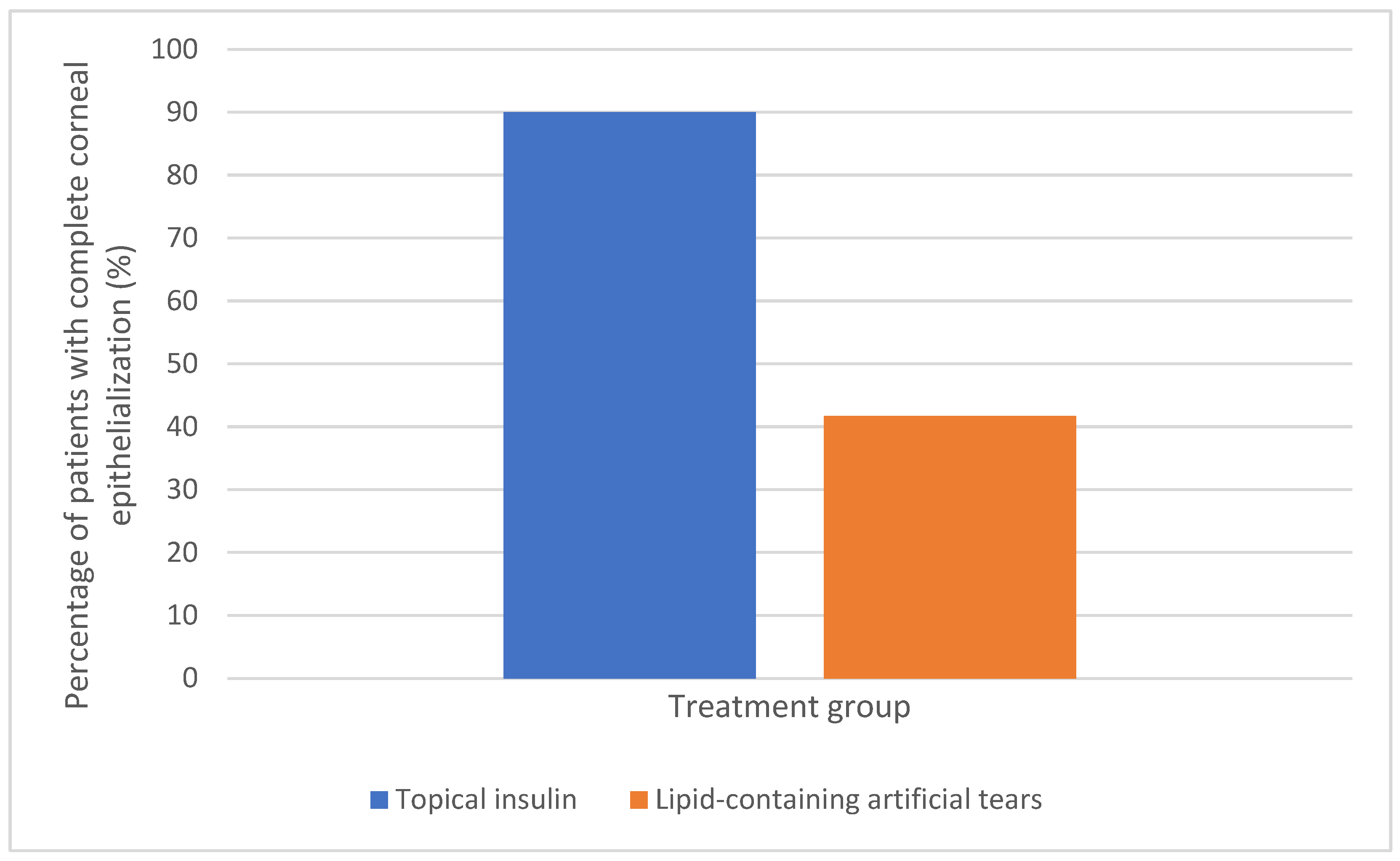
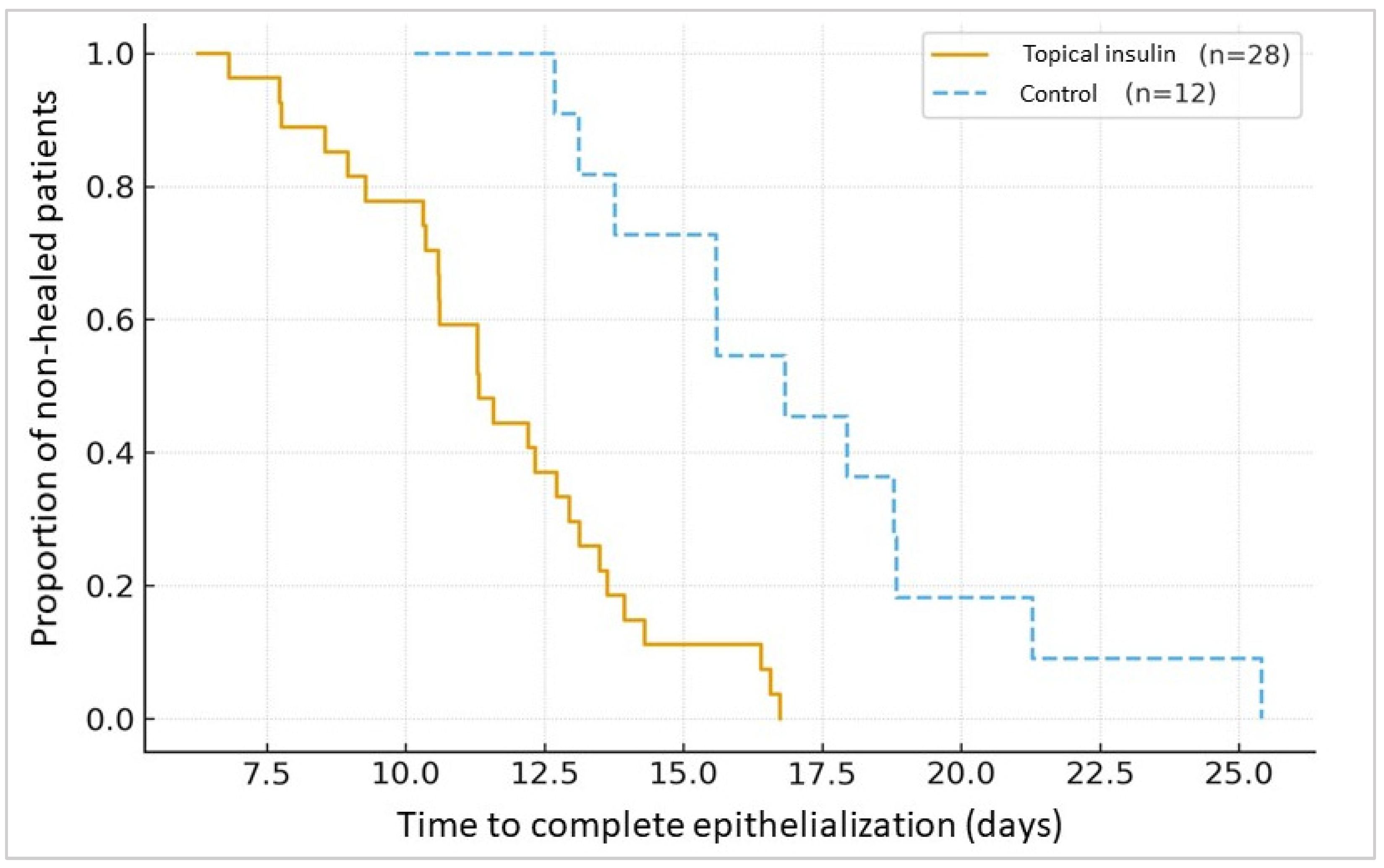
| Complete epithelialization | 90% | 41.7% | χ2 = 6.33 | 0.012 | 16–80% |
| TBUT ≥ 2 s improvement | 75% | 33% | χ2 = 4.56 | 0.033 | 6–72% |
| Schirmer I ≥ 3 mm improvement | 60% | 25% | χ2 = 3.92 | 0.048 | 2–65% |
| OSDI score reduction ≥ 20% | 70% | 33% | χ2 = 4.18 | 0.041 | 4–68% |
| Visual acuity ≥ 0.1 logMAR improvement | 50% | 25% | χ2 = 2.11 | 0.146 | −10–61% |
| Visual Acuity (logMAR) | |||||
| Parameter | Insulin Group (n = 20) | p (Within-Group) | Control Group (n = 12) | p (Within-Group) | p (Between-Groups, day-30) |
| Baseline logMAR (median [IQR]) | 0.40 [0.30–0.50] | - | 0.40 [0.30–0.60] | - | - |
| Final logMAR − day-30 (median [IQR]) | 0.20 [0.10–0.30] | 0.012 (Wilcoxon) | 0.30 [0.20–0.50] | 0.081 (Wilcoxon) | 0.045 (Mann–Whitney) |
| Change (ΔlogMAR = day-30 − baseline) | −0.20 [−0.30 to −0.10] | - | −0.10 [−0.20 to −0.00)] | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelić Vuković, M.; Matić, S.; Biuk, D.; Kalauz, M.; Kopić, A.; Nemet, V.; Strunje, I.; Maduna, I.; Vidović, S.; Kolak, E. Efficacy of Insulin Eye Drops in the Treatment of Corneal Ulcers in Patients with Facial Nerve Palsy and Lagophthalmos: A Retrospective Case–Control Study. Medicina 2025, 61, 1991. https://doi.org/10.3390/medicina61111991
Jelić Vuković M, Matić S, Biuk D, Kalauz M, Kopić A, Nemet V, Strunje I, Maduna I, Vidović S, Kolak E. Efficacy of Insulin Eye Drops in the Treatment of Corneal Ulcers in Patients with Facial Nerve Palsy and Lagophthalmos: A Retrospective Case–Control Study. Medicina. 2025; 61(11):1991. https://doi.org/10.3390/medicina61111991
Chicago/Turabian StyleJelić Vuković, Marija, Suzana Matić, Dubravka Biuk, Miro Kalauz, Andrijana Kopić, Vedran Nemet, Ivana Strunje, Ivanka Maduna, Stipe Vidović, and Ena Kolak. 2025. "Efficacy of Insulin Eye Drops in the Treatment of Corneal Ulcers in Patients with Facial Nerve Palsy and Lagophthalmos: A Retrospective Case–Control Study" Medicina 61, no. 11: 1991. https://doi.org/10.3390/medicina61111991
APA StyleJelić Vuković, M., Matić, S., Biuk, D., Kalauz, M., Kopić, A., Nemet, V., Strunje, I., Maduna, I., Vidović, S., & Kolak, E. (2025). Efficacy of Insulin Eye Drops in the Treatment of Corneal Ulcers in Patients with Facial Nerve Palsy and Lagophthalmos: A Retrospective Case–Control Study. Medicina, 61(11), 1991. https://doi.org/10.3390/medicina61111991









