Abstract
The pituitary gland is considered the conductor of the hormonal orchestra, and despite its small dimensions, numerous tumoral lesions can arise within it. Over the past decade, substantial changes have been made regarding the nomenclature, which are summarized in the 5th Edition of the World Health Organization Classification of Endocrine and Neuroendocrine Tumors. Furthermore, significant breakthroughs in biomolecular mechanisms have been uncovered, which have formed the basis for the new classification. The management of these lesions varies according to several factors such as tumoral dimensions, hormonal activity, symptomatology, and radiological findings. At the same time, the therapeutic goal is represented by normalization of hormonal hypersecretion if present, control of tumoral growth and/or relief of mass effect symptoms, and preservation or restoration of the pituitary function. The current narrative review aims to explore the link between biomolecular aspects, the extent of resectability, and the postoperative outcome.
1. A Brief Overview
1.1. Epidemiological Aspects
Out of all intracranial tumors in the adult population, pituitary neuroendocrine tumors (PitNETs) account for approximately 15% as per 2020 [1]. Earlier studies have shown that the estimated prevalence of these lesions in postmortem studies was 14.4% compared to 22.5% in radiography studies. The overall prevalence for both groups is estimated to be 16.7% [2]. Although epidemiologic studies have been used to estimate the current prevalence, their significant limitations are represented by dependence on population-specific registries and clinical diagnosis, which excludes silent incidental tumors [2]. Immunohistochemistry analyses have shown that, among all PitNETs, approximately 43% are prolactinomas, 2.8% somatotropinomas, 4.9% corticotropinomas, 1.4% gonadotropinomas, and 0.7% thyrotropinomas [2]. Functioning PitNETs, especially prolactinomas, have a clear female predominance, particularly in reproductive-age women, while non-functioning PitNETs have a male predominance and tend to be diagnosed later, at larger sizes [3]. Non-functioning PitNETs are the most common, while in functioning PitNETs, the most encountered tumors were represented by prolactinomas, somatotropinomas, corticotropinomas, and thyrotropinomas [4].
1.2. The Controversies of Nomenclature
In 1932, Harvey Cushing first introduced the term “pituitary adenoma” to describe the underlying cause of acromegaly. Notwithstanding that these tumors were subsequently treated as benign lesions, various characteristics differentiate them from typical benign-featured tumors [5]. These specific differentiating features were not only represented by a propensity for hemorrhage and necrosis, but also by the frequent invasive behavior [5].
As previously stated, pituitary tumors can invade the surrounding anatomical structures and grow rapidly despite optimized therapeutic management, and in these cases, the lesions are considered aggressive [6]. However, it is worth mentioning that invasiveness does not necessarily correlate with aggressiveness [6].
The latest changes in nomenclature were proposed in 2022 and were summarized in the 5th Edition of the World Health Organization (WHO) Classification of Endocrine and Neuroendocrine Tumors [7]. This classification introduces significant biomolecular changes, characterizing tumors beyond the conventional hormonal activity that has previously been the basis. Nowadays, tumoral lesions are classified based on cell lineage as determined by expression of transcription factors, hormones, and other biomarkers [7]. Although most scientists and physicians agreed on the new classification, others pleaded for reconsideration. The most cited reasons against the new nomenclature were represented by a more sinister connotation while removing information regarding developmental origins, a lack of distinction between endocrine and neuroendocrine cells, as well as the controversies regarding whether similar terminology should be attributed to other endocrine organs’ neoplasms [8]. Furthermore, the term PitNET asserts high-risk behavior, which is an infrequent exception [8]. The psychological burden of patients with benign pituitary lesions diagnosed as PitNET could lead to unjustified anxiety and result in more aggressive therapeutic management, even in cases of asymptomatic microadenomas [9]. However, despite ongoing debates, the new classification has been widely accepted and incorporated into clinical practice, as it raises the opportunity to implement structured and detailed reporting of these lesions [10,11]. Nevertheless, despite significant advances, especially in the biomolecular field, in most cases of PitNETs, the mechanisms behind pathogenesis are yet to be fully uncovered, and remain enigmatic in the majority of cases [12].
1.3. How to Diagnose a PitNET?
Based on their dimensions, pituitary tumors are classified into microadenomas (<10 mm), macroadenomas (10 mm), and giant adenomas (40 mm) [13]. In extensive lesions, compression on the neighboring anatomical structures can lead to various conditions like hypopituitarism, chiasmal syndrome, or diabetes insipidus. Thus, in cases where the neuroimaging examinations show tumor impingement, visual field testing and an evaluation for hypopituitarism are indicated [13]. In many instances of compression on the pituitary stalk, hyperprolactinemia can appear, known as the stalk effect. This phenomenon is due to pituitary lactotroph disinhibition. However, the stalk effect is temporary, as it resolves with tumor excision, and the prolactin level will drop postoperatively [14].
Some PitNETs can secrete excessive hormones and are known as functioning, while between 22% and 54% are not associated with clinical evidence of hormonal hypersecretion, and are called non-functioning [15].
Regarding the neuroimaging diagnosis, magnetic resonance imaging (MRI) is the most helpful imaging modality of choice (Figure 1).
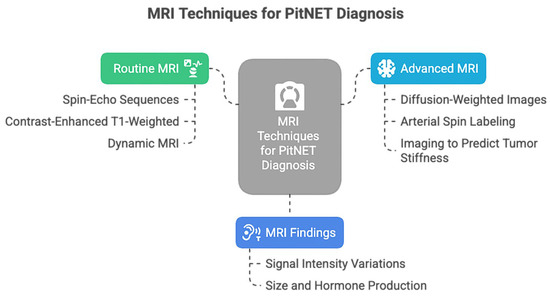
Figure 1.
MRI techniques used for PitNET diagnosis.
The routine MRI, represented by non-contrast or contrast-enhanced MRI, is useful for initial screening and lesion detection [5]. Dynamic MRI is also considered a valuable diagnostic tool in PitNETs, facilitating the detection of microadenomas and the visualization of compression and displacement of anatomical structures by macroadenomas. Furthermore, dynamic MRI could be used to predict GH-producing tumors in PitNETs [16]. However, nowadays, even more complex techniques are available, such as diffusion-weighted images (DWI), which reduce artifacts and enhance the image quality of the sellar region, supporting a precise detection of microadenomas [17]. Perfusion imaging can assess tumoral vascularity, can predict hemorrhage risk [18], and could monitor the response to medical therapy [19].
The stiffness of PitNETs can impede a complete resection, especially when using the transsphenoidal approach; thus, it could be helpful for surgical planning to predict this tumoral characteristic. Although no current imaging technology can fully evaluate the preoperative viscoelastic consistency of PitNETs, magnetic resonance elastography (MRE) can measure the propagation of mechanically induced shear waves through tissue to calculate stiffness [20,21].
Although certain assessment methods are highly suggestive, a multidisciplinary team is essential to establish a detailed and definitive diagnosis, which serves as the foundation for choosing the optimal treatment option.
1.4. Therapeutic Management
The therapeutic management of PitNETs varies according to several factors, including tumor dimensions, hormonal activity, symptomatology, and radiological findings [22]. The primary goals of therapeutic management are the normalization of hormonal hypersecretion, if present, control of tumor growth and/or relief of mass effect symptoms, and preservation or restoration of pituitary function. In functioning PitNETs, the therapeutic approach depends on the tumor subtype [23], and details about each type will be further discussed.
2. From Genes to Neurosurgical Margins
PitNETs originate from specific cells in the anterior hypophysis and are currently characterized by the expression of transcription factors, according to the 5th Edition of the WHO Classification of Endocrine and Neuroendocrine Tumors (Table 1) [12].

Table 1.
The key transcription factors in PitNETs are represented by PIT1—Pituitary Transcription Factor 1; SF1—Steroidogenic Factor 1; TPIT—T-box Pituitary Transcription Factor.
The biomolecular mechanisms underlying pituitary tumorigenesis are intricate and are still being studied. It is worth mentioning that the epigenetically mediated gene dysregulation represents a frequently encountered characteristic in tumoral development. Increasing evidence suggests that a significant contribution comes from bone morphogenic protein (BMP), Wnt, and fibroblast growth factor (FGF) families [24]. In addition, current evidence supports epigenetic alteration in other various PitNET genes, categorized based on function and alteration [25], as summarized in Figure 2.
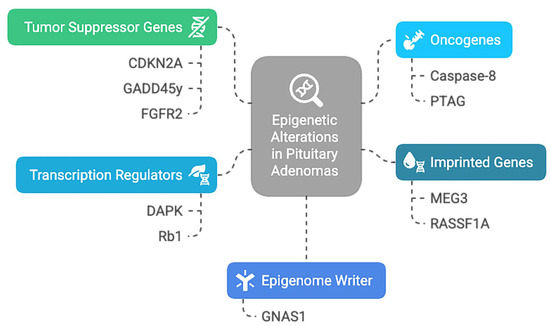
Figure 2.
Epigenetic alteration encountered in PitNETs: CDKN2A—Cyclin-Dependent Kinase Inhibitor 2A; GADD45y—Growth Arrest And DNA Damage Inducible Gamma; FGFR2—Fibroblast Growth Factor Receptor 2; PTAG—Rhomboid Domain-Containing Protein 3; DAPK—Death Associated Protein Kinase 1; Rb1—RB Transcriptional Corepressor 1; GNAS1—Guanine Nucleotide Binding Protein (G Protein), Alpha Stimulating Activity Polypeptide 1; MEG3—Maternally Expressed 3; RASSF1A—Ras Association Domain Family Member 1.
It has been stated that the DNA methyltransferases play significant roles as oncogenic factors in PitNETs development and progression. The overexpression of DNMT proteins is associated with macroadenomas, invasiveness, and aggressiveness, suggesting a poor prognosis, regardless of the therapeutic management [26].
Recent studies have revealed clinically relevant epigenetic markers that can aid in diagnosing and prognosticating patients with PitNETs, as serum and plasma cell-free DNA from these patients contains methylation fingerprints specifically related to the tumors. Furthermore, significant negative correlations between the methylation of cytosine–phosphate–guanine dinucleotide (CpG) and the expression levels of their putative target genes were discovered, concluding that these genes are epigenetically regulated by PitNET-specific differentially methylated probes [27].
A significant association between hypermethylation in PitNETs and the loss of neuronatin expression has been discovered in the last decade. It has been demonstrated that the co-localization of neuronatin to each major hormone-secreting cell type of the adenohypophysis and the decreased expression of this gene is associated with promoter hypermethylation, while the loss is irrespective of PitNET subtype. These findings suggest that epigenetic silencing of neuronatin has a significant impact on tumoral behavior and pathogenesis [28,29].
After acknowledging the main biomolecular aspects of PitNETs, a crucial question arises in the neurosurgeon’s mind: What is the connection between these genes and neurosurgery, and how can this information benefit the neurosurgeon? Although it may appear of no significant importance for the clinical practice at first glance, correlations between these two different scientific worlds have been concluded.
The most common PitNETs are represented by lactotrophinomas, followed in frequency by gonadotropinomas, somatotropinomas, corticotropinomas, and thyrotropinomas. However, sometimes no lineage differentiation, transcriptional factors, or hormone secretion can be identified, and in these cases, the tumors are considered null cell adenomas [30,31]. Similarly, tumors may produce unusual combinations of hormones and are known as plurihormonal adenomas [32].
2.1. Lactotrophinomas
Lactotroph PitNETs (LAs), also known as prolactinomas (PRLs), are the most frequently encountered functioning PitNETs in both men and women [33]. These common benign prolactin-secreting tumors derived from lactotrophs account for approximately 50% of all PitNETs, and between the second and fourth decades of life, the female-to-male ratio is up to 10:1, while after menopause, the ratio equalizes [33,34]. Microprolactinomas are very seldom proliferative and are of low concern for persistent long-term growth, being more frequent in women. Macroprolactinomas are very different and are more frequently seen in men [35]. These large tumors, although generally benign, are aggressive and invasive, often with extension into the suprasellar region and into the cavernous sinuses. In these cases, biochemical remission and tumor control can be achieved only with a multimodal therapeutic approach that includes neurosurgical excision, long-term treatment with dopamine agonists, and sometimes radiotherapy [35].
Various clinical and biological factors are associated with invasiveness and aggressiveness in LAs. Young patients, under 20 years old, tend to have tumors of larger dimensions and with a more aggressive behavior. At a young age, such a tumor should prompt exploration for a possible genetic predisposition, which is associated with a poorer prognosis [36]. Familial LAs comprise approximately 3% of the cases and are associated with genetic syndromes such as MEN1 and 4, familial isolated PitNET, Carney syndrome, and hereditary paraganglioma–pheochromocytoma syndrome [37,38]. Pediatric PitNETs report the germline aryl hydrocarbon receptor-interacting protein (AIP) gene mutation in approximately 20% of the cases [39,40], but also menin (MEN1) mutations, in both sporadic and familial cases [41,42]. Besides having more aggressive tumors, patients with AIP and MEN1 mutations tend to have cabergoline-resistant PRLs [3,43].
The tumoral dimensions also influence prolactin levels. Very high prolactin values have been correlated with poor surgical outcomes, while low levels may occasionally be observed in poorly differentiated tumors. However, low prolactin levels are also common in microprolactinomas due to the small tumor volume, and in large tumors, it is most often explained by stalk disconnection. Therefore, low prolactin levels alone should not be interpreted as a marker of poor differentiation [44,45].
Resistance to dopamine agonists has also been proven to be of major importance, as this is the first-line therapeutic option in LAs [46]. In cases with abnormal serum prolactin levels after dopamine agonist therapy, the resistant tumors exhibit a more severe clinical course, are larger in volume, more invasive, more aggressive, and have a higher Ki-67 index, especially in men [47,48].
It has been demonstrated that the human pituitary tumor-transforming gene (PTTG) is overexpressed in LAs with high invasiveness. This gene is located on chromosome 5, at position 5q33, and it induces cell transformation [49]. Thus, the PTTG abundance is a molecular marker for invasiveness in hormone-secreting PitNETs, and also plays a role in tumorigenesis [50]. It is worth mentioning that invasiveness refers primarily to radiological or intraoperative evidence of tumor extension into adjacent structures (e.g., cavernous sinus), while aggressiveness denotes biological behavior such as rapid growth, high Ki-67 index, resistance to medical therapy, or recurrence despite optimal treatment [51].
Expression of the p53 tumor suppressor gene has been controversial in PitNETs due to technical problems. However, in some LAs, elevated prolactin expression and loss of p53 have been observed, potentially influencing tumoral behavior, promoting more aggressive characteristics [51].
Chromosome abnormalities were also involved in the clinical course of the disease, with loss of heterozygosity (LOH) being the most extensively studied. Invasive tumors demonstrated a significantly higher frequency of deletions affecting 11q13, 13q12-14, and 10q26. Moreover, allelic deletion correlates with increasingly invasive behavior [52]. Integrated genomic profiling reveals the loss of chromosome 11p, which impacts transcriptomic activity in aggressive PRLs. Comparison of genomic and transcriptomic data demonstrated that allelic loss impacted upon expression of genes located in the imbalanced region, among which DGKZ, CD44, TSG101, GTF2H1, and HTATIP2 were responsible for triggering aggressive and malignant phenotypes of PRLs [53].
Adhesion molecules and metalloproteases were shown to be involved in LAs. Expression of E-cadherin and beta-catenin was significantly lower in invasive PRLs, and the reduced expression was more frequently detected in invasive tumors. In addition, expression of E-cadherin was lower in macroprolactinomas, and decreased expression predicted higher Ki-67 indexes [54]. The matrix metalloproteinases inhibitor TIMP-2 was overexpressed in noninvasive PitNETs, suggesting that it could play a role in the aggressiveness of LAs [55,56,57].
Lastly, a variety of genes related to proliferation and invasion were described in LAs. Seven genes were associated with recurrence and progression: ADAMTS6, CRMP1, PTTG, ASK, CCNB1, AURKB, and CENPE, while ADAMTS6, CRMP1, ASK, CCNB1, and CENPE were also associated with tumor recurrence and progression [58].
Table 2 summarizes the correlations between biomolecular parameters and neurosurgical aspects in lactotropinomas.

Table 2.
Correlations between biomolecular parameters and neurosurgical aspects in lactotropinomas.
2.2. Gonadotropinomas
Pituitary gonadotropinomas are usually classified as nonfunctioning PitNETs, since in the majority of cases they do not cause clinically evident hormone excess. However, approximately 35% secrete biologically active luteinizing hormone (LH) or follicle-stimulating hormone (FSH), in which case they are considered functioning gonadotropinomas [59]. These tumors are characterized by the expression of SF1, GATA3, and ERα, and often contain β-FSH or β-LH subunits. The vast majority of GAs are macroadenomas, mostly with supra—and/or parasellar extension. Usually, there is a hypersecretion of FSH, while the LH levels are normal or decreased. The hypothesis of the lack of biological activity in some cases with increased gonadotropin secretion is reported in the current literature [60].
Before 2017, most medical studies on nonfunctioning GAs were combined with null cell adenomas and other nonfunctioning PitNETs; thus, the data that specifically study GAs is rather scarce [61].
Newey et al. performed whole-exome sequencing using DNA from 7 nonfunctioning PitNETs originating from gonadotroph cells, and identified 24 mutations that occurred in independent genes with no recurrent mutations. The authors concluded that these tumors harbor few somatic mutations, consistent with their low proliferation rates and benign nature. However, it is worth mentioning that besides somatic mutations, other mechanisms are probably responsible for the etiology [62].
Falch et al. analyzed the gene expression of fast and slow-growing nonfunctioning GAs and found 350 genes that were significantly differentially expressed [63]. While metadherin (MTDH), but not endomucin (EMCN), demonstrated involvement in cell migration and association with epithelial-to-mesenchymal transition (EMT) markers, the study concluded that genes related to EMT have higher expression in fast-growing tumors. Furthermore, MTDH is an essential contributor to aggressiveness, while other genes might be useful as a biomarker tool for tumoral growth and possible therapeutic targets [63].
It has been stated that the noncoding RNA Maternally Expressed Gene (MEG), which regulates p53 gene expression, is downregulated by methylation in a series of functioning GAs. This is especially of great interest since the downregulation of MEG promotes cellular proliferation. Notwithstanding, in GAs, the proliferation is counteracted by high expression of antiproliferative genes p27/p16 [64].
The Ki-67 expression is higher in patients who require a secondary surgical intervention, while in patients with a Ki-67 index lower than 3% a second surgery was less necessary. Furthermore, the proliferative index was also higher in those who received postoperative radiotherapy than in those treated only by neurosurgical intervention [65,66].
However, given their rarity, the full biomolecular mechanisms underlying the pathogenesis of functioning GAs are yet to be fully understood.
Regarding the neurosurgical approach, the transsphenoidal resection is the initial treatment of choice and can reduce endocrine disturbances, provide tissue for analysis, and improve neurologic manifestations [67]. Because the rate of recurrence in macroadenomas is high, the optimal surgical strategy is not yet defined, leading to high morbidity and mortality. In some cases, a flexible combination between the transsphenoidal and transcranial approach can maximize the grade of resection [68].
The correlations between the biomolecular aspects of GAs and neurosurgery are summarized in Table 3.

Table 3.
Correlation between biomolecular aspects and neurosurgery in gonadotropinomas.
2.3. Somatotropinomas
Somatotroph PitNETs (SAs) comprise approximately 20% of all PitNETs and are well known for determining acromegaly, which leads to significant morbidity and mortality. Acromegaly is caused by dysregulated hypersecretion of growth hormone (GH), leading to an overproduction of insulin-like growth factor 1 (IGF-1) [69]. The most common tumor correlated with acromegaly is represented by densely granulated somatotroph adenoma (DGSA), usually highly hormonally active, in which the tumor cells express PIT1, GH, and alpha subunit of glycoprotein hormones (αSU) [70,71]. They are usually diagnosed at a younger age due to their hormonal activity and are smaller in size [70,71]. On the other hand, the sparsely granulated somatotroph tumor (SGSA) has fewer secretory granules and can be negative or only weakly positive for GH [72].
Many DGSA harbor activating mutations of the GNAS gene. The protein product of this gene, Gsα, mediates signaling from seven transmembrane domain G-protein coupled receptors to activate cyclic AMP, which explains the sensitivity to somatostatin inhibitors, unlike SGSAs, which are mainly resistant to medical therapy and respond better to surgical treatment [12].
Regarding the neurosurgical aspects, it has been demonstrated that SGSAs are much less likely to achieve remission after 3 months and during the follow-up period in comparison to DGSAs [73]. Furthermore, due to incomplete feasible resection, SGSAs are often reoperated, and approximately 14% achieve postoperative cure, in comparison to DGSAs with a percentage of 65%, independent of patients’ age and tumoral dimensions [73]. It is worth mentioning that in the preoperative settings, SGSAs tended to have higher Knosp grades, lower GH indexes, and normalized IGF-1 levels [74]. However, despite various therapeutic options, the neurosurgical approach is the main treatment, especially the endoscopic transsphenoidal approach, providing fewer complications and better outcomes [75].
In a recent study of 83 patients with SAs treated by the endoscopic transsphenoidal approach, the GH level at diagnosis and operation, tumor dimensions, and residual tumor were significantly correlated with remission results. In addition, patients with lower GH levels, smaller tumors, and no residual tumors were more likely to achieve biochemical remission. Similarly, patients with DGSAs histology were more likely to achieve GH levels less than 2.5 ng/mL than those with SGSAs [75].
A recent meta-analysis, including 1223 studies, was performed by Vuong et al. on the clinical and prognostic significance of granulation patterns in SAs [76]. The authors concluded that SGSAs had significantly larger tumoral dimensions at presentation, as the rate of macroadenomas was 3 times higher when compared to DGSAs. Furthermore, SGSAs had a significantly higher risk of cavernous sinus invasion and a higher Ki-67 index, which required a close postoperative radiologic follow-up. For tumors that needed postoperative somatostatin receptor ligand therapy and/or other medical therapy, DGSAs were correlated to a significantly better biochemical response rate. Regarding the biomolecular characteristics, it has been concluded that SGSAs were associated with a lower prevalence of the GNAS mutation [76].
Bakhtiar et al. analyzed the relationship between cytokeratin staining patterns and clinico-pathological features in SAs. They concluded that these tumors typically exhibit a perinuclear pattern (PP) and a dot pattern (DP) in cytokeratin immunostaining [77]. The authors stated that DP tumors had significantly larger dimensions and a higher Ki-67 proliferative index. Furthermore, the DGSAa typically exhibited a PP, while SGSAs exhibited a DP. The GNAS mutation was less frequent in DP tumors, and the frequency of DP was higher in younger patients. Given the more invasive and aggressive features of DP tumors, associated with diffuse growth and suprasellar extension, the endonasal approach could be limited, despite usually being reported as curative in more than 60% of SAs [78]. These characteristics could also be explained by a significantly higher expression of E-cadherin in DP tumors, which mainly influences tumoral growth and invasiveness [77].
The correlations between biomolecular, histopathological, and clinical aspects and neurosurgical implications in SAs are summarized in Table 4.

Table 4.
Correlations between biomolecular, histopathological, and clinical aspects, as well as neurosurgical implications, in SAs.
2.4. Corticotropinomas
Corticotroph PitNETs (CAs) account for approximately 15% of all PitNETs and cause ACTH oversecretion, responsible for Cushing’s disease [79]. This represents the most frequent cause of Cushing syndrome, or chronic excess of endogenous glucocorticoids. In 90% of the cases, it presents as a microadenoma, sometimes not visible on neuroimaging investigations [79]. In approximately 10% of the cases, CAs stain ATCH without causing Cushing disease and are considered silent (SCAs). These tumors are reportedly more aggressive, with increased cavernous sinus invasion and progression/recurrence [80].
From a histopathological perspective, CAs are categorized as densely granular, sparsely granular, and Crooke cell adenoma [81]. TPIT1 represents the transcriptional factor for CAs [82]. In these tumors, no common germline mutations were detected; however, somatic mutations were reported in up to 60% of the cases, and the most frequently encountered is an activating mutation in the ubiquitin-specific peptidase 8 enzyme (USP8), which promotes tumorigenesis, leading to increased cell proliferation and invasiveness [64]. USP8 mutations are strongly associated with microadenomas and are more frequent in women. The presence of these mutations may predict favorable responses to somatostatin analog pasireotide, which exhibits high affinity for SSTR5 and double SSTR2/SSTR5 positivity [83]. Moreover, in cases with USP8 wild-type status, lower surgical cure rates, more invasive behavior, and poorer response to medical therapy were recorded. No significant difference in hormonal levels was observed concerning USP8 status, but USP8-variant carriers were more likely to achieve surgical remission than wild-type PitNETs [83,84].
A recent study by Nerubenko et al. assessed the clinical significance of somatic USP8 variants in CAs derived from patients with Cushing’s disease. The study concluded that in patients with CAs harboring USP8 variants, there was a prevalence of microadenomas, a higher recurrence after successful surgery, and the prevalence of SST5 and SST2 receptors’ expression [85]. An unexpected finding of the study was positive SST2 expression in the majority of USP8-mutant CAs and in three of ten USP8 wild-type tumors [85]. Furthermore, the authors hypothesized that the USP8 mutation may result in multidirectional alterations in the regulation of cell growth and proliferation, and hormonal production in CAs [85].
Similarly, USP48 was also proposed as an essential factor in the pathogenesis of these tumors. Mutations in this gene were associated with female gender and smaller tumoral dimensions [86].
Kober et al. evaluated the expression of glucocorticoid (GR) and mineralocorticoid receptors (MR) in CAs and SCAs. They concluded that there was a correlation between the expression levels of NR3C1 and NR3C2, the genes encoding these receptors. Consistent with prior data, the authors demonstrated a higher NR3C1 expression in SCAs in comparison to tumors causing Cushing’s disease, but no difference in NR3C2 was observed [87]. In addition, in patients with Cushing’s disease, both genes were negatively correlated with the tumoral size and morning plasma ACTH. Higher NR3C2 expression was observed in patients with postoperative remission and in densely granular CAs, whereas the expression of both genes and GR protein was higher in USP8-mutated tumors. Finally, a negative correlation between GR and tumor size, and higher NR3C1 expression was demonstrated in densely granulated tumors [87].
TP53 mutations in CAs were also studied and were associated with a younger age, did not show sex predominance, and were more invasive, larger, with lower complete ressection rates. A Ki-67 index ≥ 3, together with p53 immunostaining and mitotic count, was correlated with tumor aggressiveness [88]. Uzilov et al. stated that USP8 and TP53 gene mutations are mutually exclusive, with the latter occurring only in USP8 wild-type tumors [89]. The authors suggested that USP8-mutated tumors have better surgical remission rates, whereas USP8 wild-type tumors, especially those with TP53 mutations, are correlated with high invasiveness and worse clinical outcomes. Furthermore, TP53 mutations were associated with larger tumoral volumes, higher proliferative indexes, higher Knosp grades, higher rates of subtotal resection, and lower 10-year survival rates in comparison to USP8-mutated or USP8/TP53 wild-type tumors (27% versus 86–100%) [89,90].
Mutations in the alpha thalassemia/intellectual disability syndrome X-linked (ATRX) gene were also described in up to 32% of CAs, and were correlated with aggressiveness, resistance to therapy, and potentially metastatic PitNETs [91,92].
The management of CAs, especially with high aggressiveness and invasiveness, remains a therapeutic challenge due to incomplete resection. A multimodal approach could lead to gross total resection and biochemical remission in approximately half of the patients, and if remission is not achieved by neurosurgical intervention, other therapeutic options are required [93].
A summary of correlations between biomolecular aspects and neurosurgery in corticotropinomas has been presented in Table 5.

Table 5.
Summary of correlations between biomolecular aspects and neurosurgery in corticotropinomas.
2.5. Thyrotropinomas
Tyrotroph PitNETs (TAs) account for less than 1% of all PitNETs, are characterized by excessive thyrotropin secretion, and are not linked to either germline or somatic mutations. The transcriptional factors for TAs are PIT1 and GATA3, and the hormones by immunohistochemistry are β-TSH and the α-subunit [37,61].
Although previous reports described the neurosurgical cure as difficult, given the invasive nature and larger tumoral dimensions, with the current ultrasensitive immunometric assays, these lesions are more often early diagnosed [94]. However, in those cases that were not early diagnosed, TAs are commonly invasive and large macroadenomas, and the first-line therapy is represented by transsphenoidal surgery, despite the mostly unsatisfactory results [95].
Although the biomolecular aspects of TAs have not been thoroughly studied, these tumors are known for the lack of expression of the GNAS oncogene [96,97]. The failure to detect evidence for activating mutations leaves open the search for alternative mechanisms underlying TA’s tumorigenesis [98].
From a genetic perspective, TAs are distinct from other PitNETs, as they do not have a hereditary background. A few cases were associated with multiple endocrine neoplasia type 1, which is linked to loss of heterozygosity on 11q13 and inactivating mutations of the MEN1 gene, yet data suggest that menin does not play a causative role in the tumorigenesis of TAs [99].
It is worth mentioning that in the majority of cases, TAs overexpress PIT1, which is a lineage-defining transcription factor also associated with somatotropinomas and prolactinomas. Hence, it has been demonstrated that in approximately 70% of the cases, TAs are plurihormonal [100,101]. In addition, unlike other PitNETs, the Ki-67 proliferation marker is usually low in TAs, with sporadic exceptions [102], but the tumoral development and growth are often driven by differentiation rather than proliferation [94]. Moreover, despite old studies suggesting that TAs are negative for p53, a new study by Căpraru et al. demonstrated that expression of p53 can be positive in up to 41% of these tumors [102]. The same research concludes that monohormonal tumors were larger than plurihormonal ones, but clinical and biological signs of hyperthyroidism were more frequent in the plurihormonal tumors. Similarly, monohormonal tumors had higher expression of SSTR2A and no level or low level of expression of SSTR5, while plurihormonal tumors (TSH+GH) expressed SSTR5 at high levels [102]. Regarding the neurosurgical aspects, it has been stated that while transsphenoidal surgery allows complete resection in most cases, the curative rate oscillates between 60% and 75%, and is lower in cases of macroadenomas with sinus invasion [95]. The neurosurgical approach is also recommended in recurrences, although much more attention is needed due to tumor invasive regrowth and fibrosis, which can cause perioperative complications. The most significant predictor of surgical success remains the degree of invasion into the cavernous sinuses [103].
Currently, the criteria for a complete remission of TAs comprise the disappearance of hyperthyroidism and neurological manifestations, the removal of the entire tumor demonstrated on neuroimaging studies, and normal TSH, FT3, and FT4 in the blood for at least 3 months postoperatively [104].
The correlation between TAs’ biomolecular aspects and neurosurgical implications has been summarized in Table 6.

Table 6.
Summary of the link between biomolecular aspects and neurosurgery in thyrotropinomas.
2.6. Null Cell Adenomas
Null cell adenomas (NcAs) represent approximately 0.6% of all PitNETs and are defined as immunonegative for all adenohypophyseal hormones, with a lack of cell-type-specific transcription factors [30]. Before 2017, NcAs were considered nonfunctioning, given the lack of hormone expression. Lee et al. analyzed 147 PitNETs that were previously classified as NcAs, and concluded that only 68 cases were potentially correctly diagnosed. The authors concluded that PIT1 can be utilized as a second-tier immunostain in cases of clinically nonfunctioning PitNETs that are immunonegative for all hormones and SF1, to segregate rare cases of PIT1-positive PitNETs from NcAs [105]. Thus, it is of significant importance to note that, due to recent changes in the pathological classification of these tumors, there is a scarcity of data regarding their biomolecular aspects. Hence, the current review of NcAs is mainly based on the current state of knowledge.
A recent study by Woo et al., using the 5th WHO Classification, the latest version, concluded that patients with NcAs and multiple PitNETs had less disease-free survival compared to those with GA. In addition, consistent with prior studies, the authors demonstrated that these tumors were a more aggressive subtype that was prone to invade, with a higher risk of residual disease [106].
Balogun et al. analyzed 31 patients with NcAs and concluded that the preoperative invasion into the cavernous sinus was a predictor of tumoral remnants postoperatively [107]. Moreover, preoperative invasiveness into the cavernous sinus and negative P27 expression independently predicted subsequent growth of the residual tumor. In the same study, when compared to patients with sparsely granulated adenomas in which tumors grew rapidly postoperatively and slowly after surgery, those with NcAs had tumors that grew rapidly prior to surgery and continued to exhibit rapid postoperative growth. When it comes to the Ki-67 index, NcAs had higher values, over 3%, which was consistent with prior studies [107,108,109]. A summary of the link between biomolecular and neurosurgical aspects is presented in Table 7.

Table 7.
A summary of the link between biomolecular and neurosurgical aspects in null cell adenomas.
2.7. Plurihormonal Adenomas (PAs)
PAs are categorized based on their transcription factor expression in PIT1-positive PitNETs, and with more than one transcription factor (PAwUIC) [61]. They are characterized by aggressive behavior with higher values of the percentage of invasiveness into neighboring anatomical structures. The PAs can only be identified by pathological assessment, and an initial endocrinologic diagnosis according to hormonal and clinical features is necessary [110]. A study by Aydin et al. evaluated data from 27 patients with PAs and concluded that the majority were positive for more than one transcription factor. In comparison, nine patients were diagnosed with PIT1-positive PAs. More than 80% of the patients included in the study had macroadenomas, and high aggressivity was recorded in nearly half of these cases after pathological examination. Approximately 77% of the patients with PAs with more than one transcription factor had features of non-functioning tumors, and four patients had features of functioning PitNETs. Concerning the neurosurgical aspects, PAwUIC showed a lower rate of gross total resection, while in PIT1-positive PAs, a rate of more than 77% was registered [110].
A study by Micko et al. compared the clinicopathological parameters of PAwUIC with transcription factors for gonadotropinoma (TFGA) expression with gonadotropinomas that only express TFGA [111]. The results concluded larger tumor dimensions in the TFGA-only group. Regarding the neurosurgical aspects, in TGFA-plus patients, invasive behavior was more frequent. Furthermore, in the TFGA-only group, gross-total resection was significantly higher in comparison to the non-functioning TFGA-plus group. These findings suggest that TFGA-positive tumors are more aggressive when an additional transcription factor is expressed. Moreover, the study concluded that in these lesions, shorter radiographic surveillance and earlier reintervention are recommended [111].
PIT1-positive plurihormonal adenomas were also described as very distinctive entities. They are mostly reported as macroadenomas with aggressive behavior, very invasive, and with a higher rate of recurrence [112]. These tumors are not always silent and represent poorly differentiated monomorphous plurihormonal PIT1 lineage PitNETs [113]. The only neurosurgical approach that can offer the patient the most benefits is aggressive neurosurgical intervention. Very often, due to tumor persistence, external beam radiation is required. Nevertheless, aggressive surveillance must be initiated immediately after the diagnosis, with a low threshold for radiotherapeutic intervention, even in cases of minimal recurrent diseases [112].
A summary of the link between biomolecular parameters and neurosurgical aspects in PAs is presented in Table 8.

Table 8.
Summary table linking neurosurgical aspects and biomolecular parameters in plurihormonal adenomas; EBRT—External beam radiation therapy.
A synthesis table that integrates the significant biomolecular markers across all PitNET subtypes with their direct neurosurgical implications is presented below (Table 9).

Table 9.
Biomolecular markers with direct neurosurgical relevance in PitNETs.
2.8. Postsurgical Clinical Implications and Decision-Making
An important aspect regarding advances in the molecular and histopathological fields is that all the presented biomarkers have a direct influence on intraoperative management and postoperative planning in PitNETs. Notwithstanding that the majority of these molecular and genetic biomarkers are identified after the neurosurgical intervention, their clinical relevance extends to the multidisciplinary management [70].
One of the most essential studied biomarkers in PitNETs remains the Ki-67 proliferative index, which indicates not only a higher proliferative tumoral feature but also a greater risk of recurrence. Furthermore, a higher Ki-67 index in neurosurgical practice translates to lower chances of achieving maximal resection. In addition, in the postoperative setting, a higher Ki-67 index indicates the need for a more rigorous MRI follow-up and, in some cases, the necessity of early radiotherapy adjuvant [114].
Similarly, the PTTG plays a significant role in postsurgical clinical implications and decision-making in PitNETs. This biomarker, which has an oncogenic role, is responsible for local invasion, especially in lactotroph tumors. Its overexpression is associated with an increased risk for cavernous sinus invasion, which will limit the extent of resection [115].
Similarly, p53 mutations or overexpression have been associated with a more aggressive tumor behavior in lactotroph and corticotroph PitNETs as a sign of impending recurrence after operation [97].
Cell adhesion marker changes, such as E-cadherin and β-catenin, are equally significant. Their loss suggests tumor invasiveness, which neurosurgeons can anticipate as a predictor of cavernous sinus extension and reduced likelihood of gross total resection. In these situations, long-term radiological follow-up is necessary, with adjuvant therapy being a consideration in cases of infiltrative growth [12].
Matrix metalloproteinase regulators also contribute to the invasive characteristics of the lactotroph tumor. Low TIMP-2 levels are associated with increased extracellular matrix degradation, greater surgical complexity, and a higher risk of incomplete resection. Such patients need close observation for recurrence after surgery and could be offered early adjunctive treatment [116].
Genetically, the GNAS mutations are particularly relevant in somatotroph PitNETs. Densely granular somatotroph adenomas with GNAS mutations are likely to be responsive to somatostatin analogs and tend to be less challenging to resect. However, sparsely granular variants are less responsive and are more likely to require multimodal therapy and closer follow-up. Additionally, the cytokeratin pattern (dot-like versus perinuclear) can provide insight into tumor morphology and behavior. The DP-pattern somatotroph tumors are generally larger, more invasive, and less likely to be totally resected, and thus are candidates for early consideration of radiotherapy or reoperation [117,118].
In corticotrophinomas, USP8 mutations are associated with smaller, less malignant microadenomas and higher rates of surgical remission. The same mutations also predict improved response to medical treatment such as pasireotide, which impacts endocrine follow-up management [83]. TP53 and ATRX mutations, on the other hand, are associated with tumors with a more malignant, therapy-resistant profile, where subtotal resection and early adjuvant radiotherapy or medical treatment are typically required [79,88].
Genetic syndromes such as AIP and MEN1 germline mutations are of surgical significance since they are invariably associated with large, dopamine-resistant tumors occurring in young patients. These are often so severe that they necessitate early surgery despite medical treatment, followed by genetic counseling and long-term follow-up [119].
Finally, definitions of invasiveness and aggressiveness deserve special attention. Invasiveness refers to the radiologic or intraoperative extension of the tumor into adjacent structures such as the cavernous sinus, sphenoid sinus, or suprasellar region, which signifies reduced potential for gross total resection and can necessitate a multimodal surgical approach. Aggressiveness, on the other hand, characterizes the biological behavior of tumors, as exhibited by rapid growth, an elevated Ki-67 index, resistance to treatment, or early recurrence, and directly determines postoperative treatment, specifically the indication for adjuvant radiotherapy and personalized follow-up.
Integrating these findings into postoperative decision-making enhances the precision of patient management and optimizes long-term outcomes. To provide a practical framework for clinical use, Table 10 summarizes the main biomolecular markers and concepts in PitNETs, linking them directly to neurosurgical and postsurgical decision-making.

Table 10.
Biomolecular markers in PitNETs: neurosurgical relevance, postsurgical implications, and definitions of invasiveness/aggressiveness.
3. Conclusions
The biomolecular mechanisms underlying the pathogenesis of PitNETs have become a significant area of interest in the last decade, especially since the 5th Edition of the World Health Organization Classification of Endocrine and Neuroendocrine Tumors. Although at first glance the biomolecular world may appear of no significant interest to the neurosurgeon, essential correlations between these two scientific worlds were concluded. By understanding the complex mechanisms and molecular drivers behind tumorigenesis, neurosurgeons may be able to predict prognosis and guide postoperative therapeutic management and follow-up intervals, collaborating with other specialists.
Although biomolecular research in PitNETs is currently in its infancy, it will likely help broaden the understanding of this pathology. Future research should explore the integration of molecular profiling into surgical planning, as well as into the development of personalized follow-up strategies and the selection of adjuvant therapies driven by researched biomarkers.
Ultimately, bringing biomolecular discoveries into everyday clinical care will depend on strong collaboration between neurosurgeons, endocrinologists, pathologists, and molecular scientists. Such teamwork is essential for translating research findings into practical strategies and providing patients with genuinely personalized treatment for PitNETs.
Author Contributions
Conceptualization A.K., L.G.T. and A.D.; investigation A.K., L.G.T., B.-C.C. and R.E.R.; methodology L.G.T. and A.D.; supervision L.G.T., A.D. and R.E.R.; validation L.G.T., A.D.; visualization A.K. and B.-C.C.; writing—original draft A.K., L.G.T. and R.E.R.; writing—review and editing A.K., L.G.T. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper will be supported by the University of Medicine and Pharmacy Carol Davila, Bucharest, through the institutional program Publish not Perish, subject to funds availability.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef]
- Ezzat, S.; Asa, S.L.; Couldwell, W.T.; Barr, C.E.; Dodge, W.E.; Vance, M.L.; McCutcheon, I.E. The prevalence of pituitary adenomas: A systematic review. Cancer 2004, 101, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.F.; Rixhon, M.; Adam, C.; Dempegioti, A.; Tichomirowa, M.A.; Beckers, A. High prevalence of pituitary adenomas: A cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metab. 2006, 91, 4769–4775. [Google Scholar] [CrossRef] [PubMed]
- Tjörnstrand, A.; Gunnarsson, K.; Evert, M.; Holmberg, E.; Ragnarsson, O.; Rosén, T.; Filipsson Nyström, H. The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur. J. Endocrinol. 2014, 171, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Miki, Y. Imaging of pituitary tumors: An update with the 5th WHO Classifications-part 1. Pituitary neuroendocrine tumor (PitNET)/pituitary adenoma. Jpn. J. Radiol. 2023, 41, 789–806. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R.; Li, M.; Chen, G. Pituitary adenoma or pituitary neuroendocrine tumor: A narrative review of controversy and perspective. Transl. Cancer Res. 2021, 10, 1916–1920. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Fleseriu, M.; Wass, J.; van der Lely, A.; Barkan, A.; Giustina, A.; Casanueva, F.F.; Heaney, A.P.; Biermasz, N.; Strasburger, C.; et al. A tale of pituitary adenomas: To NET or not to NET: Pituitary Society position statement. Pituitary 2019, 22, 569–573. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Fleseriu, M.; Wass, J.; van der Lely, A.; Barkan, A.; Giustina, A.; Casanueva, F.F.; Heaney, A.P.; Biermasz, N.; Strasburger, C.; et al. The tale in evolution: Clarity, consistency and consultation, not contradiction and confusion. Pituitary 2020, 23, 476–477. [Google Scholar] [CrossRef]
- Lee, C.H. Pituitary Neuroendocrine Tumor: Is It Benign or Malignant? Brain Tumor Res. Treat. 2023, 11, 173–176. [Google Scholar] [CrossRef]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): A European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Ezzat, S. Genomics and Epigenomics of Pituitary Tumors: What Do Pathologists Need to Know? Endocr. Pathol. 2021, 32, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Bergsneider, M.; Mirsadraei, L.; Yong, W.H.; Salamon, N.; Linetsky, M.; Wang, M.B.; McArthur, D.L.; Heaney, A.P. The pituitary stalk effect: Is it a passing phenomenon? J. Neurooncol. 2014, 117, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Aflorei, E.D.; Korbonits, M. Epidemiology and etiopathogenesis of pituitary adenomas. J. Neurooncol. 2014, 117, 379–394. [Google Scholar] [CrossRef]
- Amano, T.; Masumoto, T.; Akutsu, H.; Sakamoto, N.; Hoshiai, S.; Mori, K.; Nakajima, T. The utility of dynamic MRI in differentiating the hormone-producing ability of pituitary adenomas. Jpn. J. Radiol. 2021, 39, 741–748. [Google Scholar] [CrossRef]
- Yiping, L.; Hui, L.; Kun, Z.; Daoying, G.; Bo, Y. Diffusion-weighted imaging of the sellar region: A comparison study of BLADE and single-shot echo planar imaging sequences. Eur. J. Radiol. 2014, 83, 1239–1244. [Google Scholar] [CrossRef]
- Sakai, N.; Koizumi, S.; Yamashita, S.; Takehara, Y.; Sakahara, H.; Baba, S.; Oki, Y.; Hiramatsu, H.; Namba, H. Arterial spin-labeled perfusion imaging reflects vascular density in nonfunctioning pituitary macroadenomas. AJNR Am. J. Neuroradiol. 2013, 34, 2139–2143. [Google Scholar] [CrossRef]
- Sakai, N.; Yamashita, S.; Takehara, Y.; Sakahara, H.; Baba, S.; Oki, Y.; Takahashi, G.; Koizumi, S.; Sameshima, T.; Namba, H. Evaluation of the antiangiogenic effects of octreotide on growth hormone-producing pituitary adenoma using arterial spin-labeling perfusion imaging. Magn. Reson. Med. Sci. 2015, 14, 73–76. [Google Scholar] [CrossRef][Green Version]
- Hughes, J.D.; Fattahi, N.; Van Gompel, J.; Arani, A.; Ehman, R.; Huston, J., 3rd. Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas. Pituitary 2016, 19, 286–292. [Google Scholar] [CrossRef]
- Cohen-Cohen, S.; Helal, A.; Yin, Z.; Ball, M.K.; Ehman, R.L.; Van Gompel, J.J.; Huston, J. Predicting pituitary adenoma consistency with preoperative magnetic resonance elastography. J. Neurosurg. 2022, 136, 1356–1363. [Google Scholar] [CrossRef]
- Graffeo, C.S.; Yagnik, K.J.; Carlstrom, L.P.; Lakomkin, N.; Bancos, I.; Davidge-Pitts, C.; Erickson, D.; Choby, G.; Pollock, B.E.; Chamberlain, A.M.; et al. Pituitary Adenoma Incidence, Management Trends, and Long-term Outcomes: A 30-Year Population-Based Analysis. Mayo Clin. Proc. 2022, 97, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Freda, P.U.; Beckers, A.M.; Katznelson, L.; Molitch, M.E.; Montori, V.M.; Post, K.D.; Vance, M.L. Pituitary incidentaloma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S. Epigenetic control in pituitary tumors. Endocr. J. 2008, 55, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.; Ling, C.; Mack, W.J.; Wang, K.; Zada, G. The role of epigenetic modification in tumorigenesis and progression of pituitary adenomas: A systematic review of the literature. PLoS ONE 2013, 8, e82619. [Google Scholar] [CrossRef]
- Ma, H.S.; Wang, E.L.; Xu, W.F.; Yamada, S.; Yoshimoto, K.; Qian, Z.R.; Shi, L.; Liu, L.L.; Li, X.H. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med. Sci. Monit. 2018, 24, 4841–4850. [Google Scholar] [CrossRef]
- Herrgott, G.A.; Asmaro, K.P.; Wells, M.; Sabedot, T.S.; Malta, T.M.; Mosella, M.S.; Nelson, K.; Scarpace, L.; Barnholtz-Sloan, J.S.; Sloan, A.E.; et al. Detection of tumor-specific DNA methylation markers in the blood of patients with pituitary neuroendocrine tumors. Neuro-Oncology 2022, 24, 1126–1139. [Google Scholar] [CrossRef]
- Revill, K.; Dudley, K.J.; Clayton, R.N.; McNicol, A.M.; Farrell, W.E. Loss of neuronatin expression is associated with promoter hypermethylation in pituitary adenoma. Endocr.-Relat. Cancer 2009, 16, 537–548. [Google Scholar] [CrossRef]
- Usui, H.; Morii, K.; Tanaka, R.; Tamura, T.; Washiyama, K.; Ichikawa, T.; Kumanishi, T. cDNA cloning and mRNA expression analysis of the human neuronatin. J. Mol. Neurosci. 1997, 9, 55–60. [Google Scholar] [CrossRef]
- Lachkhem, A.; Yahi, A.; Kablia, S.O.; Derriche, A.; Staifi, A.; Derradji, H. Null cell adenoma: Case report. In Proceedings of the 25th European Congress of Endocrinology, Istanbul, Turkey, 13–16 May 2023. [Google Scholar]
- Peng, J.; Yuan, L.; Kang, P.; Jin, S.; Ma, S.; Zhou, W.; Jia, G.; Zhang, C.; Jia, W. Comprehensive transcriptomic analysis identifies three distinct subtypes of pituitary adenomas: Insights into tumor behavior, prognosis, and stem cell characteristics. J. Transl. Med. 2024, 22, 892. [Google Scholar] [CrossRef]
- Scheithauer, B.W.; Horvath, E.; Kovacs, K.; Laws, E.R., Jr.; Randall, R.V.; Ryan, N. Plurihormonal pituitary adenomas. Semin. Diagn. Pathol. 1986, 3, 69–82. [Google Scholar]
- Chanson, P.; Maiter, D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101290. [Google Scholar] [CrossRef]
- Yatavelli, R.K.R.; Bhusal, K. Prolactinoma. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shimon, I. Giant Prolactinomas. Neuroendocrinology 2019, 109, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Delgrange, E.; Wierinckx, A.; Vasiljevic, A.; Jouanneau, E.; Burman, P.; Raverot, G. Clinical, Pathological, and Molecular Factors of Aggressiveness in Lactotroph Tumours. Neuroendocrinology 2019, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tataranu, L.G. A Brief Overview of Molecular Biology in Pituitary Adenomas with a Focus on Aggressive Lesions. Int. J. Mol. Sci. 2025, 26, 3717. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.V.; Kasuki, L.; Coelho, M.C.; Lima, C.H.A.; Wildemberg, L.E.; Gadelha, M.R. Frequency of familial pituitary adenoma syndromes among patients with functioning pituitary adenomas in a reference outpatient clinic. J. Endocrinol. Investig. 2017, 40, 1381–1387. [Google Scholar] [CrossRef]
- Georgitsi, M.; De Menis, E.; Cannavò, S.; Mäkinen, M.J.; Tuppurainen, K.; Pauletto, P.; Curtò, L.; Weil, R.J.; Paschke, R.; Zielinski, G.; et al. Aryl hydrocarbon receptor interacting protein (AIP) gene mutation analysis in children and adolescents with sporadic pituitary adenomas. Clin. Endocrinol. 2008, 69, 621–627. [Google Scholar] [CrossRef]
- Tichomirowa, M.A.; Barlier, A.; Daly, A.F.; Jaffrain-Rea, M.L.; Ronchi, C.; Yaneva, M.; Urban, J.D.; Petrossians, P.; Elenkova, A.; Tabarin, A.; et al. High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. Eur. J. Endocrinol. 2011, 165, 509–515. [Google Scholar] [CrossRef]
- Iacovazzo, D.; Hernández-Ramírez, L.C.; Korbonits, M. Sporadic pituitary adenomas: The role of germline mutations and recommendations for genetic screening. Expert. Rev. Endocrinol. Metab. 2017, 12, 143–153. [Google Scholar] [CrossRef]
- Syro, L.V.; Scheithauer, B.W.; Kovacs, K.; Toledo, R.A.; Londoño, F.J.; Ortiz, L.D.; Rotondo, F.; Horvath, E.; Uribe, H. Pituitary tumors in patients with MEN1 syndrome. Clinics 2012, 67 (Suppl. S1), 43–48. [Google Scholar] [CrossRef]
- Cuny, T.; Pertuit, M.; Sahnoun-Fathallah, M.; Daly, A.; Occhi, G.; Odou, M.F.; Tabarin, A.; Nunes, M.L.; Delemer, B.; Rohmer, V.; et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: Besides AIP don’t forget MEN1 genetic analysis. Eur. J. Endocrinol. 2013, 168, 533–541. [Google Scholar] [CrossRef]
- Delgrange, E.; Raverot, G.; Bex, M.; Burman, P.; Decoudier, B.; Devuyst, F.; Feldt-Rasmussen, U.; Andersen, M.; Maiter, D. Giant prolactinomas in women. Eur. J. Endocrinol. 2014, 170, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Shimon, I.; Sosa, E.; Mendoza, V.; Greenman, Y.; Tirosh, A.; Espinosa, E.; Popovic, V.; Glezer, A.; Bronstein, M.D.; Mercado, M. Giant prolactinomas larger than 60 mm in size: A cohort of massive and aggressive prolactin-secreting pituitary adenomas. Pituitary 2016, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Brue, T.; Pellegrini, I.; Priou, A.; Morange, I.; Jaquet, P. Prolactinomas and resistance to dopamine agonists. Horm. Res. 1992, 38, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Vroonen, L.; Jaffrain-Rea, M.L.; Petrossians, P.; Tamagno, G.; Chanson, P.; Vilar, L.; Borson-Chazot, F.; Naves, L.A.; Brue, T.; Gatta, B.; et al. Prolactinomas resistant to standard doses of cabergoline: A multicenter study of 92 patients. Eur. J. Endocrinol. 2012, 167, 651–662. [Google Scholar] [CrossRef]
- Delgrange, E.; Sassolas, G.; Perrin, G.; Jan, M.; Trouillas, J. Clinical and histological correlations in prolactinomas, with special reference to bromocriptine resistance. Acta Neurochir. 2005, 147, 751–757, discussion 757–758. [Google Scholar] [CrossRef]
- Zhang, X.; Horwitz, G.A.; Prezant, T.R.; Valentini, A.; Nakashima, M.; Bronstein, M.D.; Melmed, S. Structure, Expression, and Function of Human Pituitary Tumor-Transforming Gene (PTTG). Mol. Endocrinol. 1999, 13, 156–166. [Google Scholar] [CrossRef]
- Zhang, X.; Horwitz, G.A.; Heaney, A.P.; Nakashima, M.; Prezant, T.R.; Bronstein, M.D.; Melmed, S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J. Clin. Endocrinol. Metab. 1999, 84, 761–767. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Rugowski, D.E.; Sullivan, R.; Schuler, L.A. Prolactin cooperates with loss of p53 to promote claudin-low mammary carcinomas. Oncogene 2014, 33, 3075–3082. [Google Scholar] [CrossRef]
- Bates, A.S.; Farrell, W.E.; Bicknell, E.J.; McNicol, A.M.; Talbot, A.J.; Broome, J.C.; Perrett, C.W.; Thakker, R.V.; Clayton, R.N. Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. J. Clin. Endocrinol. Metab. 1997, 82, 818–824. [Google Scholar] [CrossRef]
- Wierinckx, A.; Roche, M.; Raverot, G.; Legras-Lachuer, C.; Croze, S.; Nazaret, N.; Rey, C.; Auger, C.; Jouanneau, E.; Chanson, P.; et al. Integrated genomic profiling identifies loss of chromosome 11p impacting transcriptomic activity in aggressive pituitary PRL tumors. Brain Pathol. 2011, 21, 533–543. [Google Scholar] [CrossRef]
- Qian, Z.R.; Li, C.C.; Yamasaki, H.; Mizusawa, N.; Yoshimoto, K.; Yamada, S.; Tashiro, T.; Horiguchi, H.; Wakatsuki, S.; Hirokawa, M.; et al. Role of E-cadherin, alpha-, beta-, and gamma-catenins, and p120 (cell adhesion molecules) in prolactinoma behavior. Mod. Pathol. 2002, 15, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Kawamoto, K.; Mizoue, T.; Uozumi, T.; Arita, K.; Kurisu, K. Matrix metalloproteinase-9 secretion by human pituitary adenomas detected by cell immunoblot analysis. Acta Neurochir. 1996, 138, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.E.; Nagy, Z.; Esiri, M.M.; Harris, A.L.; Wass, J.A. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J. Clin. Endocrinol. Metab. 2000, 85, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Knappe, U.J.; Hagel, C.; Lisboa, B.W.; Wilczak, W.; Lüdecke, D.K.; Saeger, W. Expression of serine proteases and metalloproteinases in human pituitary adenomas and anterior pituitary lobe tissue. Acta Neuropathol. 2003, 106, 471–478. [Google Scholar] [CrossRef]
- Raverot, G.; Wierinckx, A.; Dantony, E.; Auger, C.; Chapas, G.; Villeneuve, L.; Brue, T.; Figarella-Branger, D.; Roy, P.; Jouanneau, E.; et al. Prognostic factors in prolactin pituitary tumors: Clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J. Clin. Endocrinol. Metab. 2010, 95, 1708–1716. [Google Scholar] [CrossRef]
- Zhao, Y.; Lian, W.; Xing, B.; Feng, M.; Ma, W.B. Functioning gonadotroph adenoma. Chin. Med. J. 2019, 132, 1003–1004. [Google Scholar] [CrossRef]
- Ntali, G.; Capatina, C. Updating the Landscape for Functioning Gonadotroph Tumors. Medicina 2022, 58, 1071. [Google Scholar] [CrossRef]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef]
- Newey, P.J.; Nesbit, M.A.; Rimmer, A.J.; Head, R.A.; Gorvin, C.M.; Attar, M.; Gregory, L.; Wass, J.A.H.; Buck, D.; Karavitaki, N.; et al. Whole-Exome Sequencing Studies of Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2013, 98, E796–E800. [Google Scholar] [CrossRef]
- Falch, C.M.; Sundaram, A.Y.M.; Øystese, K.A.; Normann, K.R.; Lekva, T.; Silamikelis, I.; Eieland, A.K.; Andersen, M.; Bollerslev, J.; Olarescu, N.C. Gene expression profiling of fast- and slow-growing non-functioning gonadotroph pituitary adenomas. Eur. J. Endocrinol. 2018, 178, 295–307. [Google Scholar] [CrossRef]
- Faltermeier, C.M.; Magill, S.T.; Blevins, L.S., Jr.; Aghi, M.K. Molecular Biology of Pituitary Adenomas. Neurosurg. Clin. N. Am. 2019, 30, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.; Grieve, J.; Dorward, N.; Miszkiel, K.; Kosmin, M.; Fersht, N.; Bouloux, P.M.; Jaunmuktane, Z.; Baldeweg, S.E.; Marcus, H.J. Non-functioning pituitary macroadenoma following surgery: Long-term outcomes and development of an optimal follow-up strategy. Front. Surg. 2023, 10, 1129387. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.; Guyétant, S.; Menei, P.; Rodien, P.; Illouz, F.; Vielle, B.; Rohmer, V. Relevance of Ki-67 and prognostic factors for recurrence/progression of gonadotropic adenomas after first surgery. Eur. J. Endocrinol. 2007, 157, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Smith, T.R.; Sandler, C.N.; Gupta, T.; Bale, T.A.; Bi, W.L.; Dunn, I.F.; De Girolami, U.; Woodmansee, W.W.; Kaiser, U.B.; et al. Functional Gonadotroph Adenomas: Case Series and Report of Literature. Neurosurgery 2016, 79, 823–831. [Google Scholar] [CrossRef]
- Batista, R.L.; Trarbach, E.B.; Marques, M.D.; Cescato, V.A.; da Silva, G.O.; Herkenhoff, C.G.B.; Cunha-Neto, M.B.; Musolino, N.R. Nonfunctioning Pituitary Adenoma Recurrence and Its Relationship with Sex, Size, and Hormonal Immunohistochemical Profile. World Neurosurg. 2018, 120, e241–e246. [Google Scholar] [CrossRef]
- Ogedegbe, O.J.; Cheema, A.Y.; Khan, M.A.; Junaid, S.Z.S.; Erebo, J.K.; Ayirebi-Acquah, E.; Okpara, J.; Bofah, D.; Okon, J.G.; Munir, M.; et al. A Comprehensive Review of Four Clinical Practice Guidelines of Acromegaly. Cureus 2022, 14, e28722. [Google Scholar] [CrossRef]
- Mete, O.; Cintosun, A.; Pressman, I.; Asa, S.L. Epidemiology and biomarker profile of pituitary adenohypophysial tumors. Mod. Pathol. 2018, 31, 900–909. [Google Scholar] [CrossRef]
- Asa, S.L.; Ezzat, S. An Update on Pituitary Neuroendocrine Tumors Leading to Acromegaly and Gigantism. J. Clin. Med. 2021, 10, 2254. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O. Cytokeratin profiles in pituitary neuroendocrine tumors. Hum. Pathol. 2021, 107, 87–95. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Carlson, N.E.; Borges, M.T.; Kleinschmidt-DeMasters, B.K.; Lillehei, K.O.; Kerr, J.M.; Wierman, M.E. Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 2015, 49, 231–241. [Google Scholar] [CrossRef]
- Heng, L.; Liu, X.; Jia, D.; Guo, W.; Zhang, S.; Gao, G.; Gong, L.; Qu, Y. Preoperative prediction of granulation pattern subtypes in GH-secreting pituitary adenomas. Clin. Endocrinol. 2021, 95, 134–142. [Google Scholar] [CrossRef]
- Yan, J.L.; Chen, M.Y.; Chen, Y.L.; Chuang, C.C.; Hsu, P.W.; Wei, K.C.; Chang, C.N. Surgical Outcome and Evaluation of Strategies in the Management of Growth Hormone-Secreting Pituitary Adenomas After Initial Transsphenoidal Pituitary Adenectomy Failure. Front. Endocrinol. 2022, 13, 756855. [Google Scholar] [CrossRef]
- Swanson, A.A.; Erickson, D.; Donegan, D.M.; Jenkins, S.M.; Van Gompel, J.J.; Atkinson, J.L.D.; Erickson, B.J.; Giannini, C. Clinical, biological, radiological, and pathological comparison of sparsely and densely granulated somatotroph adenomas: A single center experience from a cohort of 131 patients with acromegaly. Pituitary 2021, 24, 192–206. [Google Scholar] [CrossRef]
- Bakhtiar, Y.; Hirano, H.; Arita, K.; Yunoue, S.; Fujio, S.; Tominaga, A.; Sakoguchi, T.; Sugiyama, K.; Kurisu, K.; Yasufuku-Takano, J.; et al. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur. J. Endocrinol. 2010, 163, 531–539. [Google Scholar] [CrossRef]
- Hazer, D.B.; Işık, S.; Berker, D.; Güler, S.; Gürlek, A.; Yücel, T.; Berker, M. Treatment of acromegaly by endoscopic transsphenoidal surgery: Surgical experience in 214 cases and cure rates according to current consensus criteria. J. Neurosurg. 2013, 119, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Guignat, L.; Assie, G.; Bertagna, X.; Bertherat, J. Corticotroph adenoma. Presse Med. 2009, 38, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Wagner, J.R.; Pekmezci, M.; Hiniker, A.; Chang, E.F.; Kunwar, S.; Blevins, L.; Aghi, M.K. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery 2013, 73, 8–17, discussion 17–18. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R.; Penn, D.L.; Repetti, C.S. Advances and controversies in the classification and grading of pituitary tumors. J. Endocrinol. Investig. 2019, 42, 129–135. [Google Scholar] [CrossRef]
- Nasi-Kordhishti, I.; Hladik, M.; Kandilaris, K.; Behling, F.; Honegger, J.; Schittenhelm, J. Transcription factor-based classification of pituitary adenomas/PitNETs: A comparative analysis and clinical implications across WHO 2004, 2017 and 2022 in 921 cases. Acta Neuropathol. Commun. 2025, 13, 135. [Google Scholar] [CrossRef]
- Hayashi, K.; Inoshita, N.; Kawaguchi, K.; Ibrahim Ardisasmita, A.; Suzuki, H.; Fukuhara, N.; Okada, M.; Nishioka, H.; Takeuchi, Y.; Komada, M.; et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur. J. Endocrinol. 2016, 174, 213–226. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Pagnano, A.; Detomas, M.; Cassarino, M.F.; Pecori Giraldi, F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 2019, 63, 240–246. [Google Scholar] [CrossRef]
- Nerubenko, E.; Ryazanov, P.; Kuritsyna, N.; Paltsev, A.; Ivanova, O.; Grineva, E.; Kostareva, A.; Dmitrieva, R.; Tsoy, U. Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking. Int. J. Mol. Sci. 2024, 25, 12886. [Google Scholar] [CrossRef]
- Sbiera, S.; Perez-Rivas, L.G.; Taranets, L.; Weigand, I.; Flitsch, J.; Graf, E.; Monoranu, C.-M.; Saeger, W.; Hagel, C.; Honegger, J.; et al. Driver mutations in USP8 wild-type Cushing’s disease. Neuro-Oncology 2019, 21, 1273–1283. [Google Scholar] [CrossRef]
- Kober, P.; Rusetska, N.; Mossakowska, B.J.; Maksymowicz, M.; Pękul, M.; Zieliński, G.; Styk, A.; Kunicki, J.; Działach, Ł.; Witek, P.; et al. The expression of glucocorticoid and mineralocorticoid receptors in pituitary tumors causing Cushing’s disease and silent corticotroph tumors. Front. Endocrinol. 2023, 14, 1124646. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rivas, L.G.; Simon, J.; Albani, A.; Tang, S.; Roeber, S.; Assié, G.; Deutschbein, T.; Fassnacht, M.; Gadelha, M.R.; Hermus, A.R.; et al. TP53 mutations in functional corticotroph tumors are linked to invasion and worse clinical outcome. Acta Neuropathol. Commun. 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Uzilov, A.V.; Taik, P.; Cheesman, K.C.; Javanmard, P.; Ying, K.; Roehnelt, A.; Wang, H.; Fink, M.Y.; Lau, C.Y.; Moe, A.S.; et al. USP8 and TP53 Drivers are Associated with CNV in a Corticotroph Adenoma Cohort Enriched for Aggressive Tumors. J. Clin. Endocrinol. Metab. 2020, 106, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Pękul, M.; Szczepaniak, M.; Kober, P.; Rusetska, N.; Mossakowska, B.J.; Baluszek, S.; Kowalik, A.; Maksymowicz, M.; Zieliński, G.; Kunicki, J.; et al. Relevance of mutations in protein deubiquitinases genes and TP53 in corticotroph pituitary tumors. Front. Endocrinol. 2024, 15, 1302667. [Google Scholar] [CrossRef]
- Casar-Borota, O.; Boldt, H.B.; Engström, B.E.; Andersen, M.S.; Baussart, B.; Bengtsson, D.; Berinder, K.; Ekman, B.; Feldt-Rasmussen, U.; Höybye, C.; et al. Corticotroph Aggressive Pituitary Tumors and Carcinomas Frequently Harbor ATRX Mutations. J. Clin. Endocrinol. Metab. 2020, 106, e1183–e1194. [Google Scholar] [CrossRef]
- Lasolle, H.; Vasiljevic, A.; Jouanneau, E.; Ilie, M.D.; Raverot, G. Aggressive corticotroph tumors and carcinomas. J. Neuroendocrinol. 2022, 34, e13169. [Google Scholar] [CrossRef]
- Dai, C.; Feng, M.; Lu, L.; Sun, B.; Fan, Y.; Bao, X.; Yao, Y.; Deng, K.; Wang, R.; Kang, J. Transsphenoidal Surgery of Corticotroph Adenomas With Cavernous Sinus Invasion: Results in a Series of 86 Consecutive Patients. Front. Oncol. 2022, 12, 810234. [Google Scholar] [CrossRef]
- Sanno, N.; Teramoto, A.; Osamura, R.Y. Thyrotropin-secreting pituitary adenomas. Clinical and biological heterogeneity and current treatment. J. Neurooncol. 2001, 54, 179–186. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Lania, A.; Beckers, A.; Chatterjee, K.; Wemeau, J.L. 2013 European thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur. Thyroid. J. 2013, 2, 76–82. [Google Scholar] [CrossRef]
- Spada, A.; Vallar, L.; Faglia, G. G protein oncogenes in pituitary tumors. Trends Endocrinol. Metab. 1992, 3, 355–360. [Google Scholar] [CrossRef]
- Sumi, T.; Stefaneanu, L.; Kovacs, K.; Asa, S.L.; Rindi, G. Immunohistochemical study of p53 protein in human and animal pituitary tumors. Endocr. Pathol. 1993, 4, 95–99. [Google Scholar] [CrossRef]
- Dong, Q.; Brucker-Davis, F.; Weintraub, B.D.; Smallridge, R.C.; Carr, F.E.; Battey, J.; Spiegel, A.M.; Shenker, A. Screening of candidate oncogenes in human thyrotroph tumors: Absence of activating mutations of the G alpha q, G alpha 11, G alpha s, or thyrotropin-releasing hormone receptor genes. J. Clin. Endocrinol. Metab. 1996, 81, 1134–1140. [Google Scholar] [CrossRef][Green Version]
- Asteria, C.; Anagni, M.; Persani, L.; Beck-Peccoz, P. Loss of heterozygosity of the MEN1 gene in a large series of TSH-secreting pituitary adenomas. J. Endocrinol. Investig. 2001, 24, 796–801. [Google Scholar] [CrossRef]
- Teramoto, A.; Sanno, N.; Tahara, S.; Osamura, Y.R. Pathological study of thyrotropin-secreting pituitary adenoma: Plurihormonality and medical treatment. Acta Neuropathol. 2004, 108, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Izawa, S.; Sano, T.; Tagami, T.; Nagata, D.; Shimatsu, A.; Takahashi, J.A.; Naruse, M. Clinical and Molecular Features of a TSH-Secreting Pituitary Microadenoma. Pituitary 2005, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Căpraru, O.M.; Gaillard, C.; Vasiljevic, A.; Lasolle, H.; Borson-Chazot, F.; Raverot, V.; Jouanneau, E.; Trouillas, J.; Raverot, G. Diagnosis, pathology, and management of TSH-secreting pituitary tumors. A single-center retrospective study of 20 patients from 1981 to 2014. Ann. Endocrinol. 2019, 80, 216–224. [Google Scholar] [CrossRef]
- Yamada, S.; Fukuhara, N.; Horiguchi, K.; Yamaguchi-Okada, M.; Nishioka, H.; Takeshita, A.; Takeuchi, Y.; Ito, J.; Inoshita, N. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: A single-center study of 90 cases. J. Neurosurg. 2014, 121, 1462–1473. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, L.; Yang, L.; An, Z.; Tan, H. Progress in the Pathogenesis, Diagnosis, and Treatment of TSH-Secreting Pituitary Neuroendocrine Tumor. Front. Endocrinol. 2020, 11, 580264. [Google Scholar] [CrossRef]
- Lee, J.C.; Pekmezci, M.; Lavezo, J.L.; Vogel, H.; Katznelson, L.; Fraenkel, M.; Harsh, G.; Dulai, M.; Perry, A.; Tihan, T. Utility of Pit-1 Immunostaining in Distinguishing Pituitary Adenomas of Primitive Differentiation from Null Cell Adenomas. Endocr. Pathol. 2017, 28, 287–292. [Google Scholar] [CrossRef]
- Woo, C.S.; Ho, R.S.; Ho, G.; Lau, H.T.; Fong, C.H.; Chang, J.Y.; Leung, E.K.; Tang, L.C.; Ma, I.K.; Lee, A.C.; et al. A clinicopathological study of non-functioning pituitary neuroendocrine tumours using the World Health Organization 2022 classification. Front. Endocrinol. 2024, 15, 1368944. [Google Scholar] [CrossRef] [PubMed]
- Balogun, J.A.; Monsalves, E.; Juraschka, K.; Parvez, K.; Kucharczyk, W.; Mete, O.; Gentili, F.; Zadeh, G. Null cell adenomas of the pituitary gland: An institutional review of their clinical imaging and behavioral characteristics. Endocr. Pathol. 2015, 26, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tomono, Y.; Nose, T. Expression of P27kip1 and Ki-67 in pituitary adenomas: An investigation of marker of adenoma invasiveness. Acta Neurochir. 1999, 141, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Wunderer, J.; Zachenhofer, I.; Czech, T.; Böcher-Schwarz, H.G.; Hainfellner, J.; Knosp, E. Expression of cell proliferation markers in pituitary adenomas--correlation and clinical relevance of MIB-1 and anti-topoisomerase-IIalpha. Acta Neurochir. 2004, 146, 831–839. [Google Scholar] [CrossRef]
- Aydin, S.; Comunoglu, N.; Ahmedov, M.L.; Korkmaz, O.P.; Oz, B.; Kadioglu, P.; Gazioglu, N.; Tanriover, N. Clinicopathologic Characteristics and Surgical Treatment of Plurihormonal Pituitary Adenomas. World Neurosurg. 2019, 130, e765–e774. [Google Scholar] [CrossRef]
- Micko, A.; Rötzer, T.; Hoftberger, R.; Vila, G.; Oberndorfer, J.; Frischer, J.M.; Knosp, E.; Wolfsberger, S. Expression of additional transcription factors is of prognostic value for aggressive behavior of pituitary adenomas. J. Neurosurg. 2021, 134, 1139–1146. [Google Scholar] [CrossRef]
- Erickson, D.; Scheithauer, B.; Atkinson, J.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Young, W.F., Jr. Silent subtype 3 pituitary adenoma: A clinicopathologic analysis of the Mayo Clinic experience. Clin. Endocrinol. 2009, 71, 92–99. [Google Scholar] [CrossRef]
- Mete, O.; Gomez-Hernandez, K.; Kucharczyk, W.; Ridout, R.; Zadeh, G.; Gentili, F.; Ezzat, S.; Asa, S.L. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod. Pathol. 2016, 29, 131–142. [Google Scholar] [CrossRef]
- La Rosa, S. Diagnostic, Prognostic, and Predictive Role of Ki67 Proliferative Index in Neuroendocrine and Endocrine Neoplasms: Past, Present, and Future. Endocr. Pathol. 2023, 34, 79–97. [Google Scholar] [CrossRef]
- Voellger, B.; Zhang, Z.; Benzel, J.; Wang, J.; Lei, T.; Nimsky, C.; Bartsch, J.W. Targeting Aggressive Pituitary Adenomas at the Molecular Level-A Review. J. Clin. Med. 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bao, X.; Wang, R. Role of matrix Metalloproteinases in pituitary adenoma invasion. Chin. Neurosurg. J. 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, K.; Kim, D.; Moon, J.H.; Kim, E.H.; Kim, S.H.; Ku, C.R.; Lee, E.J. Associations of GNAS Mutations with Surgical Outcomes in Patients with Growth Hormone-Secreting Pituitary Adenoma. Endocrinol. Metab. 2021, 36, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Yang, C.; Bao, X.; Wang, R. Genetic and Epigenetic Causes of Pituitary Adenomas. Front. Endocrinol. 2021, 11, 596554. [Google Scholar] [CrossRef]
- Beckers, A.; Aaltonen, L.A.; Daly, A.F.; Karhu, A. Familial Isolated Pituitary Adenomas (FIPA) and the Pituitary Adenoma Predisposition due to Mutations in the Aryl Hydrocarbon Receptor Interacting Protein (AIP) Gene. Endocr. Rev. 2013, 34, 239–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).