Clinical Management and Prognostic Outcomes of Pancreatic Neuroendocrine Tumors: Insights from a Tertiary Care Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Classification and Grouping
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tumor Characteristics
3.3. Diagnostic Pathway
3.4. Surgical Treatment
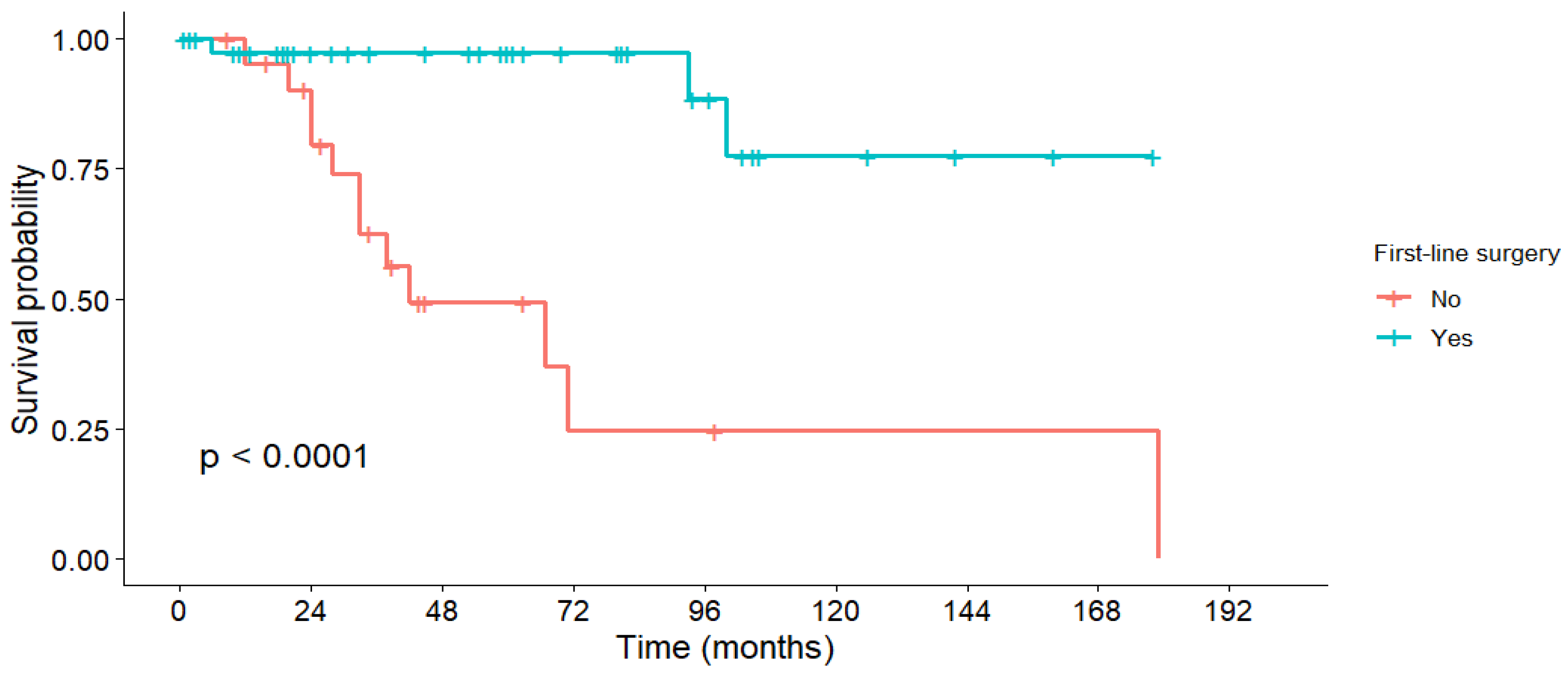
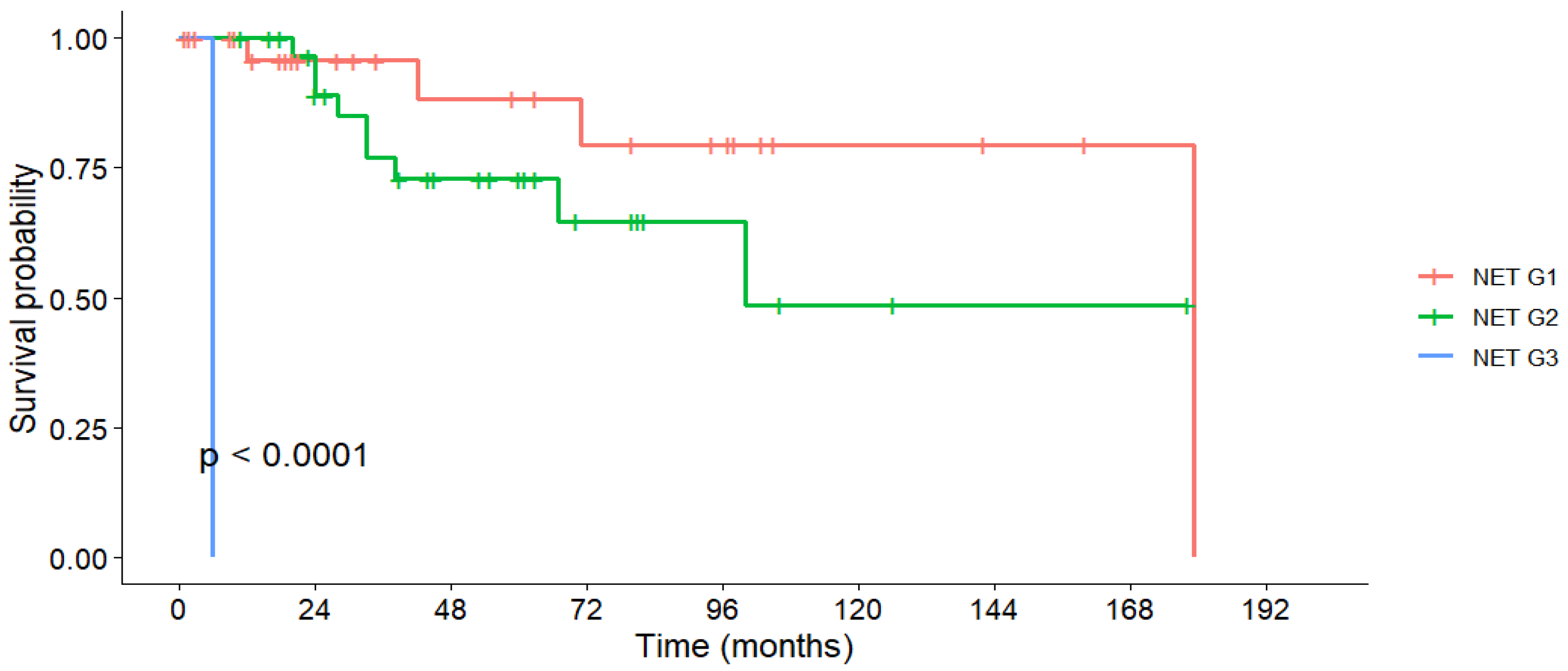
3.5. Survival Outcomes
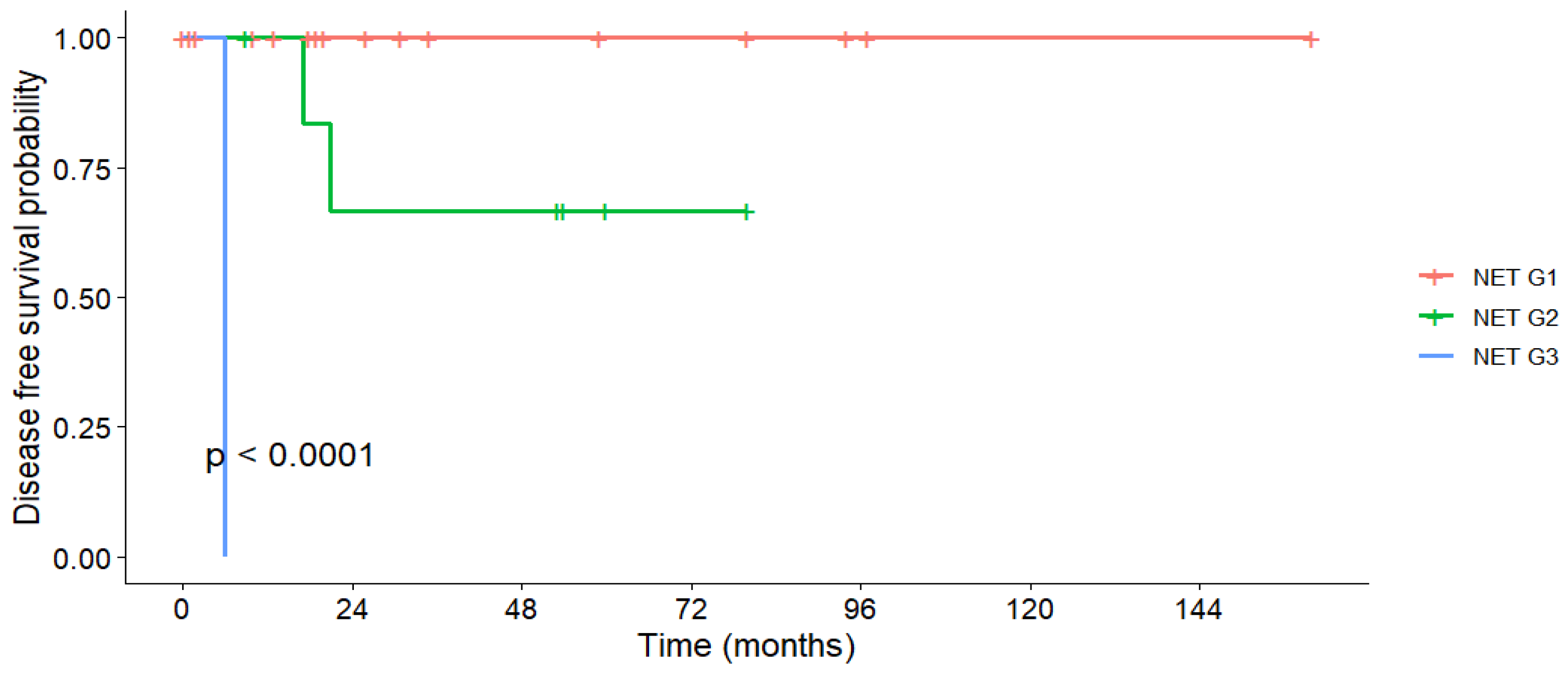
3.6. Disease-Free Survival
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rindi, G.; Inzani, F. Neuroendocrine neoplasm update: Toward universal nomenclature. Endocr. Relat. Cancer 2020, 27, R211–R218. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Totoki, Y.; Noë, M.; Nakatani, Y.; Horie, M.; Kawasaki, K.; Nakamura, H.; Saito-Adachi, M.; Suzuki, M.; Takai, E.; et al. Comprehensive Genomic Profiling of Neuroendocrine Carcinomas of the Gastrointestinal System. Cancer Discov. 2022, 12, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.; Vinagre, J.; Soares, P.; Claro, I.; Sanches, A.C.; Gomes, L.; Fernandes, I.; Catarino, A.L.; Preto, J.; Pereira, B.D.; et al. Gastroenteropancreatic Neuroendocrine Neoplasia Characterization in Portugal: Results from the NETs Study Group of the Portuguese Society of Endocrinology, Diabetes and Metabolism. Int. J. Endocrinol. 2019, 2019, 4518742. [Google Scholar] [CrossRef] [PubMed]
- Nikou, G.C.; Pazaitou-Panayiotou, K.; Dimitroulopoulos, D.; Alexandrakis, G.; Papakostas, P.; Vaslamatzis, M.; Kaldrymidis, P.; Markussis, V.; Koumarianou, A. Results of a prospective multicenter neuroendocrine tumor registry reporting on clinicopathologic characteristics of Greek patients. BMC Endocr. Disord. 2016, 16, 8. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Rikhraj, N.; Fernandez, C.J.; Ganakumar, V.; Pappachan, J.M. Pancreatic neuroendocrine tumors: A case-based evidence review. World J. Gastrointest. Pathophysiol. 2025, 16, 107265. [Google Scholar] [CrossRef]
- Danek, E.; Kavnoudias, H.; McLean, C.; Gerstenmaier, J.F.; Di Muzio, B. Radiological Variability in Pancreatic Neuroendocrine Neoplasms: A 10-Year Single-Center Study on Atypical Presentations and Diagnostic Challenges. Biomedicines 2025, 13, 496. [Google Scholar] [CrossRef]
- Magi, L.; Marasco, M.; Rinzivillo, M.; Faggiano, A.; Panzuto, F. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr. Treat. Options Oncol. 2023, 24, 725–741. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Kasumova, G.G.; Tabatabaie, O.; Eskander, M.F.; Tadikonda, A.; Ng, S.C.; Tseng, J.F. National Rise of Primary Pancreatic Carcinoid Tumors: Comparison to Functional and Nonfunctional Pancreatic Neuroendocrine Tumors. J. Am. Coll. Surg. 2017, 224, 1057–1064. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, G. Comparative outcomes of pancreatic neuroendocrine neoplasms: A population-based analysis of the SEER database. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 2181–2187. [Google Scholar] [CrossRef]
- Aysal, A.; Agalar, C.; Egeli, T.; Unek, T.; Oztop, I.; Obuz, F.; Sagol, O. Reconsideration of Clinicopathologic Prognostic Factors in Pancreatic Neuroendocrine Tumors for Better Determination of Adverse Prognosis. Endocr. Pathol. 2021, 32, 461–472. [Google Scholar] [CrossRef]
- Öberg, K. Management of functional neuroendocrine tumors of the pancreas. Gland Surg. 2018, 7, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Skogseid, B. GEP- NETS UPDATE: Genetics of neuroendocrine tumors. Eur. J. Endocrinol. 2016, 174, R275–R290. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Castanheira-Rodrigues, S.; Bastos, P.; Cristino, H.; Fernandes, A.; Rodrigues-Pinto, E.; Bispo, M.; Rio-Tinto, R.; Vilas-Boas, F. Portuguese Pancreatic Club Perspectives on Endoscopic Ultrasound-Guided and Surgical Treatment of Pancreatic Neuroendocrine Tumors. GE Port. J. Gastroenterol. 2024, 31, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef]
- Sallinen, V.J.; Le Large, T.Y.S.; Tieftrunk, E.; Galeev, S.; Kovalenko, Z.; Haugvik, S.P.; Antila, A.; Franklin, O.; Martinez-Moneo, E.; Robinson, S.M.; et al. Prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors—A multi-institutional study. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2018, 20, 251–259. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Panzuto, F.; Barbi, S.; Trama, A.; Fazio, N. The importance of education and training in neuroendocrine neoplasms: Challenges and opportunities for multidisciplinary management. Cancer Treat. Rev. 2025, 139, 102998. [Google Scholar] [CrossRef]
- Gavrilescu, M.M.; Hutanu, I.; Scripcariu, D.V.; Filip, B.; Anitei, M.G.; Radu, I.; Scripcariu, V. The Treatment of Pancreatic Neuroendocrine Tumors—A Retrospective Single-Centre Study. Chirurgia 2025, 120, 79–88. [Google Scholar] [CrossRef]
- Stoica-Mustafa, E.; Pechianu, C.; Iorgescu, A.; Hortopan, M.; Dima, S.O.; Tomulescu, V.; Dumitraşcu, T.; Ungureanu, C.; Andronesi, D.; Popescu, I.; et al. Pathological characteristics and clinical specifications in gastroenteropancreatic neuroendocrine tumors: A study of 68 cases. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2012, 53, 351–355. [Google Scholar]
- Dima, S.O.; Dumitrascu, T.; Pechianu, C.; Grigorie, R.T.; Brasoveanu, V.; Sorop, A.; Lupescu, I.; Purnichescu-Purtan, R.; Croitoru, A.; Bacalbasa, N.; et al. Prognostic Factors in Patients With Surgical Resection of Pancreatic Neuroendocrine Tumours. Acta Endocrinol. 2018, 14, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Zhang, L.; Choti, M.A.; Kulke, M.; Yao, J.C.; Nakakura, E.K.; Bloomston, M.; Benson, A.B.; Shah, M.H.; Strosberg, J.R.; et al. Recurrence Patterns After Surgical Resection of Gastroenteropancreatic Neuroendocrine Tumors: Analysis From the National Comprehensive Cancer Network Oncology Outcomes Database. Pancreas 2021, 50, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Calissendorff, J.; Bjellerup-Calissendorff, F.; Bränström, R.; Juhlin, C.C.; Falhammar, H. Characteristics, Treatment, Outcomes, and Survival in Neuroendocrine G1 and G2 Pancreatic Tumors: Experiences From a Single Tertiary Referral Center. Front. Endocrinol. 2021, 12, 657698. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Zhang, Z.; Xu, X.; Yu, X.; Zhuo, Q.; Ji, S. The clinical characteristics and survival associations of pancreatic neuroendocrine tumors: Does age matter? Gland Surg. 2021, 10, 574–583. [Google Scholar] [CrossRef]
- Luo, G.; Javed, A.; Strosberg, J.R.; Jin, K.; Zhang, Y.; Liu, C.; Xu, J.; Soares, K.; Weiss, M.J.; Zheng, L.; et al. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 274–280. [Google Scholar] [CrossRef]
- Badic, B.; Morvan, M.; Quénéhervé, L.; Bouzeloc, S.; Kermarrec, T.; Nousbaum, J.B.; Reboux, N. Real World Data for Pancreatic Adenocarcinoma from a Population-Based Study in France. Cancers 2023, 15, 525. [Google Scholar] [CrossRef]
- Yao, D.W.; Qin, M.Z.; Jiang, H.X.; Qin, S.Y. Comparison of EUS-FNA and EUS-FNB for diagnosis of solid pancreatic mass lesions: A meta-analysis of prospective studies. Scand. J. Gastroenterol. 2024, 59, 972–979. [Google Scholar] [CrossRef]
- Heidsma, C.M.; Engelsman, A.F.; van Dieren, S.; Stommel, M.W.J.; de Hingh, I.; Vriens, M.; Hol, L.; Festen, S.; Mekenkamp, L.; Hoogwater, F.J.H.; et al. Watchful waiting for small non-functional pancreatic neuroendocrine tumours: Nationwide prospective cohort study (PANDORA). Br. J. Surg. 2021, 108, 888–891. [Google Scholar] [CrossRef]
- Partelli, S.; Massironi, S.; Zerbi, A.; Niccoli, P.; Kwon, W.; Landoni, L.; Panzuto, F.; Tomazic, A.; Bongiovanni, A.; Kaltsas, G.; et al. Management of asymptomatic sporadic non-functioning pancreatic neuroendocrine neoplasms no larger than 2 cm: Interim analysis of prospective ASPEN trial. Br. J. Surg. 2022, 109, 1186–1190. [Google Scholar] [CrossRef]
- Herrera, M.F.; Åkerström, G.; Angelos, P.; Grant, C.S.; Hoff, A.O.; Pantoja, J.P.; Pérez-Johnston, R.; Sahani, D.V.; Wong, R.J.; Randolph, G. AACE/ACE disease state clinical review: Pancreatic neuroendocrine incidentalomas. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2015, 21, 546–553. [Google Scholar] [CrossRef]
- Haynes, A.B.; Deshpande, V.; Ingkakul, T.; Vagefi, P.A.; Szymonifka, J.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernández-del Castillo, C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: Short-term and long-term patient outcomes. Arch. Surg. 2011, 146, 534–538. [Google Scholar] [CrossRef]
- Liu, X.; Chin, W.; Pan, C.; Zhang, W.; Yu, J.; Zheng, S.; Liu, Y. Risk of malignancy and prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors. Gland Surg. 2021, 10, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Krampitz, G.W.; Norton, J.A.; Poultsides, G.A.; Visser, B.C.; Sun, L.; Jensen, R.T. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch. Surg. 2012, 147, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Bettini, R.; Partelli, S.; Boninsegna, L.; Capelli, P.; Crippa, S.; Pederzoli, P.; Scarpa, A.; Falconi, M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011, 150, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Grant, C.S.; Salomao, D.R.; Fletcher, J.G.; Takahashi, N.; Fidler, J.L.; Levy, M.J.; Huebner, M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): Role for nonoperative management. Surgery 2012, 152, 965–974. [Google Scholar] [CrossRef]
- Partelli, S.; Andreasi, V.; Rancoita, P.M.V.; Perez-Sanchez, E.; Muffatti, F.; Balzano, G.; Crippa, S.; Di Serio, C.; Falconi, M. Outcomes after distal pancreatectomy for neuroendocrine neoplasms: A retrospective comparison between minimally invasive and open approach using propensity score weighting. Surg. Endosc. 2021, 35, 165–173. [Google Scholar] [CrossRef]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef]
- Zhang, W.H.; Gao, H.L.; Liu, W.S.; Qin, Y.; Ye, Z.; Lou, X.; Wang, F.; Zhang, Y.; Chen, X.M.; Chen, J.; et al. A real-life treatment cohort of pancreatic neuroendocrine tumors: High-grade increase in metastases confers poor survival. Front. Endocrinol. 2022, 13, 941210. [Google Scholar] [CrossRef]
- Finkelstein, P.; Sharma, R.; Picado, O.; Gadde, R.; Stuart, H.; Ripat, C.; Livingstone, A.S.; Sleeman, D.; Merchant, N.; Yakoub, D. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2017, 21, 855–866. [Google Scholar] [CrossRef]
- Partelli, S.; Battistella, A.; Andreasi, V.; Muffatti, F.; Tamburrino, D.; Pecorelli, N.; Crippa, S.; Balzano, G.; Falconi, M. Critical appraisal of the adequacy of surgical indications for non-functioning pancreatic neuroendocrine tumours. BJS Open 2024, 8, zrae083. [Google Scholar] [CrossRef]
- Partelli, S.; Muffatti, F.; Rancoita, P.M.V.; Andreasi, V.; Balzano, G.; Crippa, S.; Doglioni, C.; Rubini, C.; Zamboni, G.; Falconi, M. The size of well differentiated pancreatic neuroendocrine tumors correlates with Ki67 proliferative index and is not associated with age. Dig. liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2019, 51, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Q.; Wang, X.; Yang, L.; Chen, Y.H.; Tan, C.L.; Zhu, X.M.; Ke, N.W.; Liu, X.B. Analysis of recurrence after resection of well-differentiated non-functioning pancreatic neuroendocrine tumors. Medicine 2020, 99, e20324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Duan, J.; Yan, S.; Zhou, J.; Zheng, S. Prognostic factors of long-term outcome in surgically resectable pancreatic neuroendocrine tumors: A 12-year experience from a single center. Oncol. Lett. 2017, 13, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.J.; Turrini, O.; Vigano, L.; Russolillo, N.; Autret, A.; Moutardier, V.; Capussotti, L.; Le Treut, Y.P.; Delpero, J.R.; Hardwigsen, J. Surgical management of advanced pancreatic neuroendocrine tumors: Short-term and long-term results from an international multi-institutional study. Ann. Surg. Oncol. 2015, 22, 1000–1007. [Google Scholar] [CrossRef]
- Souche, R.; Coignac, A.; Dupuy, M.; Bertrand, M.; Raingeart, I.; Guiu, B.; Herrero, A.; Panaro, F.; Obled, S.; Portales, F.; et al. Outcome after pancreatectomy for neuroendocrine neoplams according to the WHO 2017 grading system: A retrospective multicentric analysis of 138 consecutive patients. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 286–294. [Google Scholar] [CrossRef]
- Gudmundsdottir, H.; Möller, P.H.; Jonasson, J.G.; Björnsson, E.S. Gastroenteropancreatic neuroendocrine tumors in Iceland: A population-based study. Scand. J. Gastroenterol. 2019, 54, 69–75. [Google Scholar] [CrossRef]
- Sandvik, O.M.; Søreide, K.; Gudlaugsson, E.; Kvaløy, J.T.; Søreide, J.A. Epidemiology and classification of gastroenteropancreatic neuroendocrine neoplasms using current coding criteria. Br. J. Surg. 2016, 103, 226–232. [Google Scholar] [CrossRef]
| Characteristic | First-Line Surgery | p-Value | |
|---|---|---|---|
| YES | NO | ||
| Male gender, n (%) | 26 (56.5) | 15 (68.2) | 0.43 |
| Median age at diagnosis, years (IQR) | 56.5 (43.75–65) | 53.5 (51.25–62.75) | 0.88 |
| Urban area, n (%) | 31 (67.4) | 15 (68.2) | 1 |
| Follow-up, median (IQR) | 40 (10.25–81.75) | 34 (24–44.75) | 0.96 |
| Other significant comorbidities, n (%) | 34 (73.9) | 13 (59.1) | 0.58 |
| Performance status at diagnosis, n (%) | |||
| 18 (39.1) | 7 (31.8) | 0.72 |
| 27 (58.7) | 14 (63.6) | |
| 1 (2.2) | 1 (4.5) | |
| 0 (0) | 0 (0) | |
| Characteristic | First-Line Surgery | p-Value | ||
|---|---|---|---|---|
| YES | NO | |||
| N (%) | N (%) | |||
| Location | Head | 10 (21.7) | 6 (27.3) | 0.52 |
| Body and tail | 36 (78.3) | 12 (54.5) | ||
| Missing | 0 (0) | 3 (13.6) | ||
| Size | ≤20 mm | 18 (39.1) | 4 (18.2) | 0.15 |
| >20 mm | 24 (52.2) | 14 (63.6) | ||
| Missing | 4 (8.7) | 4 (18.2) | ||
| Tumor grading | NET G1 | 28 (60.9) | 7 (31.8) | 0.024 |
| NET G2 | 16 (34.8) | 15 (68.2) | ||
| NET G3 | 1 (2.2) | 0 (0) | ||
| Missing | 1 (2.2) | 0 (0) | ||
| T stage | T1 | 17 (37) | 0 (0) | 0.012 |
| T2 | 9 (19.6) | 4 (18.2) | ||
| T3 | 7 (15.2) | 1 (4.5) | ||
| T4 | 5 (10.9) | 4 (18.2) | ||
| Missing | 8 (17.4) | 13 (59.1) | ||
| N stage | N0 | 30 (65.2) | 5 (22.7) | 0.004 |
| N1 | 6 (13) | 8 (36.4) | ||
| Missing | 10 (21.7) | 9 (40.9) | ||
| M stage | M0 | 32 (69.6) | 6 (27.3) | <0.001 |
| M1 | 12 (26.1) | 16 (72.7) | ||
| Site of metastases | Liver | 10 (21.7) | 11 (50) | 0.66 |
| Extrahepatic | 1 (2.2) | 1 (4.5) | ||
| Both | 1 (2.2) | 4 (18.2) | ||
| TNM stage at diagnosis | Stage 1 | 20 (43.5) | 0 (0) | <0.001 |
| Stage 2 | 7 (15.2) | 3 (13.6) | ||
| Stage 3 | 6 (13) | 3 (13.6) | ||
| Stage 4 | 12 (26.1) | 16 (72.7) | ||
| Missing | 1 (2.2) | 0 (0) | ||
| Tumor functional status | Non-functional | 30 (65.2) | 10 (45.5) | 0.01 |
| Carcinoid syndrome | 4 (8.7) | 10 (45.5) | ||
| Gastrinoma | 1 (2.2) | 0 (0) | ||
| Missing | 11 (23.9) | 2 (9.1) | ||
| Tumor Grade | First-Line Surgery (n = 46) | Non-Surgery (n = 22) | ||||
|---|---|---|---|---|---|---|
| n (%) | Ki-67 (%) Median, IQR | Mitotic Index * Median, IQR | n (%) | Ki-67 (%) Median, IQR | Mitotic Index * Median, IQR | |
| G1 | 28 (60.9) | 2 (1–2) | 1 (0–1) | 7 (31.8) | 2 (2–2) | 1 (0–1) |
| G2 | 16 (34.8) | 7 (5–10) | 3 (2–5) | 15 (68.2) | 6 (5–10) | 3 (2–6) |
| G3 | 1 (2.2) | 40 (40) | 15 | 0 | - | - |
| Missing | 1 (2.2) | - | - | 0 | - | - |
| First-Line Surgery | p-Value | |||
|---|---|---|---|---|
| YES N (%) | NO N (%) | |||
| Department of referral | Gastroenterology | 14 (30.4) | 11 (50) | <0.001 |
| Surgery | 31 (67.4) | 5 (22.7) | ||
| Oncology | 1 (2.2) | 5 (22.7) | ||
| Internal medicine | 0 (0) | 1 (4.5) | ||
| Mode of diagnosis | Incidentaloma | 15 (32.6) | 14 (63.6) | <0.001 |
| Functional syndrome | 1 (2.2) | 1 (4.5) | ||
| Tumor-related symptoms | 26 (56.5) | 2 (9.1) | ||
| Screening programs | 2 (4.3) | 3 (13.6) | ||
| Missing | 2 (4.3) | 2 (9.1) | ||
| Imaging procedures | CT scan | 22 (47.8) | 13 (59.1) | 0.72 |
| MRI | 12 (26.1) | 3 (13.6) | ||
| Multiple | 10 (21.7) | 5 (22.7) | ||
| Missing | 2 (4.3) | 1 (4.5) | ||
| Histopathology sample collection | EUS with FNA | 12 (26.1) | 9 (40.9) | <0.001 |
| EUS with FNB | 2 (4.3) | 3 (13.6) | ||
| Surgery | 32 (69.6) | 3 (13.6) | ||
| Percutaneous liver biopsy | 0 (0) | 6 (27.3) | ||
| Missing | 0 (0) | 1 (4.5) | ||
| Source of histopathology sample | Primary tumor | 39 (84.8) | 11 (50) | 0.001 |
| Lymph nodes | 1 (2.2) | 0 (0) | ||
| Metastases | 2 (4.3) | 8 (36.4) | ||
| Multiple | 4 (8.7) | 2 (9.1) | ||
| Missing | 0 (0) | 1 (4.5) | ||
| Surgery | N (%) |
|---|---|
| Type of surgical procedure | |
| 5 (10.9) |
| 36 (78.3) |
| 1 (2.2) |
| 2 (4.3) |
| 1 (2.2) |
| 1 (2.2) |
| Synchronous liver metastasectomy | 13 (28.2) |
| Curative intent | 34 (73.9) |
| R0 resection | 33 (71.7) |
| Stratification | Group | 1-Year OS | 3-Year OS | 5-Year OS | Median OS (Months) | Mean OS (Months) | p-Value |
|---|---|---|---|---|---|---|---|
| Gender | Male | 94.6% | 87.8% | 80.3% | 100 | 119.4 | 0.76 |
| Female | 100% | 73.9% | 73.9% | 179 | 138.5 | ||
| Lymph node invasion (N1) | Yes | 91.7% | 67.9% | 54.3% | - | 100.5 | 0.2 |
| No | 96.2% | 91.3% | 91.3% | - | 122.1 | ||
| Metastatic disease (M1) | Yes | 96.2% | 76.3% | 70.9% | 179 | 117.9 | 0.39 |
| No | 96.8% | 89% | 84.1% | - | 130.9 | ||
| Tumor Grading | Grade 1 | 95.7% | 95.7% | 88.3% | 179 | 152.1 | <0.001 |
| Grade 2 | 100% | 76.9% | 72.8% | 100 | 116.3 | ||
| Grade 3 | 0% | 0% | 0% | 6 | 6 | ||
| Functional syndrome | Yes | 93.3% | 65.3% | 65.3% | 71 | 94.5 | 0.14 |
| No | 100% | 94.1% | 79.6% | - | 137.2 | ||
| First-line surgery | Yes | 97.3% | 97.3% | 97.3% | - | 158 | <0.001 |
| No | 95.2% | 62.6% | 49.3% | 42 | 75.9 |
| Stratification | Group | 1-Year DFS | 3-Year DFS | 5-Year DFS | Median DFS (Months) | Mean DFS (Months) | p-Value |
|---|---|---|---|---|---|---|---|
| Location | Head | 100% | 100% | 100% | - | 160 | 0.37 |
| Body and tail | 94.7% | 80.4% | 80.4% | - | 131.7 | ||
| Size | ≤20 mm | 100% | 100% | 100% | - | 160 | 0.12 |
| >20 mm | 91.7% | 78.6% | 78.6% | - | 128.4 | ||
| Lymph node invasion (N1) | Yes | 100% | 100% | 100% | - | 160 | 0.69 |
| No | 95.2% | 89.6% | 89.6% | - | 144.7 | ||
| Metastatic disease (M1) | Yes | 100% | 66.7% | 66.7% | - | 112.3 | 0.3 |
| No | 95% | 87.7% | 87.7% | - | 142.1 | ||
| Tumor Grading | Grade 1 | 100% | 100% | 100% | - | 160 | <0.001 |
| Grade 2 | 100% | 66.7% | 66.7% | - | 113 | ||
| Grade 3 | 0% | 0% | 0% | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diculescu, R.-I.; Stroie, T.; Istrătescu, D.; Croitoru, A.E.; Gheorghe, C.; Brașoveanu, V.; Dumitrașcu, T.; Eftimie, M.A.; Țuțuian, R.; Poiană, C. Clinical Management and Prognostic Outcomes of Pancreatic Neuroendocrine Tumors: Insights from a Tertiary Care Center. Medicina 2025, 61, 1955. https://doi.org/10.3390/medicina61111955
Diculescu R-I, Stroie T, Istrătescu D, Croitoru AE, Gheorghe C, Brașoveanu V, Dumitrașcu T, Eftimie MA, Țuțuian R, Poiană C. Clinical Management and Prognostic Outcomes of Pancreatic Neuroendocrine Tumors: Insights from a Tertiary Care Center. Medicina. 2025; 61(11):1955. https://doi.org/10.3390/medicina61111955
Chicago/Turabian StyleDiculescu, Rucsandra-Ilinca, Tudor Stroie, Doina Istrătescu, Adina Emilia Croitoru, Cristian Gheorghe, Vladislav Brașoveanu, Traian Dumitrașcu, Mihai Adrian Eftimie, Radu Țuțuian, and Cătălina Poiană. 2025. "Clinical Management and Prognostic Outcomes of Pancreatic Neuroendocrine Tumors: Insights from a Tertiary Care Center" Medicina 61, no. 11: 1955. https://doi.org/10.3390/medicina61111955
APA StyleDiculescu, R.-I., Stroie, T., Istrătescu, D., Croitoru, A. E., Gheorghe, C., Brașoveanu, V., Dumitrașcu, T., Eftimie, M. A., Țuțuian, R., & Poiană, C. (2025). Clinical Management and Prognostic Outcomes of Pancreatic Neuroendocrine Tumors: Insights from a Tertiary Care Center. Medicina, 61(11), 1955. https://doi.org/10.3390/medicina61111955






