Increases in Strain, Strain Rate, Displacement and Velocity in the Thoracic Aorta After Bench Pressing
Abstract
1. Introduction
2. Materials and Methods
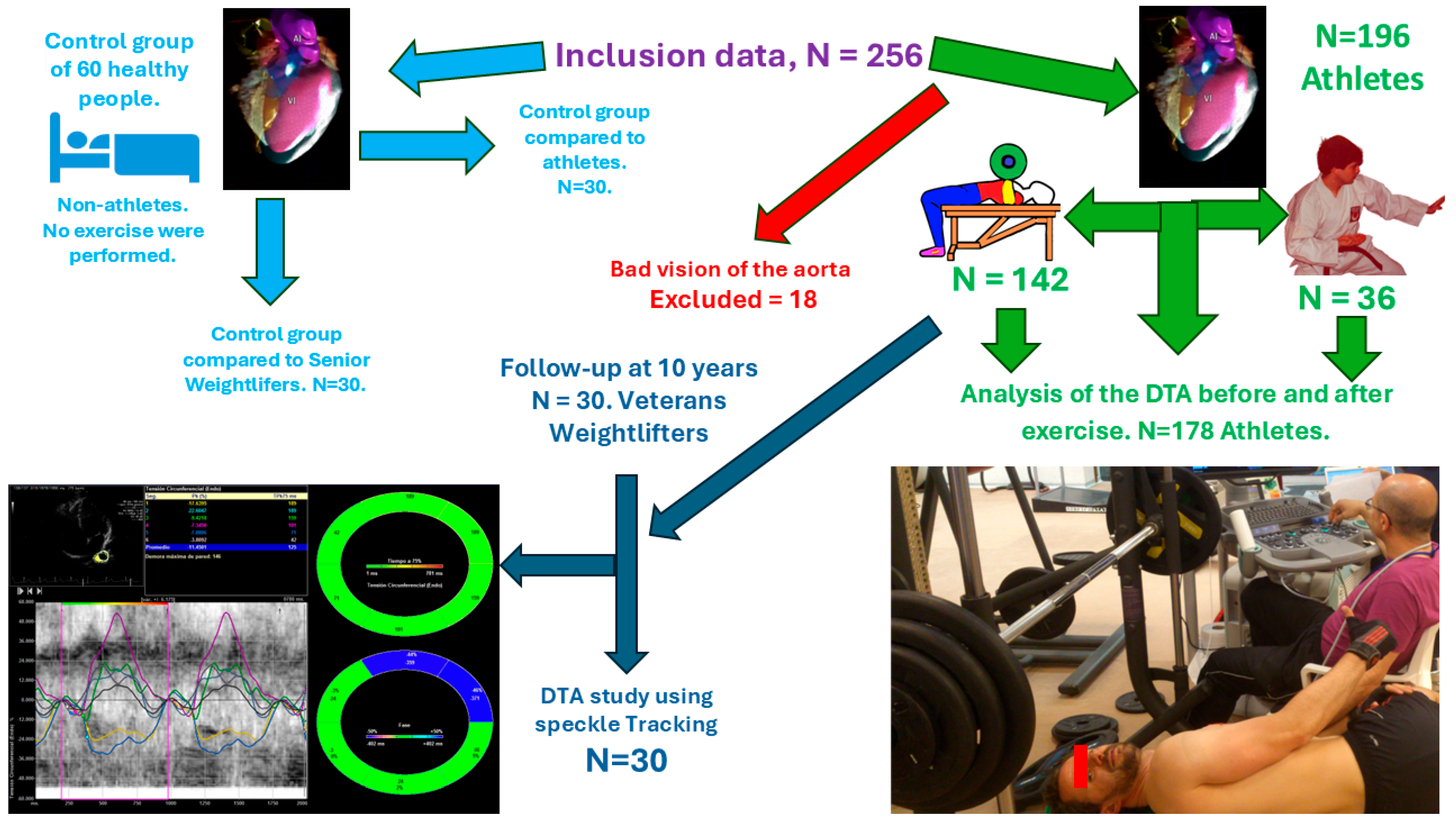
2.1. Clinical Cohort
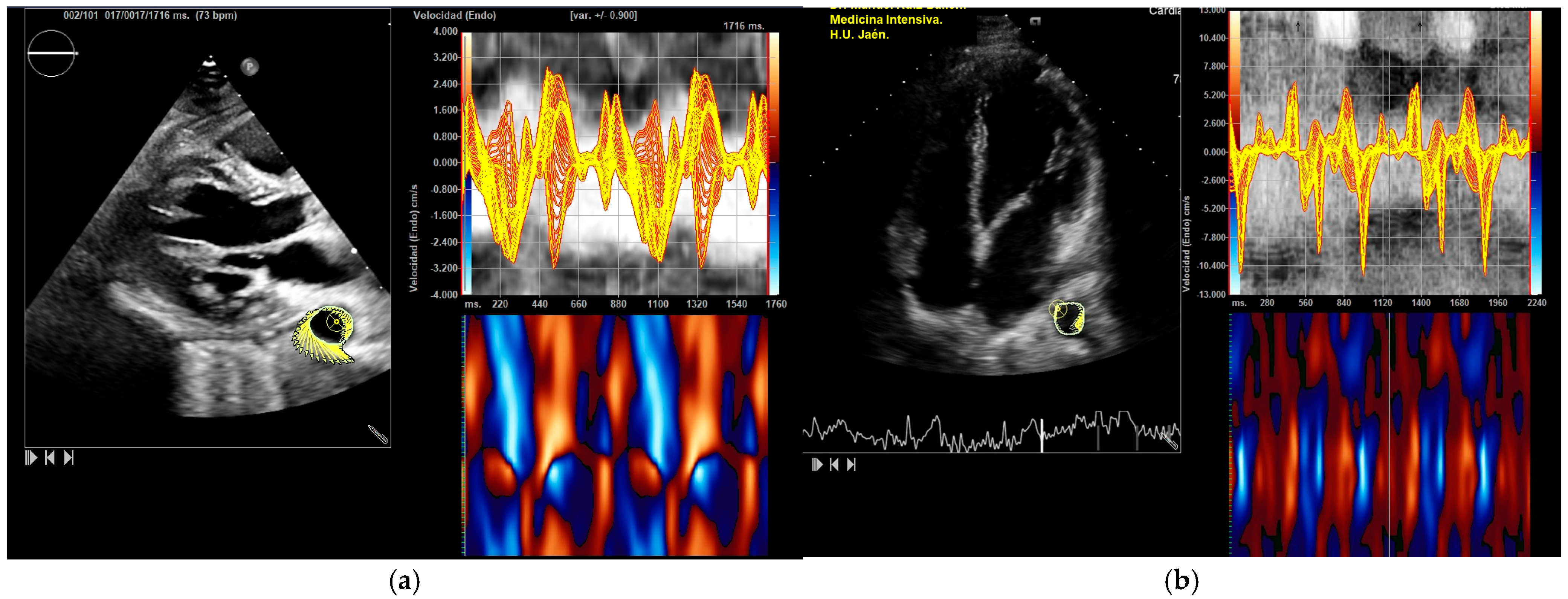
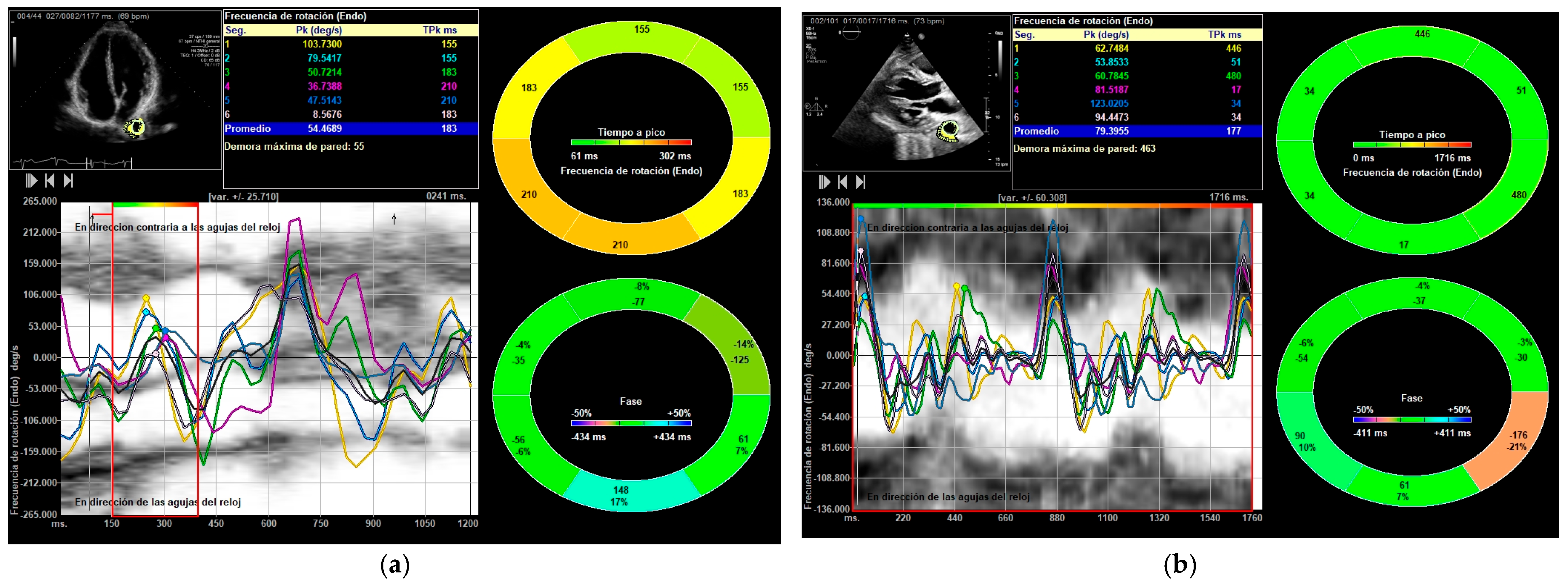
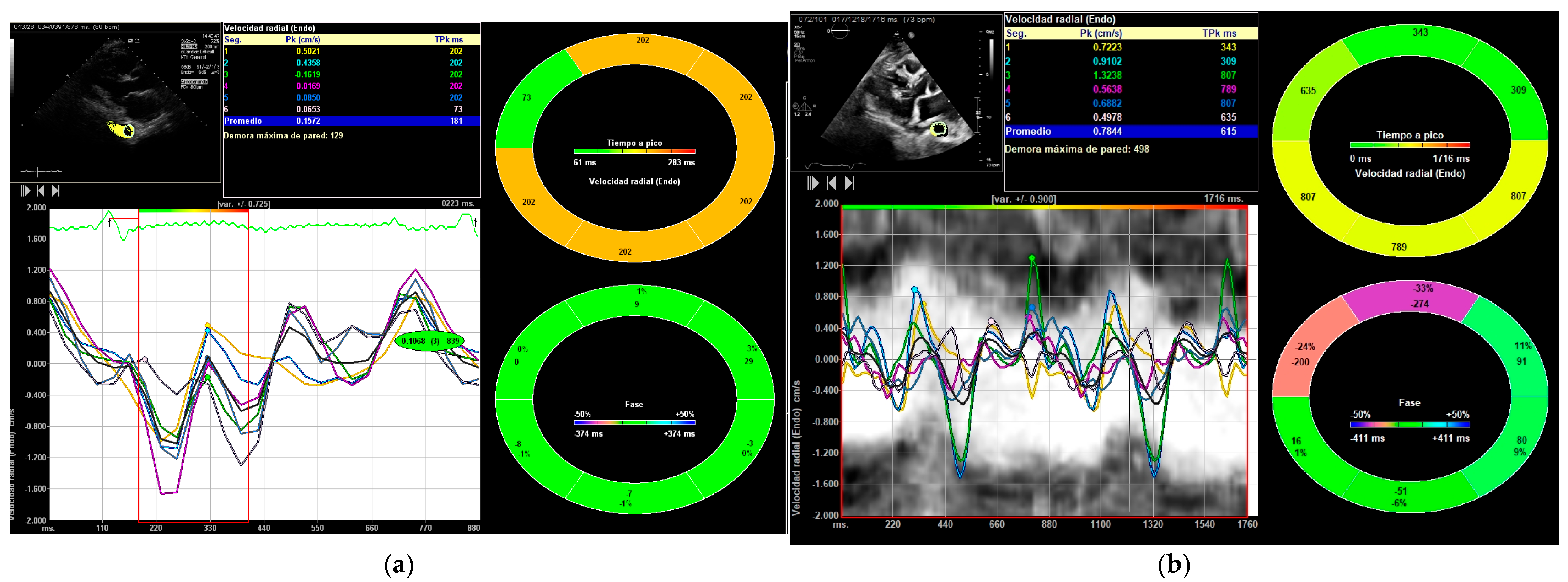
2.2. Image Acquisition and Processing
2.3. Bench Press Exercise
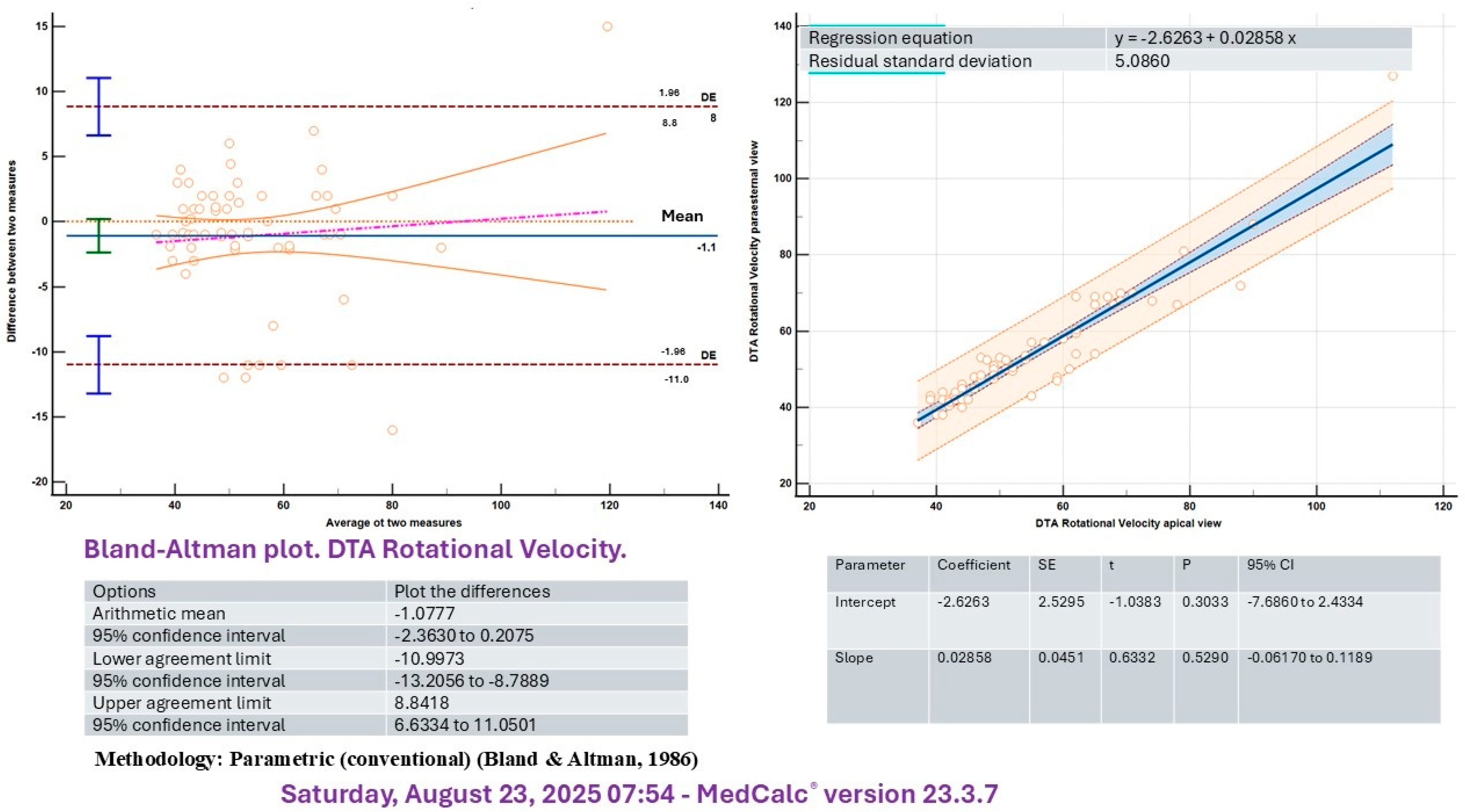
2.4. Statistical Analysis
3. Results
3.1. The Control Group and Athletes at the Beginning of the Study
3.2. Differences Between Before and After Bench Press Exercise
3.3. Karate Subgroup
3.4. Follow-Up at 10 Years
3.5. Nutrition and DTA Speckle Tracking
4. Discussion
4.1. Clinical Applicability
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DTA | Descending thoracic aorta |
| PUFAs | Polyunsaturated fatty acids |
| S | Strain |
| SR | Strain rate |
| RM | Repetition maximal |
| VVI | Velocity vector analysis |
References
- Mazzolai, L.; Belch, J.; Venermo, M.; Aboyans, V.; Brodmann, M.; Bura-Rivière, A.; Debus, S.; Espinola-Klein, C.; Harwood, A.E.; Hawley, J.A.; et al. Exercise Therapy for Chronic Symptomatic Peripheral Artery Disease: A Clinical Consensus Document of the European Society of Cardiology Working Group on Aorta and Peripheral Vascular Diseases in Collaboration With the European Society of Vascular Medicine and the European Society for Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Galloo, X.; Cosyns, B. Tell me the name of your sport, and I will tell you the size of your aorta. Eur. J. Prev. Cardiol. 2020, 27, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jiang, S.; Hu, C.; Shen, B.; Gu, J. The effects of circuit-based resistance training on blood pressure, arterial stiffness, and body composition in community-dwelling older adults: A systematic review and meta-analysis. Front. Physiol. 2025, 16, 1609013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.; Zhou, K.; Shang, H.; Du, M.; Wu, L.; Chen, Y. Effects of concurrent aerobic and resistance training on vascular health in type 2 diabetes: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1216962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaman, S.; Selva Raj, I.; Yang, A.W.H.; Lindner, R.; Denham, J. Exercise training reduces arterial stiffness in women with high blood pressure: A systematic review and meta-analysis. J. Hypertens. 2024, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Churchill, T.W.; Groezinger, E.; Kim, J.H.; Loomer, G.; Guseh, J.S.; Wasfy, M.M.; Isselbacher, E.M.; Lewis, G.D.; Weiner, R.B.; Schmied, C.; et al. Association of Ascending Aortic Dilatation and Long-term Endurance Exercise Among Older Masters-Level Athletes. JAMA Cardiol. 2020, 5, 522–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Limongelli, G.; Monda, E.; Lioncino, M.; Di Paolo, F.; Ferrara, F.; Vriz, O.; Calabro, P.; Bossone, E.; Pelliccia, A. Aortic Root Diameter in Highly-Trained Competitive Athletes: Reference Values According to Sport and Prevalence of Aortic Enlargement. Can. J. Cardiol. 2023, 39, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Maceira, A.; Santos-Lozano, A.; García-González, M.P.; Higueras Ortega, L.; Díaz-Gonzalez, L.; Boraita, A.; Barranco-Gil, D.; Lucia, A. Aortic Diameters and Calcifications in Former World-Class Cyclists. Med. Sci. Sports Exerc. 2023, 55, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Rubies, C.; Batlle, M.; Sanz-de la Garza, M.; Dantas, A.P.; Jorba, I.; Fernandez, G.; Sangüesa, G.; Abuli, M.; Brugada, J.; Sitges, M.; et al. Long-Term Strenuous Exercise Promotes Vascular Injury by Selectively Damaging the Tunica Media: Experimental Evidence. JACC Basic Transl. Sci. 2022, 7, 681–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, H.; Yang, S.; Yu, S.; Hu, X.; Meng, X.; Wu, K. Prognostic Value of Left Ventricular Global Longitudinal Strain for Major Adverse Cardiovasc.ular Events in Patients with Aortic Valve Disease: A Meta-Analysis. Cardiology 2024, 149, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Bailén, M.; Cobo Molinos, J.; Espada-Fuentes, J.C.; Castillo-Rivera, A.M.; Martínez-Amat, A.; Martínez Ramírez, M.J. Left Ventricular Function Improve after Bench Press: A Speckle Tracking and 3D Echocardiography Study. J. Sports Med. Doping Stud. 2017, 7, 1. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.B.; Bi, X.J.; Liu, Y.N.; Zhang, J.; Li, L.; Chen, B. Evaluation of myocardial strain and aortic elasticity in patients with bicuspid aortic valve. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Petrini, J.; Eriksson, M.J.; Caidahl, K.; Larsson, M. Circumferential strain by velocity vector imaging and speckle-tracking echocardiography: Validation against sonomicrometry in an aortic phantom. Clin. Physiol. Funct. Imaging 2017, 38, 269–277. [Google Scholar] [CrossRef]
- Bell, V.; Sigurdsson, S.; Westenberg, J.J.; Gotal, J.D.; Torjesen, A.A.; Aspelund, T. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility—Reykjavik Study. Circ. Cardiovasc. Imaging. 2015, 8, e003039. [Google Scholar] [CrossRef]
- Bird, S.P.; Nienhuis, M.; Biagioli, B.; De Pauw, K.; Meeusen, R. Supplementation Strategies for Strength and Power Athletes: Carbohydrate, Protein, and Amino Acid Ingestion. Nutrients 2024, 16, 1886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Wang, C.; Jiang, H.; Zhu, J.; Wu, R.; Niu, Y.; Chen, F.; Jin, Y. Geriatric nutritional risk index predicts perioperative cardiovascular events in older patients with coronary artery disease undergoing non-cardiac surgery: A multicenter retrospective cohort study. Front. Nutr. 2025, 12, 1652742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz-Bailén, M.; Hidalgo-Martín, J.; Manetsberger, J.; Clau-Terré, F.; Martínez-Gámez, J.; Dagomar Lohman, J.; Lavilla-Lerma, M.L.; Matallana-Zapata, D.F.; Ballesteros-Barroso, M.; Rivera-Fernández, R.; et al. Usefulness of vector velocity imaging in the descending thoracic aorta. Med. Intensiv. Engl. Ed. 2025, 502224. [Google Scholar] [CrossRef]
- Vizzardi, E.; Pina, P.; Caretta, G.; Bonadei, I.; Sciatti, E.; Lombardi, C. The effect of aldosterone-antagonist therapy on aortic elastic properties in patients with nonischemic dilated cardiomyopathy. J. Cardiovasc. Med. 2015, 16, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Qiu, H.Q.; Wang, C.; Tang, Y.T.; Zhang, C.R.; Fan, Y.Y.; Jiao, X.Y. Levosimendan Relaxes Thoracic Aortic Smooth Muscle in Mice by Inhibiting PKC and Activating Inwardly Rectifying Potassium Channels. J. Cardiovasc. Pharmacol. 2024, 83, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; McCabe, E.L.; Larson, M.G.; Rong, J.; Merz, A.A.; Osypiuk, E. Relations Between Aortic Stiffness and Left Ventricular Mechanical Function in the Community. J. Am. Heart Associ. 2017, 6, e004903. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, A.O.; Zachariah, J.P.; Tuomainen, T.P. Arterial stiffness but not carotid intima-media thickness progression precedes premature structural and functional cardiac damage in youth: A 7-year temporal and mediation longitudinal study. Atherosclerosis 2023, 380, 117197. [Google Scholar] [CrossRef] [PubMed]
- Zapolski, T.; Fumaga, J.; Jaroszynski, A.; Wysocka, A.; Rudzki, S.; Wysokinski, A. The reverse remodeling of the aorta in patients after renal transplantation—The value of aortic stiffness index: Prospective echocardiographic study. BMC Nephrol. 2017, 18, 33. [Google Scholar] [CrossRef]
- Bieseviciene, M.; Vaskelyte, J.J.; Mizariene, V.; Karaliute, R.; Lesauskaite, V.; Verseckaite, R. Two-dimensional speckle-tracking echocardiography for evaluation of dilative ascending aorta biomechanics. BMC Cardiovasc. Disord. 2017, 17, 27. [Google Scholar] [CrossRef]
- Martínez-Lechuga, M.B.; Hidalgo-Martín, J.; Ruiz-Bailén, M. Right Ventricular Function Improves After Bench Press: A Speckle Tracking Echocardiography Study. Medicina 2025, 61, 1469. [Google Scholar] [CrossRef]
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019, 1, CD003331, Erratum in Cochrane Database Syst. Rev. 2024, 3, CD003331. https://doi.org/10.1002/14651858.CD003331.pub6. PMID: 30695817; PMCID: PMC6492482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nemes, A.; Domsik, P.; Kalapos, A.; Forster, T. An alternative way to assess aortic elasticity: Three- dimensional speckle-tracking echocardiography-derived strain assessment (From the MAGYAR-Healthy Study). Int. J. Cardiol. 2016, 203, 109–110. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults-A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gruson, D.; Pouleur, A.C.; Makris, K.; Chrysohoou, C. Systematic vitamin D supplementation and monitoring: Improving outcomes in heart failure? Eur. J. Heart Fail. 2017, 19, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Gil-Izquierdo, A.; González-Rodríguez, L.G.; Alférez, M.J.M.; San Juan, A.F.; Sánchez-Gómez, Á.; Calvo-Ayuso, N.; Ramos-Álvarez, J.J.; Fernández-Lázaro, D.; Lopez-Grueso, R.; et al. Nutritional Strategies for Optimizing Health, Sports Performance, and Recovery for Female Athletes and Other Physically Active Women: A Systematic Review. Nutr. Rev. 2024, 83, e1068–e1089. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Mawandji, N.B.S.; Schereider, I.R.G.; de Oliveira, E.C.; Vieira, J.M.; Bolsoni-Lopes, A.; Graceli, J.B.; Dantas, J.A.; Cardoso, L.S.; Vassallo, D.V.; et al. A Refined Carbohydrate-Rich Diet Reduces Vascular Reactivity Through Endothelial Oxidative Stress and Increased Nitric Oxide: The Involvement of Inducible Nitric Oxide Synthase. Nutrients 2025, 17, 2395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zou, K.; Wang, Y.; Zhang, Y.; Zhong, J.; Zhou, W.; Tang, F.; Peng, L.; Liu, X.; Deng, L. Exploring the link between A Body Shape Index and abdominal aortic calcification in chronic kidney disease: A cross-sectional analysis from 2013–2014 National Health and Nutrition Examination Survey. Ren. Fail. 2025, 47, 2517403. [Google Scholar] [CrossRef] [PubMed]
- Bezuglov, E.; Vinogradov, M.; Anishchenko, I.; Vakhidov, T.; Usmanova, E.; Malyakin, G.; Kapralova, E. Vitamin D Deficiency in Young Elite Soccer Players Residing Permanently in Regions above 55 Degrees North Latitude. J. Bone Metab. 2025, 32, 114–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cistulli, D.C.; Tong, B.K.; Currie, P.J.; Bin, Y.S.; Stewart, G.M.; Bannon, P.G.; Ugander, M.; Cistulli, P.A. Association between obstructive sleep apnea and thoracic aortic diameter: A cross-sectional study in a clinical sample. J. Clin. Sleep Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Migacz, E.; Olejarz, W.; Łoś, A.; Bednarek-Rajewska, K.; Smith, D.F.; Kluk, A.; Kukwa, W. S100a8 and s100a9 are elevated in aortic wall from patients with moderate-and-severe obstructive sleep apnea syndrome. Sleep Breath 2025, 29, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hidalgo-Martín, J.; Del Carmen Ruiz-Iniesta, M.; Pola-Gallego-de-Guzmán, M.D.; Manetsberger, J.; Martínez-Lechuga, M.B.; Ramos-Cuadra, J.Á.; Castillo-Portellano, C.; Manzano-Moreno, J.L.; Colmenero-Aguilar, C.; Castellanos-Hernández, M.; et al. Detecting heart failure in severe asthma patients using speckle tracking echocardiography. Crit. Care 2025, 29, 374. [Google Scholar] [CrossRef] [PubMed]

| Step | Instruction |
|---|---|
| 1 | Light-load warm-up set, 10 reps |
| 2 | Rest 1 min |
| 3 | Increase load 5–10%, perform 3–5 reps |
| 4 | Rest 2 min |
| 5 | Increase load 5–10%, perform 2–3 reps |
| 6 | Rest 4 min |
| 7 | Increase load 5–10%, perform 1 rep |
| 8 | Rest 4 min, increase load 5–10%, repeat |
| 9 | If unable to lift, rest 4 min, reduce 2.5–5%, repeat |
| 10 | Continue adjusting to find 1 RM |
| 11 | After 1 RM: estimate 10 reps |
| 12 | Training: 10 sets of 10 reps at 75% 1 RM, 1′30″ break |
| Variables | Control Group | 178 Athletes | p-Value | |
|---|---|---|---|---|
| Do Not Perform Bench Presses (n = 30) | Before Bench Press (n = 178) | After Bench Press (n = 178) | ||
| Systolic blood pressure (mmHg) | 117.35 ± 14.56 | 128.88 ± 09.28 | 139.78 ± 16.37 | <0.05 |
| Diastolic blood pressure (mmHg) | 78.11 ± 17.36 | 88.98 ± 10.31 | 98.76 ± 25.32 | <0.05 |
| Heart rate (B/min) | 71.21 ± 13.15 | 55.14 ± 09.32 | 110.55 ± 26.41 | <0.05 |
| Breathing rate (B/m) | 16.38 ± 5.44 | 15.72 ± 6.87 | 26.88 ± 6.38 | <0.05 |
| SpO2 (%) | 97.05 ± 0.65 | 95.42 ± 1.22 | 98.98 ± 1.24 | N.S. |
| Variables | Control Group | 178 Athletes | p-Value | |
|---|---|---|---|---|
| Do Not Perform Bench Presses n = 30 | Before Bench Press N = 178 | After Bench Press N = 178 | ||
| 3D LVEF (%) | 65.33 ± 1.19 | 53.04 ± 1.31 | 74.78 ± 1.87 | <0.05 |
| 2D LVEF (%) | 56.21 ± 3.22 | 46.21 ± 1.18 | 68.44 ± 1.28 | <0.05 |
| Right ventricular global longitudinal strain (%) | −27.77 ± 3.27 | −23.88 ± 2.88 | −30.88 ± 11.02 | <0.05 |
| Left ventricular global longitudinal strain (%) | −21.88 ± 4.11 | −18.11 ± 3.47 | −25.21 ± 3.27 | <0.05 |
| Left ventricular global longitudinal rate (1/s) | −1.59 ± 0.93 | −1.44 ± 0.45 * | −2.93 ± 0.56 * | <0.05 |
| Right ventricular area shortening fraction (%) | 55.78 ± 3.22 | 47.56 ± 1.28 | 65.68 ± 2.52 | <0.05 |
| Variable | Before Bench Press | After Bench Press |
|---|---|---|
| Right ventricular fractional shortening Area (%) | N = 178; 44.12 ± 1.21 | N = 178; 62.36 ± 0.09 |
| DTA rotational velocity (°/s) | N = 178; 55.44 ± 16.15 | N = 178; 88.98 ± 10.31 |
| DTA radial velocity (cm/s) | N = 144; 1.02 ± 0.36 | N = 144; 1.56 ± 0.42 |
| DTA circumferential strain (%) | N = 149; −8.52 ± 0.31 | N = 149; −12.55 ± 1.13 |
| DTA strain-rate circumferential aorta (1/s) | N = 156; −1.55 ± 0.72 | N = 138; −2.28 ± 0.56 |
| DTA rotational displacement (°) | N = 168; 6.22 ± 0.36 | N = 168; 14.91 ± 0.85 |
| DTA aorta radial displacement (mm) | N = 178; 0.89 ± 0.31 | N = 164; 1.19 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Lechuga, M.B.; Hidalgo-Martín, J.; Ruiz-Bailén, M. Increases in Strain, Strain Rate, Displacement and Velocity in the Thoracic Aorta After Bench Pressing. Medicina 2025, 61, 1950. https://doi.org/10.3390/medicina61111950
Martínez-Lechuga MB, Hidalgo-Martín J, Ruiz-Bailén M. Increases in Strain, Strain Rate, Displacement and Velocity in the Thoracic Aorta After Bench Pressing. Medicina. 2025; 61(11):1950. https://doi.org/10.3390/medicina61111950
Chicago/Turabian StyleMartínez-Lechuga, María Belén, Javier Hidalgo-Martín, and Manuel Ruiz-Bailén. 2025. "Increases in Strain, Strain Rate, Displacement and Velocity in the Thoracic Aorta After Bench Pressing" Medicina 61, no. 11: 1950. https://doi.org/10.3390/medicina61111950
APA StyleMartínez-Lechuga, M. B., Hidalgo-Martín, J., & Ruiz-Bailén, M. (2025). Increases in Strain, Strain Rate, Displacement and Velocity in the Thoracic Aorta After Bench Pressing. Medicina, 61(11), 1950. https://doi.org/10.3390/medicina61111950







