Prolonged Corrected QT Interval Is Associated with Lower Incidence of Maternal Hypotension During Spinal Anesthesia in Cesarean Delivery: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
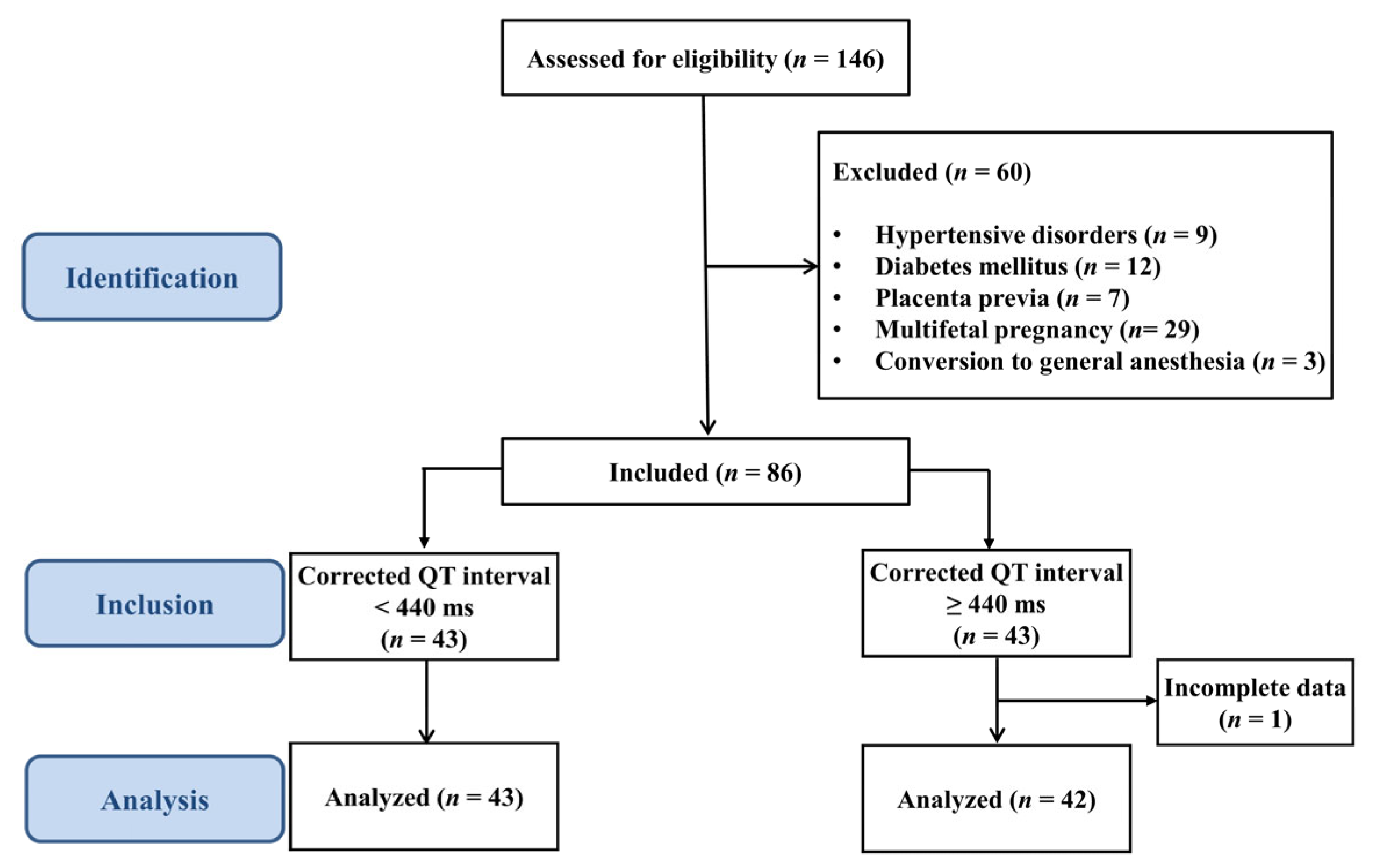
2.1. Patients and Eligibility Criteria
2.2. Data Sources
2.3. CSEA and Management in the Operating Room
2.4. Sample Size
2.5. Statistical Analysis
3. Results
3.1. Comparisons of Hemodynamic Parameters Between Groups
3.2. Predictive Performance According to the Baseline QTc
3.3. Vasopressor Requirement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSEA | Combined spinal–epidural anesthesia |
| QTc | Corrected QT interval |
References
- Sumikura, H.; Niwa, H.; Sato, M.; Nakamoto, T.; Asai, T.; Hagihira, S. Rethinking general anesthesia for cesarean section. J. Anesth. 2016, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Lieberman, E.S.; Camann, W.R. Regional anesthesia and analgesia for labor and delivery. N. Engl. J. Med. 2003, 348, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Klohr, S.; Roth, R.; Hofmann, T.; Rossaint, R.; Heesen, M. Definitions of hypotension after spinal anaesthesia for caesarean section: Literature search and application to parturients. Acta Anaesthesiol. Scand. 2010, 54, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Lato, K.; Bekes, I.; Widschwendter, P.; Friedl, T.W.P.; Janni, W.; Reister, F.; Froeba, G.; Friebe-Hoffmann, U. Hypotension due to spinal anesthesia influences fetal circulation in primary caesarean sections. Arch. Gynecol. Obstet. 2018, 297, 667–674. [Google Scholar] [CrossRef]
- Sakata, K.; Yoshimura, N.; Tanabe, K.; Kito, K.; Nagase, K.; Iida, H. Prediction of hypotension during spinal anesthesia for elective cesarean section by altered heart rate variability induced by postural change. Int. J. Obstet. Anesth. 2017, 29, 34–38. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.Q. Role of pleth variability index for predicting hypotension after spinal anesthesia for cesarean section. Int. J. Obstet. Anesth. 2014, 23, 324–329. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Noel, A.; Duclos, G.; Haddam, M.; Delmas, A.; Bechis, C.; Loundou, A.; Blanc, J.; Mignon, A.; Bouvet, L.; et al. Can point-of-care ultrasound predict spinal hypotension during caesarean section? A prospective observational study. Anaesthesia 2018, 73, 15–22. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Circulation 2009, 119, e241–e250. [Google Scholar] [CrossRef]
- Sen, S.; Ozmert, G.; Turan, H.; Caliskan, E.; Onbasili, A.; Kaya, D. The effects of spinal anesthesia on QT interval in preeclamptic patients. Anesth. Analg. 2006, 103, 1250–1255. [Google Scholar] [CrossRef]
- Gupta, S.D.; Roy, S.; Mitra, K.; Kundu, S.B.; Maji, S.; Sarkar, A.; Bhattacharya, S.; Roy, C.D.; Sen, S.; Basu, M. Effect of QTc interval on prediction of hypotension following subarachnoid block in patients undergoing cesarean section: A comparative study. J. Obstet. Anaesth. Crit. Care 2012, 2, 79–85. [Google Scholar] [CrossRef]
- Fischer, T.; Schobel, H.P.; Frank, H.; Andreae, M.; Schneider, K.T.; Heusser, K. Pregnancy-induced sympathetic overactivity: A precursor of preeclampsia. Eur. J. Clin. Investig. 2004, 34, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, J.; Bennett, K. Pre-eclampsia: Fluids, drugs, and anesthetic management. Anesthesiol. Clin. N. Am. 2003, 21, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Almaghamsi, A.; Almalki, M.H.; Buhary, B.M. Hypocalcemia in Pregnancy: A Clinical Review Update. Oman Med. J. 2018, 33, 453–462. [Google Scholar] [CrossRef]
- Merri, M.; Benhorin, J.; Alberti, M.; Locati, E.; Moss, A.J. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989, 80, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Moss, A.J.; Vincent, G.M.; Crampton, R.S. Diagnostic criteria for the long QT syndrome. An update. Circulation 1993, 88, 782–784. [Google Scholar] [CrossRef]
- Booker, P.D.; Whyte, S.D.; Ladusans, E.J. Long QT syndrome and anaesthesia. Br. J. Anaesth. 2003, 90, 349–366. [Google Scholar] [CrossRef]
- Goldenberg, I.; Moss, A.J.; Zareba, W. QT interval: How to measure it and what is “normal”. J. Cardiovasc. Electrophysiol. 2006, 17, 333–336. [Google Scholar] [CrossRef]
- Taggart, N.W.; Haglund, C.M.; Tester, D.J.; Ackerman, M.J. Diagnostic miscues in congenital long-QT syndrome. Circulation 2007, 115, 2613–2620. [Google Scholar] [CrossRef]
- Vetter, V.L. Clues or miscues? How to make the right interpretation and correctly diagnose long-QT syndrome. Circulation 2007, 115, 2595–2598. [Google Scholar] [CrossRef]
- Aboshady, O.A.; Raffa, J.Z.; Quinney, S.K.; Tisdale, J.E.; Overholser, B.R. QTc Interval Changes in Preeclampsia vs. Normal Pregnancy: A Systematic Review and Meta-Analysis. Clin. Pharmacol. Ther. 2025, 117, 1650–1660. [Google Scholar] [CrossRef]
- Vandenberk, B.; Vandael, E.; Robyns, T.; Vandenberghe, J.; Garweg, C.; Foulon, V.; Ector, J.; Willems, R. Which QT Correction Formulae to Use for QT Monitoring? J. Am. Heart Assoc. 2016, 5, e003264. [Google Scholar] [CrossRef]
- Clunies-Ross, N.; Roston, T.M.; Taylor, J.; Whyte, S.; Albert, A.; Gorges, M.; Chau, A. The Effect of Carbetocin Dose on Transmural Dispersion of Myocardial Repolarization in Healthy Parturients Scheduled for Elective Cesarean Delivery Under Spinal Anesthesia: A Prospective, Randomized Clinical Trial. Anesth. Analg. 2021, 132, 485–492. [Google Scholar] [CrossRef]
- Magnano, A.R.; Holleran, S.; Ramakrishnan, R.; Reiffel, J.A.; Bloomfield, D.M. Autonomic nervous system influences on QT interval in normal subjects. J. Am. Coll. Cardiol. 2002, 39, 1820–1826. [Google Scholar] [CrossRef]
- Raffaelli, R.; Antonia Prioli, M.; Parissone, F.; Prati, D.; Carli, M.; Bergamini, C.; Cacici, G.; Balestreri, D.; Vassanelli, C.; Franchi, M. Pre-eclampsia: Evidence of altered ventricular repolarization by standard ECG parameters and QT dispersion. Hypertens. Res. 2014, 37, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Lechmanova, M.; Kittnar, O.; Mlcek, M.; Slavicek, J.; Dohnalova, A.; Havranek, S.; Kolarik, J.; Parizek, A. QT dispersion and T-loop morphology in late pregnancy and after delivery. Physiol. Res. 2002, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, S.M.; Carvalho, B.; Dyer, R.A.; Fernando, R.; McDonnell, N.; Mercier, F.J.; Palanisamy, A.; Sia, A.T.H.; Van de Velde, M.; Vercueil, A.; et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018, 73, 71–92. [Google Scholar] [CrossRef]
- Langesaeter, E.; Rosseland, L.A.; Stubhaug, A. Continuous invasive blood pressure and cardiac output monitoring during cesarean delivery: A randomized, double-blind comparison of low-dose versus high-dose spinal anesthesia with intravenous phenylephrine or placebo infusion. Anesthesiology 2008, 109, 856–863. [Google Scholar] [CrossRef]
- George, R.B.; McKeen, D.; Columb, M.O.; Habib, A.S. Up-down determination of the 90% effective dose of phenylephrine for the treatment of spinal anesthesia-induced hypotension in parturients undergoing cesarean delivery. Anesth. Analg. 2010, 110, 154–158. [Google Scholar] [CrossRef]
- Postema, P.G.; Wilde, A.A. The measurement of the QT interval. Curr. Cardiol. Rev. 2014, 10, 287–294. [Google Scholar] [CrossRef]
- Uyarel, H.; Okmen, E.; Cobanoglu, N.; Karabulut, A.; Cam, N. Effects of anxiety on QT dispersion in healthy young men. Acta Cardiol. 2006, 61, 83–87. [Google Scholar] [CrossRef]
- Piccirillo, G.; Viola, E.; Nocco, M.; Santagada, E.; Durante, M.; Bucca, C.; Marigliano, V. Autonomic modulation and QT interval dispersion in hypertensive subjects with anxiety. Hypertension 1999, 34, 242–246. [Google Scholar] [CrossRef]
- Xiao, F.; Shen, B.; Xu, W.P.; Feng, Y.; Ngan Kee, W.D.; Chen, X.Z. Dose-Response Study of 4 Weight-Based Phenylephrine Infusion Regimens for Preventing Hypotension During Cesarean Delivery Under Combined Spinal-Epidural Anesthesia. Anesth. Analg. 2020, 130, 187–193. [Google Scholar] [CrossRef]
- Dahlgren, G.; Granath, F.; Wessel, H.; Irestedt, L. Prediction of hypotension during spinal anesthesia for cesarean section and its relation to the effect of crystalloid or colloid preload. Int. J. Obstet. Anesth. 2007, 16, 128–134. [Google Scholar] [CrossRef]
- Xiao, F.; Wei, C.; Chang, X.; Zhang, Y.; Xue, L.; Shen, H.; Ngan Kee, W.D.; Chen, X. A Prospective, Randomized, Double-Blinded Study of the Effect of Intravenous Ondansetron on the Effective Dose in 50% of Subjects of Prophylactic Phenylephrine Infusions for Preventing Spinal Anesthesia-Induced Hypotension During Cesarean Delivery. Anesth. Analg. 2020, 131, 564–569. [Google Scholar] [CrossRef]

| QTc < 440 ms | QTc ≥ 440 ms | ||
|---|---|---|---|
| Variables | (n = 43) | (n = 42) | p-Value |
| Age (years) | 35 (3.3) | 34 (3.3) | 0.468 |
| Weight (kg) | 69.0 (8.5) | 69.0 (7.9) | 0.995 |
| Height (cm) | 161.6 (5.6) | 161.4 (5.4) | 0.850 |
| Gestational age (weeks) | 38.5 (0.9) | 38.6 (0.7) | 0.704 |
| Diagnosis, n (%) | |||
| Cephalopelvic disproportion | 13 (30.2) | 18 (42.9) | 0.227 |
| Previous cesarean delivery | 15 (34.9) | 12 (28.6) | 0.532 |
| Uterine myoma | 5 (11.6) | 5 (11.9) | 1.000 |
| Breech presentation | 6 (14.0) | 2 (4.8) | 0.265 |
| Others | 8 (18.6) | 8 (19.1) | 0.958 |
| Potassium (mmol/L) | 4.2 (0.3) | 4.2 (0.3) | 0.586 |
| Calcium (mg/dL) | 9.0 (0.4) | 9.1 (0.4) | 0.631 |
| Surgical time (min) | 42.3 (8.3) | 43.5 (7.6) | 0.483 |
| Anesthesia time (min) | 61.6 (7.6) | 62.2 (6.3) | 0.701 |
| CSEA to delivery interval (min) | 17.0 (4.5) | 16.6 (3.7) | 0.615 |
| Apgar score at 1 min | 8 [8, 9] | 8 [8, 9] | 0.132 |
| Apgar score at 5 min | 9 [9] | 9 [9–9) | 0.128 |
| QTc < 440 ms | QTc ≥ 440 ms | ||
|---|---|---|---|
| Variables | (n = 43) | (n = 42) | p-Value |
| Baseline HR (beat/min) | 76.3 (11.5) | 78.0 (9.6) | 0.463 |
| Baseline SBP (mmHg) | 118.1 (15.3) | 114.3 (11.1) | 0.196 |
| Baseline DBP (mmHg) | 74.1 (13.0) | 72.8 (10.3) | 0.602 |
| Baseline MBP (mmHg) | 87.3 (12.8) | 85.2 (10.0) | 0.390 |
| Baseline QT interval (ms) | 378.6 (25.2) | 401.3 (24.4) | <0.001 |
| Sensory block level, n (%) | 1.000 | ||
| T4 | 41 (95.4) | 41 (97.6) | |
| T6 | 2 (4.7) | 1 (2.4) |
| 440 ms Cut-Off | 450 ms Cut-Off | 460 ms Cut-Off | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | |
| Study subjects | ||||||
| Sensitivity | 0.685 (0.561–0.809) | 0.007 | 0.759 (0.645–0.873) | <0.001 | 0.852 (0.757–0.947) | <0.001 |
| Specificity | 0.807 (0.667–0.946) | <0.001 | 0.710 (0.550–0.870) | 0.020 | 0.452 (0.276–0.627) | 0.590 |
| PPV | 0.861 (0.757–0.964) | 0.820 (0.714–0.927) | 0.730 (0.621–0.840) | |||
| NPV | 0.595 (0.447–0.744) | 0.629 (0.469–0.789) | 0.636 (0.435–0.837) | |||
| External validation | ||||||
| Sensitivity | 0.684 (0.564–0.805) | 0.005 | 0.772 (0.663–0.881) | <0.001 | 0.930 (0.864–0.996) | <0.001 |
| Specificity | 0.720 (0.544–0.896) | 0.028 | 0.400 (0.208–0.592) | 0.317 | 0.280 (0.104–0.456) | 0.028 |
| PPV | 0.848 (0.744–0.952) | 0.746 (0.635–0.857) | 0.747 (0.645–0.848) | |||
| NPV | 0.500 (0.337–0.663) | 0.435 (0.232–0.637) | 0.636 (0.352–0.921) | |||
| Univariate Regression | Multivariate Stepwise Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Baseline QTc | 0.964 | 0.944–0.984 | <0.001 | 0.961 | 0.940–0.983 | <0.001 |
| Age | 1.188 | 1.028–1.374 | 0.020 | 1.219 | 1.041–1.427 | 0.014 |
| Weight | 0.995 | 0.942–1.051 | 0.865 | |||
| Height | 0.968 | 0.892–1.049 | 0.423 | |||
| Gestational age | 0.696 | 0.385–1.261 | 0.232 | |||
| Calcium | 0.479 | 0.095–2.411 | 0.372 | |||
| Potassium | 0.491 | 0.145–1.666 | 0.254 | |||
| Baseline SBP | 0.991 | 0.959–1.024 | 0.578 | |||
| Baseline HR | 0.959 | 0.917–1.002 | 0.062 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Jang, D.-M.; Park, J.Y.; Koh, W.U.; Choi, W.-J. Prolonged Corrected QT Interval Is Associated with Lower Incidence of Maternal Hypotension During Spinal Anesthesia in Cesarean Delivery: A Prospective Observational Study. Medicina 2025, 61, 1925. https://doi.org/10.3390/medicina61111925
Park H-S, Jang D-M, Park JY, Koh WU, Choi W-J. Prolonged Corrected QT Interval Is Associated with Lower Incidence of Maternal Hypotension During Spinal Anesthesia in Cesarean Delivery: A Prospective Observational Study. Medicina. 2025; 61(11):1925. https://doi.org/10.3390/medicina61111925
Chicago/Turabian StylePark, Hee-Sun, Dong-Min Jang, Jong Yeon Park, Won Uk Koh, and Woo-Jong Choi. 2025. "Prolonged Corrected QT Interval Is Associated with Lower Incidence of Maternal Hypotension During Spinal Anesthesia in Cesarean Delivery: A Prospective Observational Study" Medicina 61, no. 11: 1925. https://doi.org/10.3390/medicina61111925
APA StylePark, H.-S., Jang, D.-M., Park, J. Y., Koh, W. U., & Choi, W.-J. (2025). Prolonged Corrected QT Interval Is Associated with Lower Incidence of Maternal Hypotension During Spinal Anesthesia in Cesarean Delivery: A Prospective Observational Study. Medicina, 61(11), 1925. https://doi.org/10.3390/medicina61111925






