Comparison of Cryoballoon Ablation Methods in Pulmonary Vein Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Data Analysis
2.2. Procedure Description
3. Results
Procedural Data Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC), with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial Fibrillation: Epidemiology, Screening and Digital Health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Hachem, A.H.; Marine, J.E.; Tahboub, H.A.; Kamdar, S.; Kanjwal, S.; Soni, R.; Kanjwal, K. Radiofrequency Ablation versus Cryoablation in the Treatment of Paroxysmal Atrial Fibrillation: A Meta-Analysis. Cardiol. Res. Pract. 2018, 2018, 6276241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, J.; Han, Y.; Han, S.; Li, P.; Liao, H.; Guo, J. Global Burden of Atrial Fibrillation/Atrial Flutter and Its Attributable Risk Factors from 1990 to 2021. EP Eur. 2024, 26, euae195. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kuniss, M.; Pavlovic, N.; Velagic, V.; Hermida, J.S.; Healey, S.; Arena, G.; Badenco, N.; Meyer, C.; Chen, J.; Iacopino, S.; et al. Cryoballoon Ablation vs. Antiarrhythmic Drugs: First-Line Therapy for Patients with Paroxysmal Atrial Fibrillation. EP Eur. 2021, 23, 1033–1041. [Google Scholar] [CrossRef]
- Gupta, D.; Ding, W.Y.; Calvert, P.; Williams, E.; Das, M.; Tovmassian, L.; Tayebjee, M.H.; Haywood, G.; Martin, C.A.; Rajappan, K.; et al. Cryoballoon Pulmonary Vein Isolation as First-Line Treatment for Typical Atrial Flutter. Heart 2023, 109, 364–371. [Google Scholar] [CrossRef]
- Andrade, J.G.; Dubuc, M.; Guerra, P.G.; Macle, L.; Rivard, L.; Roy, D.; Talajic, M.; Thibault, B.; Khairy, P. Cryoballoon Ablation for Atrial Fibrillation. Indian Pacing Electrophysiol. J. 2012, 12, 39–53. [Google Scholar] [CrossRef]
- Ozcan, C.; Ruskin, J.; Mansour, M. Cryoballoon Catheter Ablation in Atrial Fibrillation. Cardiol. Res. Pract. 2011, 2011, 256347. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Yacoub, M.; Sheppard, R.C. Cryoballoon Pulmonary Vein Catheter Ablation of Atrial Fibrillation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534804/ (accessed on 20 September 2024).
- Martin, C.A.; Tilz, R.R.R.; Anic, A.; Defaye, P.; Luik, A.; de Asmundis, C.; Champ-Rigot, L.; Iacopino, S.; Sommer, P.; Albrecht, E.M.; et al. Acute Procedural Efficacy and Safety of a Novel Cryoballoon for the Treatment of Paroxysmal Atrial Fibrillation: Results from the POLAR ICE Study. J. Cardiovasc. Electrophysiol. 2023, 34, 833–840. [Google Scholar] [CrossRef]
- Boston Scientific. Frozen-AF US IDE Clinical Trial Results. 2023. Available online: https://www.bostonscientific.com/content/dam/bostonscientific/ep/Electrophysiology%20Portfolio/polarx/polarx-product-page/EP-1576508-AA%20PX%20FROzEN-AF%20US%20IDE%20Clinical%20Trial%20Results%20Data%20Sheet_Final%20(1).pdf (accessed on 6 February 2025).
- Ellenbogen, K.A.; Mittal, S.; Varma, N.; Aryana, A.; Marrouche, N.; Anić, A.; Nair, D.; Champagne, J.; Iacopino, S.; de Asmundis, C.; et al. One-Year Outcomes of Pulmonary Vein Isolation with a Novel Cryoballoon: Primary Results of the FROZEN AF Trial. J. Cardiovasc. Electrophysiol. 2024, 35, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Tomaiko-Clark, E.; Bai, R.; Khokhar, M.; Su, W.W. A Tale of Two Balloons: Technical and Procedural Difference between Cryoballoon Systems. Curr. Opin. Cardiol. 2022, 37, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hamada, K.; Iwasaki, K.; Takada, J.; Murakami, M.; Saito, S. Difference in Tissue Temperature Change between Two Cryoballoons. Open Heart 2023, 10, e002426. [Google Scholar] [CrossRef]
- Creta, A.; Kanthasamy, V.; Schilling, R.J.; Rosengarten, J.; Khan, F.; Honarbakhsh, S.; Earley, M.J.; Hunter, R.J.; Finlay, M. First Experience of POLARxTM versus Arctic Front AdvanceTM: An Early Technology Comparison. J. Cardiovasc. Electrophysiol. 2021, 32, 925–930. [Google Scholar] [CrossRef]
- Su, W.; Aryana, A.; Passman, R.; Singh, G.; Hokanson, R.; Kowalski, M.; Andrade, J.; Wang, P. Cryoballoon Best Practices II: Practical Guide to Procedural Monitoring and Dosing during Atrial Fibrillation Ablation from the Perspective of Experienced Users. Heart Rhythm 2018, 15, 1348–1355. [Google Scholar] [CrossRef]
- Aryana, A.; Mugnai, G.; Singh, S.M.; Pujara, D.K.; de Asmundis, C.; Singh, S.K.; Bowers, M.R.; Brugada, P.; d’Avila, A.; O’Neill, P.G.; et al. Procedural and Biophysical Indicators of Durable Pulmonary Vein Isolation during Cryoballoon Ablation of Atrial Fibrillation. Heart Rhythm 2016, 13, 424–432. [Google Scholar] [CrossRef]
- Tilz, R.R.; Meyer-Saraei, R.; Eitel, C.; Fink, T.; Sciacca, V.; Lopez, L.D.; Kirstein, B.; Schlüter, M.; Vogler, J.; Kuck, K.-H.; et al. Novel Cryoballoon Ablation System for Single Shot Pulmonary Vein Isolation―The Prospective ICE-AGE-X Study―. Circ. J. 2021, 85, 1296–1304. [Google Scholar] [CrossRef]
- Rodríguez Muñoz, D.; Marco del Castillo, Á.; Rajjoub Al-Mahdi, E.A.; Lázaro Rivera, C.; Guisasola Cienfuegos, M.; Ramos Jiménez, J.; Borrego Bernabé, L.; Arribas Ynsaurriaga, F.; Salguero-Bodes, R. Systematic Workflow and Electrogram Guidance to Reduce X-Ray Exposure Time during Cryoballoon Ablation of Atrial Fibrillation: The SWEET-Cryo Strategy. EP Eur. 2023, 25, euad231. [Google Scholar] [CrossRef]
- Assaf, A.; Bhagwandien, R.E.; Szili-Torok, T.; Yap, S.-C. Comparison of the Acute Outcome of Two Cryoballoon Technologies for Pulmonary Vein Isolation: An Updated Systematic Review and Meta-Analysis. IJC Heart Vasc. 2022, 42, 101115. [Google Scholar] [CrossRef] [PubMed]
- Kochi, A.N.; Moltrasio, M.; Tundo, F.; Riva, S.; Ascione, C.; Dessanai, M.A.; Pizzamiglio, F.; Vettor, G.; Cellucci, S.; Gasperetti, A.; et al. Cryoballoon Atrial Fibrillation Ablation: Single-Center Safety and Efficacy Data Using a Novel Cryoballoon Technology Compared to a Historical Balloon Platform. J. Cardiovasc. Electrophysiol. 2021, 32, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.; Anic, A.; Breskovic, T.; Haas, A.; Bhagwandien, R.E.; Jurisic, Z.; Szili-Torok, T.; Luik, A. Comparison of Procedural Efficacy and Biophysical Parameters between Two Competing Cryoballoon Technologies for Pulmonary Vein Isolation: Insights from an Initial Multicenter Experience. J. Cardiovasc. Electrophysiol. 2021, 32, 580–587. [Google Scholar] [CrossRef]
- Reichlin, T.; Kueffer, T.; Knecht, S.; Madaffari, A.; Badertscher, P.; Maurhofer, J.; Krisai, P.; Jufer, C.; Asatryan, B.; Heg, D.; et al. PolarX vs Arctic Front for Cryoballoon Ablation of Paroxysmal AF. JACC Clin. Electrophysiol. 2024, 10, 1367–1376. [Google Scholar] [CrossRef]
- Knappe, V.; Lahrmann, C.; Funken, M.; Zietzer, A.; Gestrich, C.; Nickenig, G.; Schrickel, J.W.; Beiert, T. Comparison of Arctic Front Advance Pro and POLARx Cryoballoons for Ablation Therapy of Atrial Fibrillation: An Intraprocedural Analysis. Clin. Res. Cardiol. 2025, 114, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Lucas, P.; Isgandarova, K.; El Hamriti, M.; Bergau, L.; Fink, T.; Sciacca, V.; Imnadze, G.; Braun, M.; Khalaph, M.; et al. News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2022, 9, 16. [Google Scholar] [CrossRef]
- Moser, F.; Rottner, L.; Moser, J.; Schleberger, R.; Lemoine, M.; Münkler, P.; Dinshaw, L.; Kirchhof, P.; Reissmann, B.; Ouyang, F.; et al. The Established and the Challenger: A Direct Comparison of Current Cryoballoon Technologies for Pulmonary Vein Isolation. J. Cardiovasc. Electrophysiol. 2022, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cordes, F.; Ellermann, C.; Dechering, D.G.; Frommeyer, G.; Kochhäuser, S.; Lange, P.S.; Pott, C.; Lenze, F.; Schmidt, H.; Ullerich, H.; et al. Time-to-Isolation-Guided Cryoballoon Ablation Reduces Oesophageal and Mediastinal Alterations Detected by Endoscopic Ultrasound: Results of the MADE-PVI Trial. EP Eur. 2019, 21, 1325–1333. [Google Scholar] [CrossRef]
- Vogt, J.; Heintze, J.; Gutleben, K.J.; Muntean, B.; Horstkotte, D.; Nölker, G. Long-Term Outcomes After Cryoballoon Pulmonary Vein Isolation: Results from a Prospective Study in 605 Patients. J. Am. Coll. Cardiol. 2013, 61, 1707–1712. [Google Scholar] [CrossRef]
| POLARx | Arctic Front | p-Value | |
|---|---|---|---|
| Age | 62.3 ± 10.1 | 61.4 ± 10.2 | 0.254 |
| Male gender | 42 (39.3) | 35 (37.6) | |
| BMI, kg/m2 | 29.94 ± 5.29 | 31.58 ± 5.82 | 0.041 |
| LAVI, mL/m2 | 35.9 ± 8.1 | 35.5 ± 8.8 | 0.676 |
| CHA2DS2VASC | 1.9 ± 1.7 | 1.7 ± 1.6 | 0.881 |
| 0 | 19 | 27 | |
| 1 | 18 | 22 | |
| 2 | 26 | 19 | |
| 3 | 16 | 13 | |
| 4 | 1 | 8 | |
| 5 | 4 | 2 | |
| 6 | 1 | 0 | |
| 7 | 2 | 2 | |
| Use of AADs | 85 (79.4) | 71 (76.3) | |
| EHRA | 2.4 ± 0.7 | 2.5 ± 0.7 | 0.546 |
| POLARx | Arctic Front | p Value | |
|---|---|---|---|
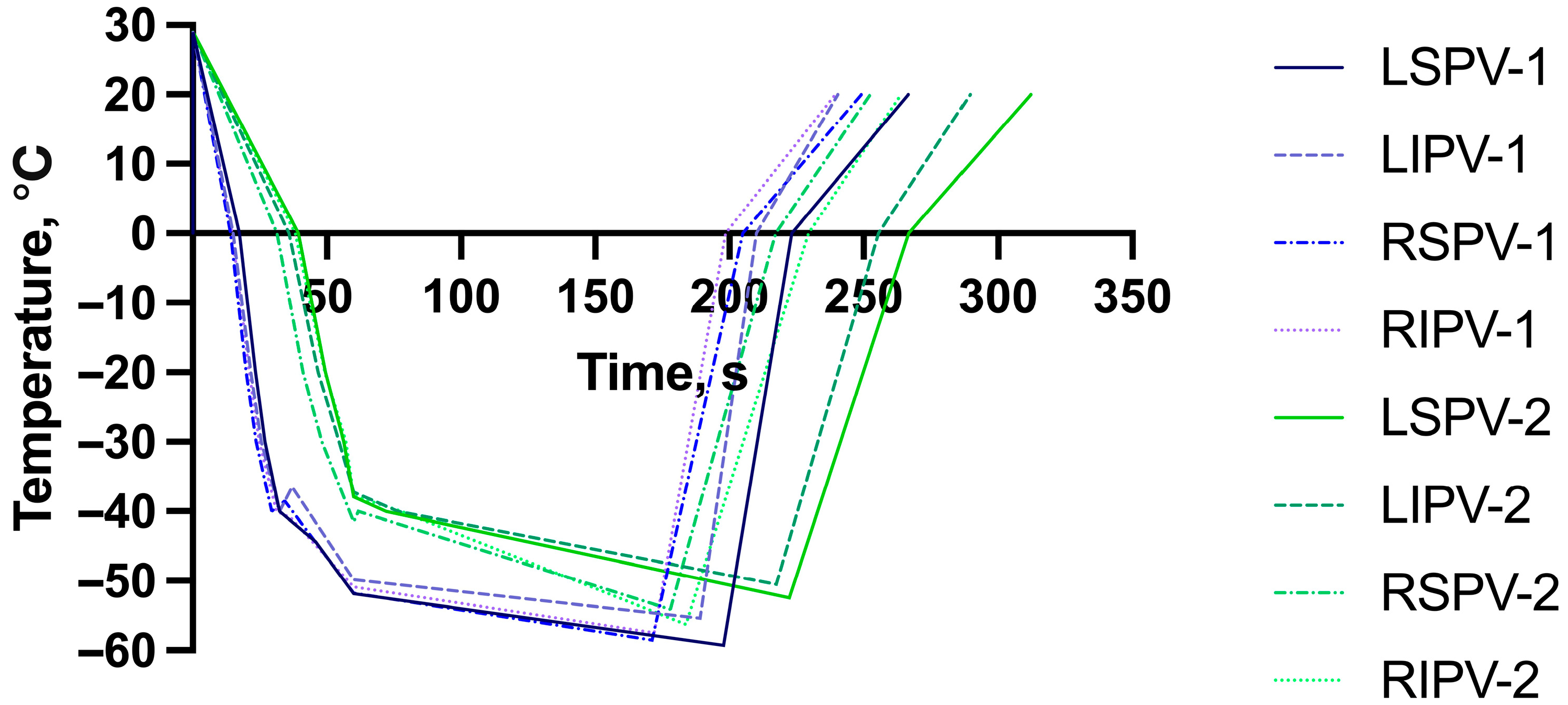
| LSPV FT, s | 197.8 ± 37.7 | 222.3 ± 32.6 | <0.001 |
| LSPV 0 °C, s | 17.3 ± 1.7 | 39.5 ± 15.1 | <0.001 |
| LSPV −40 °C, s | 32.5 ± 3.8 | 72.1 ± 24.5 | <0.001 |
| LSPV temp at 60 s | −51.9 ± 4.6 | −37.9 ± 15.5 | <0.001 |
| LSPV isolation time, s | 45.8 ± 22.1 | 48.7 ± 39.4 | 0.578 |
| LSPV isolation temperature, °C | −44.4 ± 9.8 | −37.3 ± 16.3 | 0.002 |
| LSPV minimal temperature, °C | −59.3 ± 5.8 | −52.5 ± 19.1 | <0.001 |
| LSPV RT to 0 °C, s | 223.3 ± 38.5 | 269.9 ± 39.2 | <0.001 |
| LIPV FT, s | 189.2 ± 34.4 | 217.5 ± 38.0 | <0.001 |
| LIPV 0 °C, s | 15.1 ± 1.9 | 35.8 ± 14.0 | <0.001 |
| LIPV −40 °C, s | 32.3 ± 4.4 | 76.5 ± 29.5 | <0.001 |
| LIPV temp at 60 s | −49.9 ± 4.4 | −37.3 ± 12.3 | <0.001 |
| LIPV isolation time, s | 37.0 ± 25.0 | 41.8 ± 26.7 | 0.286 |
| LIPV isolation temperature, °C | −36.5 ± 13.3 | −33.7 ± 11.7 | 0.328 |
| LIPV minimal temperature, °C | −55.4 ± 5.7 | −50.5 ± 20.8 | 0.034 |
| LIPV RT to 0 °C, s | 210.0 ± 36.5 | 255.6 ± 42.9 | <0.001 |
| RSPV FT, s | 171.2 ± 34.3 | 178.0 ± 20.5 | 0.102 |
| RSPV 0 °C, s | 14.0 ± 1.5 | 31.5 ± 11.2 | <0.001 |
| RSPV −40 °C, s | 29.5 ± 5.4 | 61.7 ± 19.5 | <0.001 |
| RSPV temp at 60 s | −51.8 ± 11.4 | −41.6 ± 12.6 | <0.001 |
| RSPV isolation time, s | 34.3 ± 18.9 | 31.0 ± 17.7 | 0.290 |
| RSPV isolation temperature, °C | −38.5 ± 11.0 | −31.1 ± 12.0 | <0.001 |
| RSPV minimal temperature, °C | −58.6 ± 13.4 | −54.2 ± 14.4 | 0.028 |
| RSPV RT to 0 °C, s | 205.1 ± 23.7 | 217.3 ± 28.7 | 0.002 |
| RIPV FT, s | 172.1 ± 30.8 | 183.8 ± 33.4 | 0.011 |
| RIPV 0 °C, s | 14.6 ± 1.4 | 38.1 ± 19.0 | <0.001 |
| RIPV −40 °C, s | 31.3 ± 4.2 | 77.7 ± 29.4 | <0.001 |
| RIPV temp at 60 s | −50.9 ± 5.0 | −37.8 ± 12.1 | <0.001 |
| RIPV isolation time, s | 36.6 ± 14.2 | 47.2 ± 30.0 | 0.008 |
| RIPV isolation temperature, °C | −40.9 ± 9.9 | −37.7 ± 10.8 | 0.075 |
| RIPV minimal temperature, °C | −57.5 ± 6.6 | −56.3 ± 42.7 | 0.768 |
| RIPV RT to 0 °C, s | 198.7 ± 22.7 | 229.6 ± 33.8 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupics, K.; Bricis, R.; Jubele, K.; Ansaberga, I.; Kalējs, O.; Erglis, A. Comparison of Cryoballoon Ablation Methods in Pulmonary Vein Isolation. Medicina 2025, 61, 1920. https://doi.org/10.3390/medicina61111920
Kupics K, Bricis R, Jubele K, Ansaberga I, Kalējs O, Erglis A. Comparison of Cryoballoon Ablation Methods in Pulmonary Vein Isolation. Medicina. 2025; 61(11):1920. https://doi.org/10.3390/medicina61111920
Chicago/Turabian StyleKupics, Kaspars, Raivis Bricis, Kristine Jubele, Ieva Ansaberga, Oskars Kalējs, and Andrejs Erglis. 2025. "Comparison of Cryoballoon Ablation Methods in Pulmonary Vein Isolation" Medicina 61, no. 11: 1920. https://doi.org/10.3390/medicina61111920
APA StyleKupics, K., Bricis, R., Jubele, K., Ansaberga, I., Kalējs, O., & Erglis, A. (2025). Comparison of Cryoballoon Ablation Methods in Pulmonary Vein Isolation. Medicina, 61(11), 1920. https://doi.org/10.3390/medicina61111920






