The Impact of Postoperative Intravenous Iron Therapy on Clinical Outcomes in Surgical Patients with Iron-Deficiency Anemia: A Comparative Analysis by Frailty Status in the Setting of Elective Cardiac Surgery
Abstract
1. Introduction
2. Methods
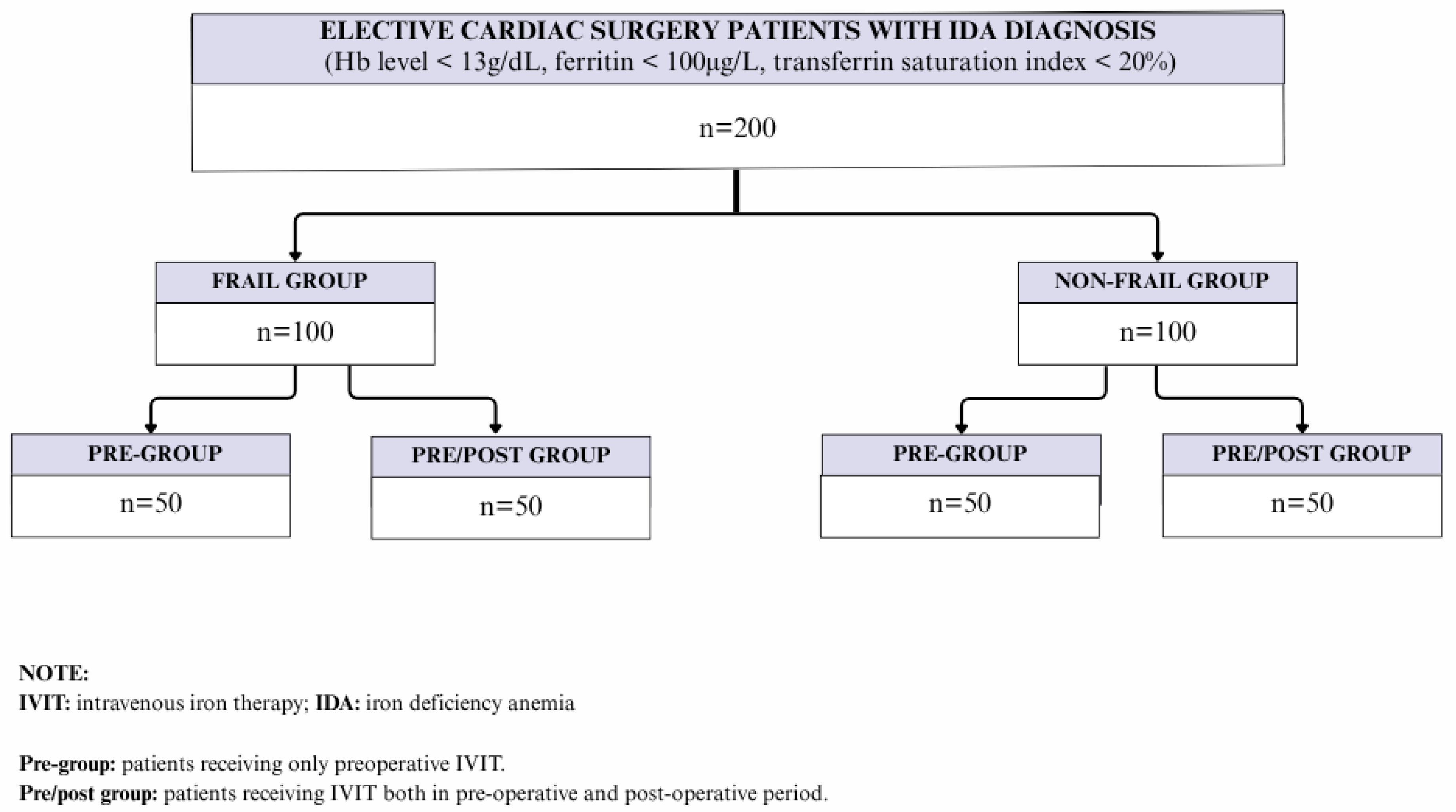
2.1. Study Population
2.2. Assessments
2.3. Frailty Diagnosis
2.4. IDA Diagnosis and IVIT Protocol
2.5. Statistical Analysis
3. Results
Patient Demographics, Surgical Intervention and Postoperative Outcome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venturini, E.; Iannuzzo, G.; Di Lorenzo, A.; Cuomo, G.; D’Angelo, A.; Merone, P.; Cudemo, G.; Pacileo, M.; D’Andrea, A.; Vigorito, C.; et al. Short-term treatment of iron deficiency anemia after cardiac surgery. Int. J. Cardiol. Heart Vasc. 2022, 40, 101038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramezani, F.; Khalaf-Adeli, E.; Mehr, A.Z. Prevalence of Iron Deficiency Anemia in Patients Scheduled for Cardiac Surgery: A Survey at a Cardiovascular Medical and Research Center in Iran. Iran. Heart J. 2024, 25, 21–26. [Google Scholar]
- Kim, H.H.; Park, E.H.; Lee, S.H.; Yoo, K.J.; Youn, Y.N. Effect of Preoperative Administration of Intravenous Ferric Carboxymaltose in Patients with Iron Deficiency Anemia after Off-Pump Coronary Artery Bypass Grafting: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 1737. [Google Scholar] [CrossRef]
- Rossler, J.; Schoenrath, F.; Seifert, B.; Kaserer, A.; Spahn, G.H.; Falk, V.; Spahn, D.R. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: A prospective study. Br. J. Anaesth. 2020, 124, 25–34. [Google Scholar] [CrossRef]
- Bartoszko, J.; Miles, S.; Ansari, S.; Grewal, D.; Li, M.; Callum, J.; McCluskey, S.A.; Lin, Y.; Karkouti, K. Postoperative intravenous iron to treat iron-deficiency anaemia in patients undergoing cardiac surgery: A protocol for a pilot, multicentre, placebo-controlled randomized trial (the POAM trial). BJA Open 2024, 11, 100303. [Google Scholar] [CrossRef]
- Graham, F.J.; Masini, G.; Lakhal-Littleton, S.; Clark, A.L.; Cleland, J.G.F.; Pellicori, P. Iron Deficiency in Cardiovascular Disease. Circ. J. 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramirez, S.; Jericó, C.; Muñoz, M. Perioperative anemia: Prevalence, consequences and pathophysiology. Transfus. Apher. Sci. 2019, 58, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Smoor, R.M.; Rettig, T.C.D.; Vernooij, L.M.; Groenewegen, E.M.; van Dongen, H.P.A.; Noordzij, P.G. The effect of postoperative intravenous iron in anaemic, older cardiac surgery patients on disability-free survival (AGE ANEMIA study): Study protocol for a multi-centre, double-blind, randomized, placebo-controlled trial. Trials 2023, 24, 693. [Google Scholar] [CrossRef]
- Mandal, S.; Smith, D.L.; Peter, P.J.; Louv, V.J.; Sil, S.; Ibrahim, I.N.; Maji, M.; Nath, S. Perioperative anaemia management. Ann. Blood 2023, 8, 30. [Google Scholar] [CrossRef]
- Cespedes, I.C.; Figueiredo, M.S.; Santos, A.A.; Hossne, N.A., Jr. Patient Blood Management in Cardiovascular Surgery. Braz. J. Cardiovasc. Surg. 2024, 39, e20240994. [Google Scholar] [CrossRef]
- Şanal, L.; Günaydın, S.; Tatar, M. Cost-Effectiveness and Budget Impact Analyses of Patient Blood Management in a Cardiovascular Surgery Department at Ankara Bilkent City Hospital in Turkey. Adv. Ther. 2024, 41, 716–729. [Google Scholar]
- Tatar, M.; Alkis, N.; Yildirim Guclu, C.; Bermede, O.; Erdemli, B.; Gunaydin, S. Cost-effectiveness and budget impact of comprehensive anemia management, the first pillar of patient blood management, on the Turkish healthcare system. Clin. Outcomes Res. 2022, 14, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, S.; Najafizadeh, H.; Teimouri, H. A Restrictive Blood Transfusion Strategy and Patient Blood Management Improved Clinical Outcomes in Cardiac Surgery Patients. J. Arch. Mil. Med. 2021, 9, e114661. [Google Scholar] [CrossRef]
- Gunaydin, S.; Spahn, D.R.; Ozisik, K.; Demir, A.; Aşkın, G.; Sert, D.E.; Bozkurt, H.; Şampiyon, A.; Kazancı, D.; Kılıçlı, A.B.; et al. Building a patient blood management program in a large-volume tertiary hospital setting: Problems and solutions. Turk. J. Thorac. Cardiovasc. Surg. 2020, 28, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Budak, A.B.; McCusker, K.; Gunaydin, S. A cardiopulmonary bypass based blood management strategy in adult cardiac surgery. Heart Surg. Forum 2017, 20, E195–E198. [Google Scholar]
- Munoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gomez-Ramirez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
- Hare, G.M.T.; Mazer, C.D. Anemia: Perioperative risk and treatment opportunity. Anesthesiology 2021, 135, 520–530. [Google Scholar] [CrossRef]
- Richards, T.; Baikady, R.R.; Clevenger, B.; Butcher, A.; Abeysiri, S.; Chau, M.; Macdougall, I.C.; Murphy, G.; Swinson, R.; Collier, T.; et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): A randomised, double-blind, controlled trial. Lancet 2020, 396, 1353–1361. [Google Scholar] [CrossRef]
- Elhenawy, A.M.; Meyer, S.R.; Bagshaw, S.M.; MacArthur, R.G.; Carroll, L.J. Role of preoperative intravenous iron therapy to correct anemia before major surgery: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 36. [Google Scholar] [CrossRef]
- Spahn, D.R.; Schoenrath, F.; Spahn, G.H.; Seifert, B.; Stein, P.; Theusinger, O.M.; Kaserer, A.; Hegemann, I.; Hofmann, A.; Maisano, F.; et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: A prospective randomised trial. Lancet 2019, 393, 2201–2212. [Google Scholar] [CrossRef]
- Tankard, K.A.; Park, B.; Brovman, E.Y.; Bader, A.M.; Urman, R.D. The impact of preoperative intravenous iron therapy on perioperative outcomes in cardiac surgery: A systematic review. J. Hematol. 2020, 9, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Hutchinson, N.; Hill, A.; Ingoldby, F.; Skipper, N.; Jones, C.; Bremner, S.; Bruce, C.; Wright, J.; Lewis, M.; et al. Randomised open-label trial comparing intravenous iron and an erythropoiesis-stimulating agent versus oral iron to treat preoperative anaemia in cardiac surgery (INITIATE trial). Br. J. Anaesth. 2022, 128, 796–805. [Google Scholar] [PubMed]
- Gupta, S.; Panchal, P.; Gilotra, K.; Wilfred, A.M.; Hou, W.; Siegal, D.; Whitlock, R.P.; Belley-Cote, E.P. Intravenous iron therapy for patients with preoperative iron deficiency or anaemia undergoing cardiac surgery reduces blood transfusions: A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Van Remoortel, H.; Laermans, J.; Bekkering, G.; Fergusson, D.; Georgsen, J.; Manzini, P.M.; Ozier, Y.; De Buck, E.; Compernolle, V.; et al. Lack of cost-effectiveness of preoperative erythropoiesis-stimulating agents and/or iron therapy in anaemic, elective surgery patients: A systematic review and updated analysis. Pharmacoeconomics 2021, 39, 1123–1139. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.; Wei, Q.; Zhang, Y.; Ouyang, X.; Wang, S. Intravenous iron and erythropoietin therapy for postoperative anemia among orthopedic surgery patients. J. Orthop. Surg. Res. 2023, 18, 510. [Google Scholar] [CrossRef]
- Qin, X.; Liu, H.; Tao, X.; Zhou, Z.; Mei, G.; Zhang, M.; Zou, S. Risk prediction model of frailty and its associated factors in older adults: A cross-sectional study in Anhui Province, China. Front. Nutr. 2025, 12, 1611914. [Google Scholar] [CrossRef]
- Jiang, H.; Wong, J.J.; Tan, R.S.; Gao, F.; Teo, L.L.; Strom, J.B.; Lang, C.C.; Koh, A.S. Effect of Frailty on Cardiovascular Clinical Trials: A Systematic Review and Meta-Analysis. JACC Adv. 2025, 4, 101889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, D.T.T.; Tu, J.V.; Dupuis, J.Y.; Bader Eddeen, A.; Sun, L.Y. Association of Frailty and Long-Term Survival in Patients Undergoing Coronary Artery Bypass Grafting. J. Am. Heart Assoc. 2018, 7, e009882. [Google Scholar] [CrossRef]
- Yanagawa, B.; Graham, M.M.; Afilalo, J.; Hassan, A.; Arora, R.C. Frailty as a risk predictor in cardiac surgery: Beyond the eyeball test. J. Thorac. Cardiovasc. Surg. 2019, 157, 1905–1909. [Google Scholar] [CrossRef]
- Lee, D.H.; Buth, K.J.; Martin, B.J.; Yip, A.M.; Hirsch, G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010, 121, 973–978. [Google Scholar] [CrossRef]
- Wleklik, M.; Czapla, M.; Denfeld, Q.; Przybylski, R.; Reczuch, K.; Uchmanowicz, I. The how and why of assessing frailty syndrome in cardiac surgery. Adv. Clin. Exp. Med. 2022, 31, 1061–1064. [Google Scholar] [CrossRef]
- Fehlmann, C.A.; Bezzina, K.; Mazzola, R.; Visintini, S.M.; Guo, M.H.; Rubens, F.D.; Wells, G.A.; McGuinty, C.; Huang, A.; Khoury, L.; et al. Influence of preoperative frailty on quality of life after cardiac surgery: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2023, 71, 3278–3286. [Google Scholar] [CrossRef]
- Wishahi, M.; Kamal, N.M.; Hedaya, M.S. Enhanced recovery after surgery: Progress in adapted pathways for implementation in standard and emerging surgical settings. World J. Clin. Cases 2024, 12, 5636–5641. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, P.I.; Afferi, L.; Antonelli, A.; Cerruto, M.A.; Mordasini, L.; Mattei, A.; Baumeister, P.; Marra, G.; Krajewski, W.; Mari, A.; et al. Frailty impact on postoperative complications and early mortality rates in patients undergoing radical cystectomy for bladder cancer: A systematic review. Arab. J. Urol. 2020, 19, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Babaroğlu, S.; Aksöyek, A.; Demirbağ, A.E.; Günaydın, İ. Which frailty score in cardiac surgery patients? Turk. J. Thorac. Cardiovasc. Surg. 2025, 33, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Artz, A.S. Anemia and the frail elderly. Semin. Hematol. 2008, 45, 261–266. [Google Scholar] [CrossRef]
- Munoz, M.; Acheson, A.G.; Auerbach, M.; Besser, M.; Habler, O.; Kehlet, H.; Liumbruno, G.M.; Lasocki, S.; Meybohm, P.; Baikady, R.R.; et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017, 72, 233–247. [Google Scholar]
- Herpich, C.; Göger, L.; Faust, L.; Kalymon, M.; Ott, C.; Walter, S.; Lehmkuhl, E.; Grune, T.; Moskiou, V.; Müller-Werdan, U.; et al. Disentangling Anemia in Frailty: Exploring the Role of Inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae243. [Google Scholar] [CrossRef]
- Qaseem, A.; Humphrey, L.L.; Fitterman, N.; Starkey, M.; Shekelle, P.; Clinical Guidelines Committee of the American College of Physicians. Treatment of anemia in patients with heart disease: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2013, 159, 770–779. [Google Scholar] [CrossRef]
- Marchitto, N.; Curcio, A.; Iannarelli, N.; Petrucci, A.; Romano, A.; Pironti, M.; Paparello, P.T.; Raimondi, G. A pilot study on secondary anemia in “frailty” patients treated with Ferric Sodium EDTA in combination with vitamin C, folic acid, copper gluconate, zinc gluconate and selenomethionine: Safety of treatment explored by HRV non-linear analysis as predictive factor of cardiovascular tolerability. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7776–7783. [Google Scholar]
- Patel, N.; Evans, C.R. Pre-operative Intravenous Iron to Optimise Patients Before Cardiac Surgery. J. Clin. Haematol. 2021, 2, 24–35. [Google Scholar]
- Howell, K.; Garvan, C.; Amini, S.; Kamyszek, R.W.; Tighe, P.; Price, C.C.; Spiess, B.D.; PeCAN Program Study Group. Association Between Preoperative Anemia and Cognitive Function in a Large Cohort Study of Older Patients Undergoing Elective Surgery. Anesth. Analg. 2025, 140, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.V.; Zierentz, P.; Hof, L.; Kloka, J.A.; Messroghli, L.; Zacharowski, K.; Meybohm, P.; Choorapoikayil, S. The impact of intravenous iron supplementation in elderly patients undergoing major surgery. BMC Geriatr. 2022, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Froessler, B.; Palm, P.; Weber, I.; Hodyl, N.A.; Singh, R.; Murphy, E.M. The Important Role for Intravenous Iron in Perioperative Patient Blood Management in Major Abdominal Surgery: A Randomized Controlled Trial. Ann. Surg. 2016, 264, 41–46. [Google Scholar] [CrossRef]
- Meybohm, P.; Goehring, M.H.; Choorapoikayil, S.; Fischer, D.; Rey, J.; Herrmann, E.; Mueller, M.; Geisen, C.; Schmitz-Rixen, T.; Zacharowski, K. Feasibility and efficiency of a preoperative anaemia walk-in clinic: Secondary data from a prospective observational trial. Br. J. Anaesth. 2017, 118, 625–626. [Google Scholar] [CrossRef]
- Triphaus, C.; Judd, L.; Glaser, P.; Goehring, M.H.; Schmitt, E.; Westphal, S.; Füllenbach, C.; Lindau, S.; Zacharowski, K.; Meybohm, P.; et al. Effectiveness of Preoperative Iron Supplementation in Major Surgical Patients with Iron Deficiency: A Prospective Observational Study. Ann. Surg. 2021, 274, e212–e219. [Google Scholar] [CrossRef]
- Friedman, T.; Dann, E.J.; Bitton-Worms, K.; Makhoul, M.; Glam, R.; Weis, A.; Tam, D.Y.; Bolotin, G. Intravenous iron administration before cardiac surgery reduces red blood cell transfusion in patients without anaemia. Br. J. Anaesth. 2023, 131, 981–988. [Google Scholar] [CrossRef]
- Evans, C.R.; Jones, R.; Phillips, G.; Greene, G.; Phillips, M.; Morris-Clarke, R. Observational study of pre-operative intravenous iron given to anaemic patients before elective cardiac surgery. Anaesthesia 2021, 76, 639–646. [Google Scholar]
- Saricaoglu, M.C.; Bermede, O. Impact of intravenous iron supplementation before coronary artery bypass grafting. JARSS 2023, 31, 357–362. [Google Scholar]
- Colson, P.H.; Gaudard, P.; Meunier, C.; Seguret, F. Impact of Red Blood Cell Transfusion on In-hospital Mortality of Isolated Coronary Artery Bypass Graft Surgery: A Retrospective Observational Study of French Nationwide 3-year Cohort. Ann. Surg. 2023, 278, e184–e189. [Google Scholar] [CrossRef]
- Madi-Jebara, S.N.; Sleilaty, G.S.; Achouh, P.E.; Yazigi, A.G.; Haddad, F.A.; Hayek, G.M.; Antakly, M.C.; Jebara, V.A. Postoperative intravenous iron used alone or in combination with low- dose erythropoietin is not effective for correction of anemia after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 59–63. [Google Scholar] [CrossRef]
| Frail Pre/Post (n = 50) | Non-Frail Pre/Post (n = 50) | Frail Pre (n = 50) | Non-Frail Pre (n = 50) | p Value | |
|---|---|---|---|---|---|
| Patient demographics | |||||
| Age (year), mean ± SD | 77.0 ± 8.0 | 71.4 ± 7.0 | 75.1 ± 6.0 | 68.2 ± 6.0 | >0.05 |
| Gender, n (%) | |||||
| Female | 36 (72.0) | 31 (62.0) | 33 (66.0) | 38 (76.0) | >0.05 |
| Male | 14 (28.0) | 19 (38.0) | 17 (34.0) | 12 (24.0) | |
| Surgical intervention | |||||
| Type of operation, n (%) | |||||
| CABG | 39 (78.0) | 41 (82.0) | 37 (74.0) | 42 (84.0) | >0.05 |
| Valve replacement | 6 (12.0) | 6 (12.0) | 8 (16.0) | 5 (10.0) | |
| CABG + valve replacement | 5 (10.0) | 3 (6.0) | 5 (10.0) | 3 (6.0) | |
| CBP time (min), mean ± SD | 89.0 ± 10.0 | 84.0 ± 8.0 | 92.0 ± 10.0 | 86.0 ± 8.0 | >0.05 |
| X-clamp time (min), mean ± SD | 66.0 ± 7.0 | 69.0 ± 8.0 | 77.0 ± 8.0 | 71.0 ± 8.0 | >0.05 |
| Perioperative parameters | |||||
| Perioperative bleeding (mL), mean ± SD | 655.0 ± 60.0 | 590.0 ± 55.0 | 715.0 ± 60.0 | 550.0 ± 50.0 | >0.05 |
| RBC transfusion (U), mean ± SD | 2.0 ± 0.2 | 2.2 ± 0.3 | 2.6 ± 0.3 | 2.5 ± 0.3 | >0.05 |
| Postoperative complications | |||||
| Reoperation for bleeding, n (%) | 2 (4.0) | 3 (6.0) | 3 (6.0) | 2 (4.0) | >0.05 |
| Surgical site infection, n (%) | 1 (2.0) | 2 (4.0) | 1 (2.0) | 0 (0.0) | >0.05 |
| Hospital outcome | |||||
| Length of ICU stay (hours), mean ± SD | 44.0 ± 10.0 | 45.0 ± 10.0 | 51.0 ± 10.0 | 46.0 ± 10.0 | >0.05 |
| Length of hospital stay (day), mean ± SD | 5.8 ± 3.0 | 5.9 ± 3.0 | 6.1 ± 3.0 | 6.2 ± 3.0 | >0.05 |
| Follow-up outcome | |||||
| Hospital readmission, n (%) | 2 (4.0) | 2 (4.0) | 4 (8.0) | 3 (6.0) | >0.05 |
| Mortality, n (%) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (2.0) | >0.05 |
| Frail Pre/Post (n = 50) | Non-Frail Pre/Post (n = 50) | Frail Pre (n = 50) | Non-Frail Pre (n = 50) | |
|---|---|---|---|---|
| Hemoglobin concentration (g/dL), mean ± SD | ||||
| Baseline | 10.6 ± 1.2 | 11.3 ± 1.3 | 10.8 ± 1.2 | 11.5 ± 1.2 |
| Operation day | 11.5 ± 1.3 | 12.1 ± 1.3 | 10.9 ± 1.3 | 11.9 ± 1.3 |
| PO day 1 | 10.8 ± 1.1 | 11.0 ± 1.2 | 10.5 ± 1.1 | 11.1 ± 1.1 |
| PO day 7 | 11.9 ± 1.4 | 12.5 ± 1.4 | 11.1 ± 1.3 | 12.3 ± 1.4 |
| PO 1st month | 12.6 ± 1.4 a | 12.8 ± 1.4 | 11.9 ± 1.4 | 12.5 ± 1.4 |
| PO 3rd month | 13.4 ± 1.4 b | 12.9 ± 1.4 | 12.2 ± 1.4 | 12.8 ± 1.4 |
| Reticulocyte count (×1012/L), mean ± SD | ||||
| Baseline | 0.035 ± 0.005 | 0.04 ± 0.005 | 0.035 ± 0.005 | 0.04 ± 0.005 |
| Operation day | 0.044 ± 0.005 | 0.043 ± 0.005 | 0.04 ± 0.005 | 0.045 ± 0.005 |
| PO day 1 | 0.045 ± 0.005 | 0.04 ± 0.005 | 0.045 ± 0.005 | 0.05 ± 0.005 |
| PO day 7 | 0.050 ± 0.005 | 0.055 ± 0.005 | 0.045 ± 0.005 | 0.05 ± 0.005 |
| PO 1st month | 0.075 ± 0.005 c | 0.055 ± 0.005 | 0.05 ± 0.005 | 0.055 ± 0.005 |
| PO 3rd month | 0.085 ± 0.005 d | 0.045 ± 0.005 | 0.065 ± 0.005 b | 0.05 ± 0.005 |
| Measurement Items | |
|---|---|
| 1. Malnutrition | Nutritional marasmus Other severe protein–calorie malnutrition |
| 2. Dementia | Senile dementia with delusional or depressive features Senile dementia with delirium |
| 3. Vision impairment | Profound impairment, both eyes Better eye: moderate or severe impairment Lesser eye: profound |
| 4. Decubitus ulcer | Decubitus ulcer |
| 5. Urinary incontinence | Incontinence without sensory awareness Continuous leakage |
| 6. Loss of weight | Abnormal loss of weight and underweight Feed difficulties and mismanagement |
| 7. Fecal incontinence | Incontinence of feces |
| 8. Poverty and social support needs | Lack of housing Inadequate housing Inadequate material resources |
| 9. Difficulty in walking | Difficulty in walking Abnormality of gait |
| 10. Fall | Fall on stairs or steps Fall from wheelchair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şanal, L.; Şimşek, E.; Günaydın, S. The Impact of Postoperative Intravenous Iron Therapy on Clinical Outcomes in Surgical Patients with Iron-Deficiency Anemia: A Comparative Analysis by Frailty Status in the Setting of Elective Cardiac Surgery. Medicina 2025, 61, 1919. https://doi.org/10.3390/medicina61111919
Şanal L, Şimşek E, Günaydın S. The Impact of Postoperative Intravenous Iron Therapy on Clinical Outcomes in Surgical Patients with Iron-Deficiency Anemia: A Comparative Analysis by Frailty Status in the Setting of Elective Cardiac Surgery. Medicina. 2025; 61(11):1919. https://doi.org/10.3390/medicina61111919
Chicago/Turabian StyleŞanal, Laser, Erdal Şimşek, and Serdar Günaydın. 2025. "The Impact of Postoperative Intravenous Iron Therapy on Clinical Outcomes in Surgical Patients with Iron-Deficiency Anemia: A Comparative Analysis by Frailty Status in the Setting of Elective Cardiac Surgery" Medicina 61, no. 11: 1919. https://doi.org/10.3390/medicina61111919
APA StyleŞanal, L., Şimşek, E., & Günaydın, S. (2025). The Impact of Postoperative Intravenous Iron Therapy on Clinical Outcomes in Surgical Patients with Iron-Deficiency Anemia: A Comparative Analysis by Frailty Status in the Setting of Elective Cardiac Surgery. Medicina, 61(11), 1919. https://doi.org/10.3390/medicina61111919






