Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. TMA Construction and Ethical Approval
2.3. Immunohistochemical (IHC) Staining and Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
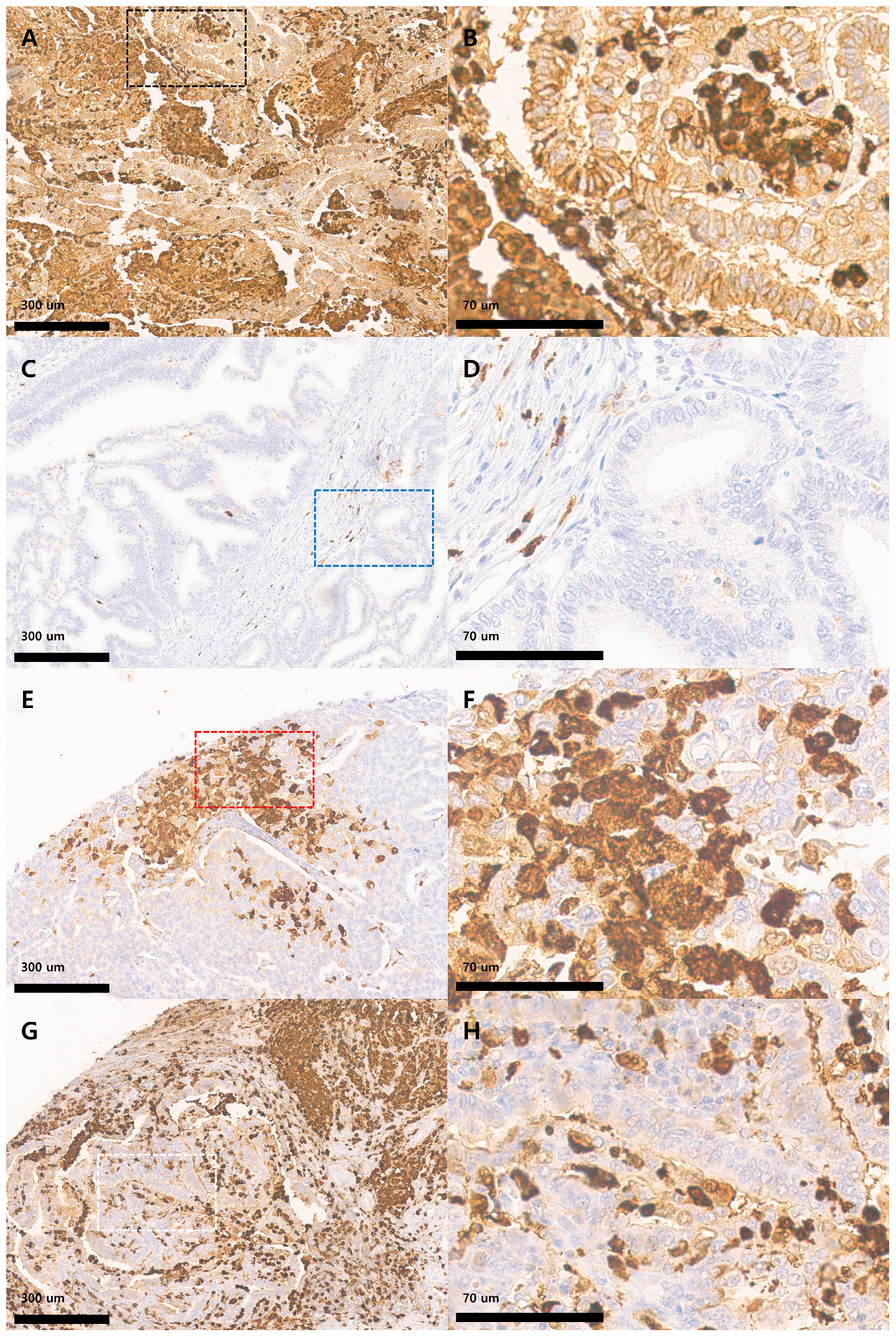
3.2. IHC Patterns of S100A8 Expression
3.3. Correlation of S100A8 Expression and Clinicopathological Features
3.4. Correlation Among TPS, TI, and IPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Shim, S.-H.; Lim, J.; Kim, J.H.; Lee, Y.J.; Ha, H.I.; Lim, M.C.; Won, Y.-J. Trends in the incidence and survival outcomes of endometrial cancer in Korea: A nationwide population-based cohort study. J. Gynecol. Oncol. 2024, 35, e32. [Google Scholar] [CrossRef]
- Ha, H.I.; Chang, H.K.; Park, S.J.; Lim, J.; Won, Y.J.; Lim, M.C. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet. Gynecol. Sci. 2021, 64, 444–453. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Campos, S.M.; Amarnath, S.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines(R) Insights: Uterine Neoplasms, Version 3.2025. J. Natl. Compr. Cancer Netw. 2025, 23, 284–291. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Cibula, D.; Colombo, N.; Creutzberg, C.L.; Ledermann, J.; Mirza, M.R.; Vergote, I.; Abu-Rustum, N.R.; Bosse, T.; et al. ESGO-ESTRO-ESP guidelines for the management of patients with endometrial carcinoma: Update 2025. Lancet Oncol. 2025, 26, e423–e435. [Google Scholar] [CrossRef]
- Palmieri, E.; Mariani, A.; Coleman, R.; Croce, S.; Hui, P.; Lax, S.; Matias-Guiu, X.; Mutch, D.; Scambia, G.; Sehouli, J.; et al. The new 2023 endometrial cancer FIGO staging system: Balancing innovation with complexity. Int. J. Gynecol. Cancer 2025, 35, 101823. [Google Scholar] [CrossRef]
- Moore, B.W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965, 19, 739–744. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 2013, 53, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, J. Advances in S100 protein family for gynecological malignancies. Discov. Oncol. 2025, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Abdi, W.; Romasco, A.; Alkurdi, D.; Santacruz, E.; Okinedo, I.; Zhang, Y.; Kannan, S.; Shakiba, S.; Richmond, J.M. An overview of S100 proteins and their functions in skin homeostasis, interface dermatitis conditions and other skin pathologies. Exp. Dermatol. 2024, 33, e15158. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xing, G.; Wu, C.; Zhu, J.; Wei, M.; Liu, D.; Ge, Y.; Chen, Y.; Lei, T.; Yang, Y. Inhibition of Expression of the S100A8 Gene Encoding the S100 Calcium-Binding Protein A8 Promotes Apoptosis by Suppressing the Phosphorylation of Protein Kinase B (Akt) in Endometrial Carcinoma and HEC-1A Cells. Med. Sci. Monit. 2018, 24, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Safari, E.; Ghorghanlu, S.; Ahmadi-Khiavi, H.; Mehranfar, S.; Rezaei, R.; Motallebnezhad, M. Myeloid-derived suppressor cells and tumor: Current knowledge and future perspectives. J. Cell. Physiol. 2019, 234, 9966–9981. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.W.; Chung, Y.R.; Kim, M.; Choi, H.Y.; Ahn, S.; Park, S.Y. Prognostic significance of S100A8-positive immune cells in relation to other immune cell infiltration in pre-invasive and invasive breast cancers. Cancer Immunol. Immunother. 2021, 70, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Takano, S.; Teratani, T.; Ito, Y.; Yamada, T.; Nozawa, R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr. Cancer Drug Targets 2008, 8, 243–252. [Google Scholar] [CrossRef]
- Yun, S.J.; Yan, C.; Jeong, P.; Kang, H.W.; Kim, Y.-H.; Kim, E.-A.; Lee, O.-J.; Kim, W.T.; Moon, S.-K.; Kim, I.Y.; et al. Comparison of mRNA, Protein, and Urinary Nucleic Acid Levels of S100A8 and S100A9 between Prostate Cancer and BPH. Ann. Surg. Oncol. 2015, 22, 2439–2445. [Google Scholar] [CrossRef]
- Yasar, O.; Akcay, T.; Obek, C.; Turegun, F.A. Significance of S100A8, S100A9 and calprotectin levels in bladder cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 437–441. [Google Scholar] [CrossRef]
- Wang, C.; Luo, J.; Rong, J.; He, S.; Zhang, L.; Zheng, F. Distinct prognostic roles of S100 mRNA expression in gastric cancer. Pathol. Res. Pract. 2019, 215, 127–136. [Google Scholar] [CrossRef]
- Koh, H.M.; An, H.J.; Ko, G.H.; Lee, J.H.; Lee, J.S.; Kim, D.C.; Yang, J.W.; Kim, M.H.; Kim, S.H.; Jeon, K.N.; et al. Prognostic Role of S100A8 and S100A9 Protein Expressions in Non-small Cell Carcinoma of the Lung. J. Pathol. Transl. Med. 2019, 53, 13–22. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Zhang, C.; Zhang, Q.; Li, J.; Xiao, F.; Li, Y.; Zhang, R.; Dou, D.; Liang, J.; et al. Methylation of S100A8 is a promising diagnosis and prognostic marker in hepatocellular carcinoma. Oncotarget 2016, 7, 56798–56810. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, T.; Qi, C.; Du, J.; Ye, C. High expression of S100A2 predicts poor prognosis in patients with endometrial carcinoma. BMC Cancer 2022, 22, 77. [Google Scholar] [CrossRef]
- Xie, R.; Schlumbrecht, M.P.; Shipley, G.L.; Xie, S.; Bassett, R.L., Jr.; Broaddus, R.R. S100A4 mediates endometrial cancer invasion and is a target of TGF-beta1 signaling. Lab. Investig. 2009, 89, 937–947. [Google Scholar] [CrossRef]
- Nakagawa, M.; Higuchi, S.; Hashimura, M.; Oguri, Y.; Matsumoto, T.; Yokoi, A.; Ishibashi, Y.; Ito, T.; Saegusa, M. Functional interaction between S100A1 and MDM2 may modulate p53 signaling in normal and malignant endometrial cells. BMC Cancer 2022, 22, 184. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Bertero, L.; Massa, F.; Metovic, J.; Zanetti, R.; Castellano, I.; Ricardi, U.; Papotti, M.; Cassoni, P. Eighth Edition of the UICC Classification of Malignant Tumours: An overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch. 2018, 472, 519–531. [Google Scholar] [CrossRef]
- Walsh, C.S.; Hacker, K.E.; Secord, A.A.; DeLair, D.F.; McCourt, C.; Urban, R. Molecular testing for endometrial cancer: An SGO clinical practice statement. Gynecol. Oncol. 2023, 168, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Huang, A.; Fan, W.; Liu, J.; Huang, B.; Cheng, Q.; Wang, P.; Duan, Y.; Ma, T.; Chen, L.; Wang, Y.; et al. Prognostic Role of S100A8 in Human Solid Cancers: A Systematic Review and Validation. Front. Oncol. 2020, 10, 564248. [Google Scholar] [CrossRef]
- Gebhardt, C.; Nemeth, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G. S100A8 and S100A9: New insights into their roles in malignancy. J. Innate Immun. 2012, 4, 31–40. [Google Scholar] [CrossRef] [PubMed]
| Variables | Value (Median or Proportion) | |
|---|---|---|
| Age (years) | 35–78 (51) | |
| Tumor size (cm) | 0.7–10 (3.5) | |
| Invasion depth (cm) | 0.01–4 (0.3) | |
| T stage | 1a | 36 (69.2%) |
| 1b | 12 (23.1%) | |
| 2 | 2 (3.8%) | |
| 3a | 1 (1.9%) | |
| 3b | 1 (1.9%) | |
| N stage | 0 | 47 (90.4%) |
| 1 | 3 (5.8%) | |
| 2 | 2 (3.8%) | |
| FIGO histologic grade | G1 | 35 (67.3%) |
| G2 | 13 (25.0%) | |
| G3 | 4 (7.7%) | |
| S100A8 TPS * | 0–30% | 27 (51.9%) |
| 31–60% | 16 (30.8%) | |
| 61–100% | 9 (17.3%) | |
| S100A8 TI ** | Negative | 6 (11.5%) |
| Weak | 13 (25.0%) | |
| Moderate | 21 (40.4%) | |
| Severe | 12 (23.1%) | |
| S100A8 IPS *** | 0–30% | 22 (42.3%) |
| 31–60% | 20 (38.5%) | |
| 61–100% | 10 (19.2%) |
| Variable | S100A8 TPS | S100A8 TI | S100A8 IPS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low * | High | p-Value | Low ** | High | p-Value | Low *** | High | p-Value | ||
| Age (years) | ≤51 | 14 | 13 | 0.991 | 11 | 16 | 0.513 | 15 | 12 | 0.044 |
| >51 | 13 | 12 | 8 | 17 | 7 | 18 | ||||
| Tumor size | ≤3.5 cm | 12 | 15 | 0.262 | 8 | 19 | 0.282 | 9 | 18 | 0.173 |
| >3.5 cm | 15 | 10 | 11 | 14 | 13 | 12 | ||||
| Invasion depth | ≤0.3 cm | 12 | 10 | 0.746 | 7 | 15 | 0.545 | 12 | 10 | 0.126 |
| >0.3 cm | 15 | 15 | 12 | 18 | 10 | 20 | ||||
| Histologic grade | G1 | 16 | 19 | 0.199 | 14 | 21 | 0.457 | 19 | 16 | 0.012 |
| G2.3 | 11 | 6 | 5 | 12 | 3 | 14 | ||||
| T stage | ≤1a | 16 | 20 | 0.105 | 12 | 24 | 0.472 | 18 | 18 | 0.092 |
| >1a | 11 | 5 | 7 | 9 | 4 | 12 | ||||
| N stage | ≤0 | 25 | 22 | 0.462 | 17 | 30 | 0.610 | 20 | 27 | 0.648 |
| N1,2 | 2 | 3 | 2 | 3 | 2 | 3 | ||||
| S100A8 IPS | ||||
|---|---|---|---|---|
| Low *** | High | p-Value | ||
| S100A8_TPS | Low * | 15 | 12 | 0.044 |
| High | 7 | 18 | ||
| S100A8 TI | Low ** | 10 | 9 | 0.253 |
| High | 12 | 21 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.H.; Kim, M.H.; Yang, J.; Jo, H.C.; Park, J.E.; Baek, J.C. Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance. Medicina 2025, 61, 1918. https://doi.org/10.3390/medicina61111918
Song DH, Kim MH, Yang J, Jo HC, Park JE, Baek JC. Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance. Medicina. 2025; 61(11):1918. https://doi.org/10.3390/medicina61111918
Chicago/Turabian StyleSong, Dae Hyun, Min Hye Kim, Juseok Yang, Hyen Chul Jo, Ji Eun Park, and Jong Chul Baek. 2025. "Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance" Medicina 61, no. 11: 1918. https://doi.org/10.3390/medicina61111918
APA StyleSong, D. H., Kim, M. H., Yang, J., Jo, H. C., Park, J. E., & Baek, J. C. (2025). Differential Expression of S100A8 in Tumor and Immune Compartments of Endometrial Carcinoma and Its Clinical Relevance. Medicina, 61(11), 1918. https://doi.org/10.3390/medicina61111918





