Uterine Ectopic Pregnancies and Live Births: Systematic Review of the Literature and Concepts Underlying Favorable Outcomes
Abstract
1. Introduction
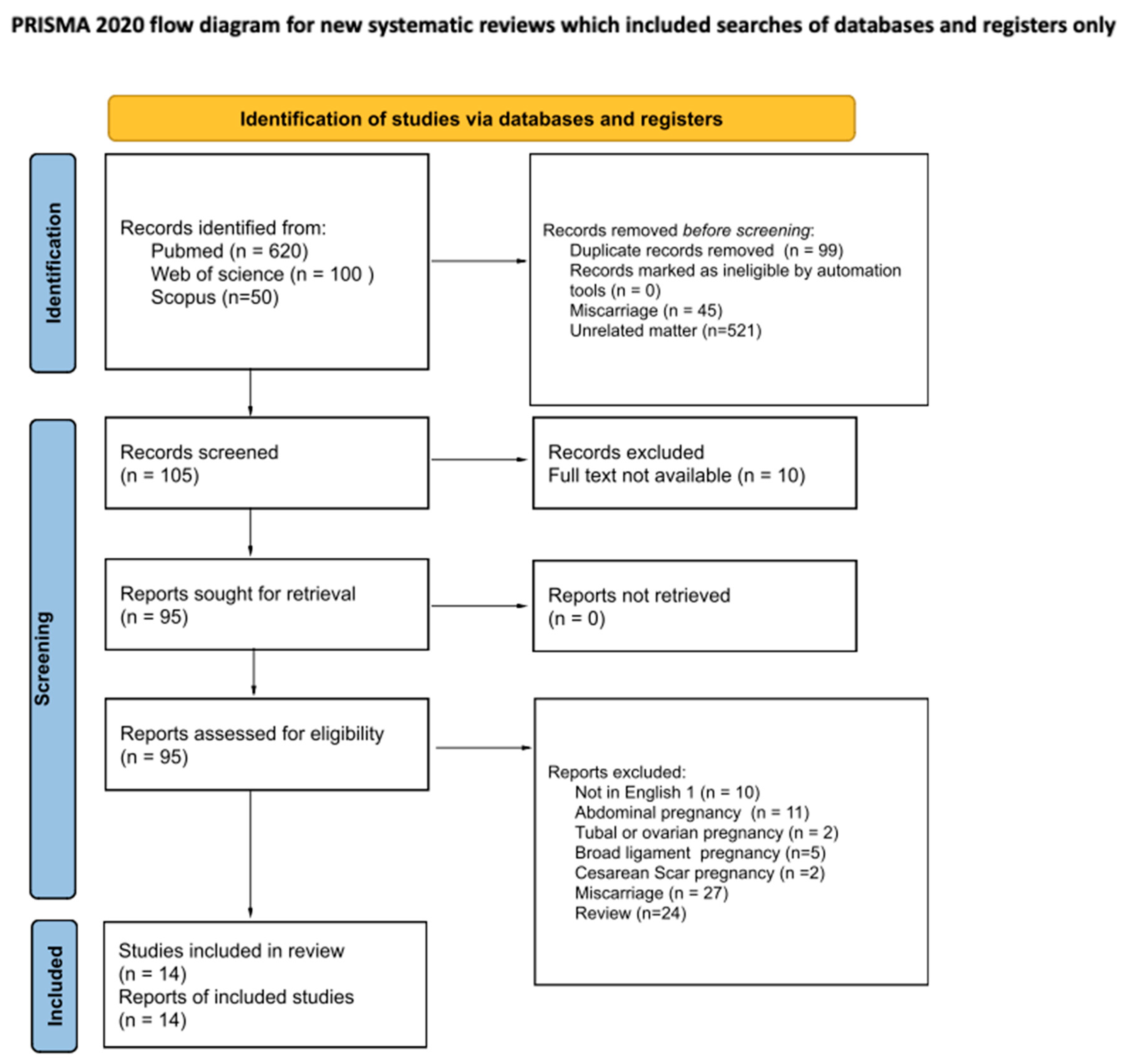
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Risk of Bias Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
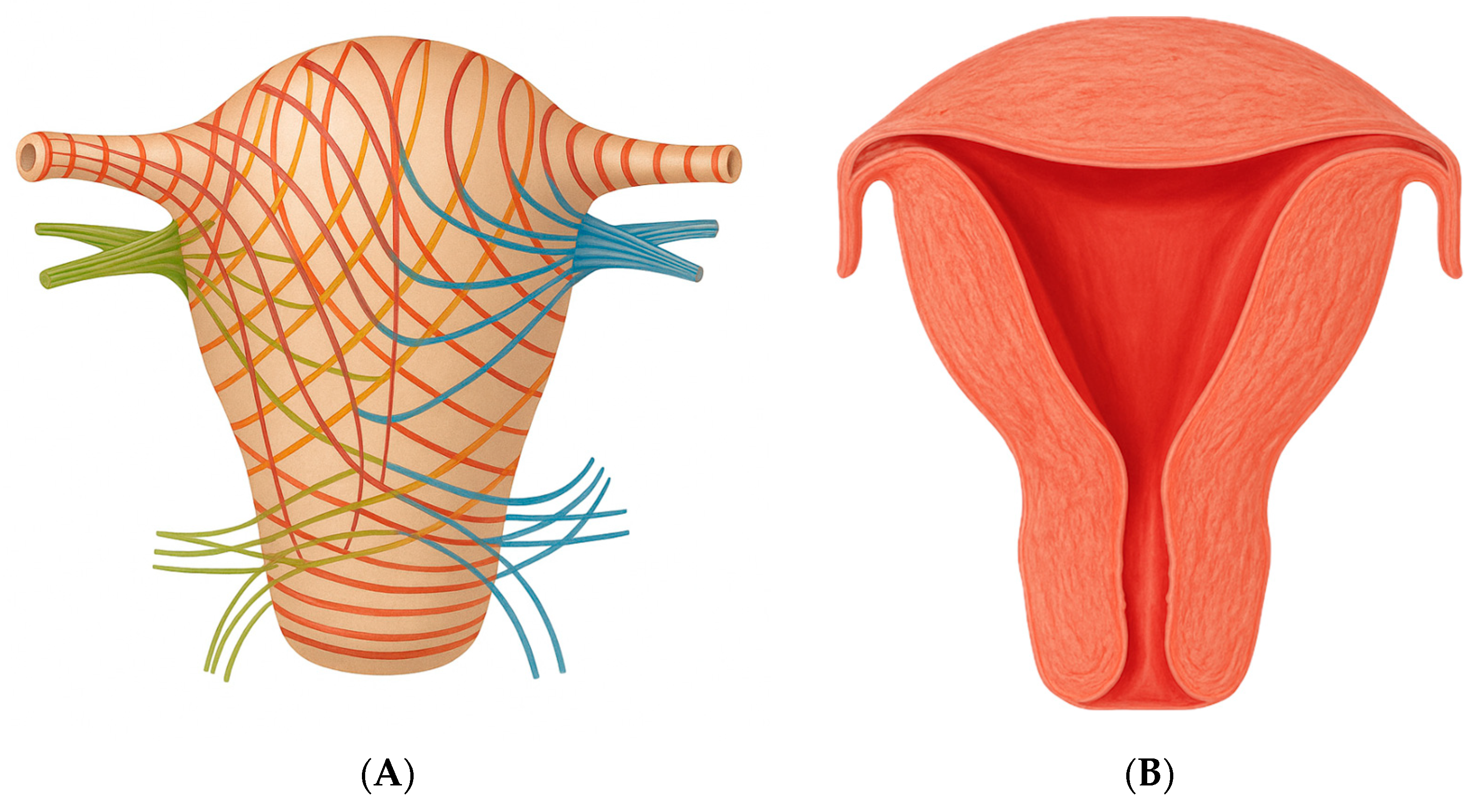
4. Discussion
- The amount of endometrium present at the implantation site, which enables proper development of the syncytiotrophoblast;
- The ability of the myometrium in that uterine region to expand.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ESHRE working group on Ectopic Pregnancy; Kirk, E.; Ankum, P.; Jakab, A.; Le Clef, N.; Ludwin, A.; Small, R.; Tellum, T.; Töyli, M.; Van den Bosch, T.; et al. Terminology for describing normally sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice. Hum. Reprod. Open 2020, 2020, hoaa055. [Google Scholar] [CrossRef]
- Jurkovic, D.; Hillaby, K.; Woelfer, B.; Lawrence, A.; Salim, R.; Elson, C.J. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound. Obstet. Gynecol. 2003, 21, 220–227. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Khatib, N.; Monteagudo, A.; Ramos, J.; Berg, R.; Kovacs, S. Cesarean scar pregnancies: Experience of 60 cases. J. Ultrasound. Med. 2015, 34, 601–610. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Banco, R.; Turrisi, A.; Airoud, M.; De Leo, R.; Stabile, G.; Ceccarello, M.; Restaino, S.; De Santo, D. Pelvic inflammatory disease (PID) from Chlamydia trachomatis versus PID from Neisseria gonorrhea: From clinical suspicion to therapy. G. Ital. Dermatol. Venereol. 2012, 147, 423–430. [Google Scholar] [PubMed]
- Hoyos, L.R.; Tamakuwala, S.; Rambhatla, A.; Brar, H.; Vilchez, G.; Allsworth, J.; Rodriguez-Kovacs, J.; Awonuga, A. Risk factors for cervical ectopic pregnancy. J. Gynecol. Obstet. Hum. Reprod. 2019, 49, 101665. [Google Scholar] [CrossRef] [PubMed]
- Vadakekut, E.S.; Gnugnoli, D.M. Ectopic Pregnancy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Elson, C.J.; Salim, R.; Potdar, N.; Chetty, M.; Ross, J.A.; Kirk, E.J.; on behalf of the Royal College of Obstetricians and Gynaecologists. Diagnosis and Management of Ectopic Pregnancy: Green-top Guideline No. 21. BJOG 2016, 123, e15–e55. [Google Scholar]
- Stabile, G.; Mangino, F.P.; Romano, F.; Zinicola, G.; Ricci, G. Ectopic Cervical Pregnancy: Treatment Route. Medicina 2020, 56, 293. [Google Scholar] [CrossRef]
- Ushakov, F.B.; Elchalal, U.; Aceman, P.J.; Schenker, J.G. Cervical pregnancy: Past and future. Obstet. Gynecol. Surv. 1997, 52, 45–59. [Google Scholar] [CrossRef]
- Dicker, D.; Feldberg, D.; Samuel, N.; Goldman, J.A. Etiology of cervical pregnancy. association with abortion, pelvic pathology, IUDs and Asherman’s syndrome. J. Reprod. Med. 1985, 30, 25–27. [Google Scholar]
- Stabile, G.; Cracco, F.; Zinicola, G.; Carlucci, S.; Mangino, F.P.; Stampalija, T.; Ricci, G. Subserosal pregnancy: Systematic review with proposal of new diagnostic criteria and ectopic pregnancy classification. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 297, 254–259. [Google Scholar] [CrossRef]
- Ginsburg, K.A.; Quereshi, F.; Thomas, M.; Snowman, B. Intramural ectopic pregnancy implanting in adenomyosis. Fertil. Steril. 1989, 51, 354–356. [Google Scholar] [CrossRef]
- Bannon, K.; Fernandez, C.; Rojas, D.; Levine, E.M.; Locher, S. Diagnosis and management of intramural ectopic pregnancy. J. Minim. Invasive Gynecol. 2013, 20, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Nijjar, S.; Bottomley, C.; Jauniaux, E.; Jurkovic, D. Imaging in gynecological disease (25): Clinical and ultrasound characteristics of intramural pregnancy. Ultrasound. Obstet. Gynecol. 2023, 62, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Y.; Zhu, H.; Zheng, F.Y. Interstitial pregnancy after ipsilateral salpingectomy: Analysis of 46 cases and a literature review. J. Minim. Invasive Gynecol. 2020, 27, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Takahashi, H.; Tozawa, S.; Narumi, R.; Usui, R.; Ohkuchi, A.; Matsubara, S. Interstitial pregnancy in the third trimester with severe preeclampsia: A case report and literature review. Case Rep. Obstet. Gynecol. 2020, 2020, 9408501. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Tulandi, T. Conservative medical and surgical manage- ment of interstitial ectopic pregnancy. Fertil. Steril. 1999, 72, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Liu, Y.-L. Interstitial and Cornual Ectopic Pregnancy: A Review of the Management Options. Clin. Exp. Obstet. Gynecol. 2023, 50, 47. [Google Scholar] [CrossRef]
- Ackerman, T.E.; Levi, C.S.; Dashefsky, S.M.; Holt, S.C.; Lindsay, D.J. Interstitial line: Sonographic finding in interstitial (cornual) ectopic pregnancy. Radiology 1993, 189, 83–87. [Google Scholar] [CrossRef]
- Moawad, N.S.; Mahajan, S.T.; Moniz, M.H.; Taylor, S.E.; Hurd, W.W. Current diagnosis and treatment of interstitial pregnancy. Am. J. Obstet. Gynecol. 2010, 202, 15–29. [Google Scholar] [CrossRef]
- Timor-Tritsch, I.E.; Monteagudo, A.; Santos, R.; Tsymbal, T.; Pineda, G.; Arslan, A.A. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am. J. Obstet. Gynecol. 2012, 207, 44.e1–44.e13. [Google Scholar] [CrossRef]
- Ash, A.; Smith, A.; Maxwell, D. Caesarean scar pregnancy. BJOG 2007, 114, 253–263. [Google Scholar] [CrossRef]
- Stabile, G.; Vona, L.; Carlucci, S.; Zullo, F.; Laganà, A.S.; Etrusco, A.; Restaino, S.; Nappi, L. Conservative treatment of cesarean scar pregnancy with the combination of methotrexate and mifepristone: A systematic review. Womens Health 2024, 20, 17455057241290424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartels, H.C.; Brennan, D.J.; Timor-Tritsch, I.E.; Agten, A.K. Global variation and outcomes of expectant management of CSP. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 89, 102353. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ugwumadu, A.H.; Hamid, R.; Ross, L.D. Live infant salvaged from a ruptured cornual (interstitial) pregnancy at 33-weeks gestation. Int. J. Gynaecol. Obstet. 1997, 58, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Kakigano, A.; Matsuzaki, S.; Jitsumori, M.; Mimura, K.; Endo, M.; Kimura, T. An evident asymmetrical uterus during cesarean delivery. Clin. Case Rep. 2018, 6, 2281–2282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scarella, A.; Marquez, R.; Schilling, H.; Palomino, A. Antenatal diagnosis of a third trimester interstitial pregnancy: A case report. J. Obstet. Gynaecol. Res. 2012, 38, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Lecointre, C.; Ducarme, G. Intramural ectopic pregnancy with live birth at 37 weeks of gestation. Arch. Gynecol. Obstet. 2013, 287, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Altaras, M.; Siegal, A.; Ben-Aderet, N. Cervico-isthmic pregnancy ending with the delivery of a live-born infant in late second trimester. Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 20, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.M.; Urdl, W.; Höfler, H.; Hönigl, W.; Tamussino, K. Cervical pregnancy: Case reports and current concepts in diagnosis and treatment. Arch. Gynecol. Obstet. 1987, 241, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Najib, F.S.; Vafaei, H.; Foroughi, A.A.; Namazi, N. Diagnosis pitfall of interstitial pregnancy: A case report of a term pregnancy with abnormal placentation. BMC Pregnancy Childbirth 2021, 21, 699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, A.J.; Van Winden, K.R.; Cook, C.R. A true cornual (interstitial) pregnancy resulting in a viable fetus. Obstet. Gynecol. 2013, 121 Pt 2 (Suppl. 1), 427–430. [Google Scholar] [CrossRef] [PubMed]
- Köninger, A.; Nguyen, B.P.; Schwenk, U.; Vural, M.; Iannaccone, A.; Theysohn, J.; Kimmig, R. Cervical ectopic pregnancy—The first case of live birth and uterus-conserving management. BMC Pregnancy Childbirth 2023, 23, 664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesogitis, S.A.; Daskalakis, G.J.; Doublis, D.G.; Antsaklis, A.J.; Papantoniou, N.E.; Michalas, S.P. Cervico-isthmic pregnancy: An extremely rare case diagnosed during labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mimura, K.; Kanagawa, T.; Nakayama, M.; Matsuzaki, S.; Kinugasa-Taniguchi, Y.; Endo, M.; Kimura, T. Interstitial pregnancy resulting in a viable infant coexistent with massive perivillous fibrin deposition: A case report and literature review. AJP Rep. 2014, 4, 29–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Ng, P.H.; Nor Azlin, M.I.; Nasri, N.I. Term interstitial pregnancy with uterine conservation. Int. J. Gynaecol. Obstet. 2007, 99, 251. [Google Scholar] [CrossRef] [PubMed]
- Idama, T.O.; Tuck, C.S.; Ivory, C.; Ellerington, M.C.; Travis, S. Survival of cornual (interstitial) pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 84, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Chukus, A.; Tirada, N.; Restrepo, R.; Reddy, N.I. Uncommon Implantation Sites of Ectopic Pregnancy: Thinking beyond the Complex Adnexal Mass. Radiographics 2015, 35, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Collins, S.; Burton, G.J. Placenta accreta spectrum: Pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am. J. Obstet. Gynecol. 2018, 218, 75–87. [Google Scholar] [CrossRef]
- Netter, F.H. Atlas of Human Anatomy, 8th ed.; Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Fruscalzo, A.; Londero, A.P.; Salvador, S.; Bertozzi, S.; Biasioli, A.; Della Martina, M.; Driul, L.; Marchesoni, D. New and old predictive factors for breech presentation: Our experience in 14 433 singleton pregnancies and a literature review. J. Matern. Fetal Neonatal Med. 2014, 27, 167–172. [Google Scholar] [CrossRef]
- Mattuizzi, A. Présentation du siège. Recommandations pour la pratique clinique du CNGOF—Épidémiologie, facteurs de risque et complications. Breech Presentation: CNGOF Guidelines for Clinical Practice—Epidemiology, Risk Factors and Complications. Gynecol. Obstet. Fertil. Senol. 2020, 48, 70–80. (In French) [Google Scholar] [CrossRef] [PubMed]
- Malinowski, A.; Bates, S.K. Semantics and pitfalls in the diagnosis of cornual/interstitial pregnancy. Fertil. Steril. 2006, 86, 1764.e11–1764.e14. [Google Scholar] [CrossRef]
- Spuentrup, C.; Wendt, E.; Banerjee, M.; Schmitz, J.; Hellmich, M.; Noé, G.K. The influence on resection line during supracervical hysterectomy: Physiological extension of endometrial cells in the cervix uteri. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manuale di Ecografia Ostetrica e Ginecologica, 3rd ed.; Società Italiana di Ecografia Ostetrico-Ginecologica: Palermo, Italy, 2023.

| Author, Year | Ectopic Pregnancy Type | Cases (n) | General Anamnesis Obstetric Anamnesis | GA at the Diagnosis (Weeks) | Complications During the Pregnancy | Delivery Way and Complications | GA at Delivery (Weeks) | Fetal Weight (g) and APGAR | Complications After the Delivery | Pathological Examination |
|---|---|---|---|---|---|---|---|---|---|---|
| Angela Köninger et al., 2023 [34] | Cervical Pregnancy | 1 | 37 yo 1 IIIG IIP 2 CS 2 | US 3 (7 + 3) | Cerclage at 12 weeks Pelvic Pain at 30 weeks | CS UAE 4 was planned during the surgery | 30 | 1620 8/8/9 | The placenta was left in situ. Hematuria. Re-laparotomy and cervicotomy to evacuate the placenta and repair the bladder injury. Transfusion: 8 Blood units | No |
| Spryros A. Mesogitis et al., 2001 [35] | Cervical Pregnancy | 1 | 27 yo IG 0P | At delivery (37 + 2) | Vaginal Bleeding at 9 and 33 weeks | Vaginal vacuum extractor | 37 + 2 | 2750 9/10 | Excessive Hemorrhage, anuria—Hemorrhagic Shock Total Hysterectomy Transfusion: 4 blood units and 8 platelet units | Yes |
| H. M. H. Hofmann et al., 1987 [31] | Cervical Pregnancy | 1 | 42 yo IIIG IIP | At delivery (32 + 3) | 32 weeks: severe preeclampsia and vaginal bleeding 32 + 3 weeks: Profuse painless bleeding | CS (emergency) | 32 + 3 | 1930 6/7 | Placenta Increta Hysterectomy | Yes |
| I. Cohen et al., 1985 [30] | Cervical Pregnancy | 1 | 36 yo IG 0P | At delivery (26 + 2) | 12 weeks: cerclage for severe degree of cervical effacement 25 weeks: Painless vaginal bleeding 26 + 2 weeks: Preterm Premature Rupture of Membranes (pPROM). Cervical cerclage was removed | CS (breech presentation) | 26 + 2 | 1050 9/10 | Placenta accreta Hysterectomy Transfusion: 5 blood Units | Yes |
| Fatemeh Sadat Najib et al., 2021 [32] | Interstitial Pregnancy | 1 | 32 yo IG 0P | At delivery (38) | 26 weeks: acute abdomen and hemoperitoneum—interstitial pregnancy was mistaken with a degenerated and bleeding posterior myoma. Blood Transfusion was performed. | CS (breech presentation) | 38 | 2840 8/9 | Placenta increta. Hysterectomy | Yes |
| Shiho Nagayama et al., 2020 [16] | Interstitial Pregnancy | 1 | 41 yo IVG IP | At delivery (28 + 1) | 11 weeks: subchorionic hematoma 26 + 6 weeks: early onset preeclampsia 27 + 2 weeks: preeclampsia; lung edema; normal umbilical Doppler; suspect of PAS 5. 28 + 1 weeks: severe headache and PA 180/100mmHg | CS (emergency) | 28 + 1 | 926 (−1.4 SD) 3/6 | Placenta accreta Hysterectomy 10 blood units and 8 plasma units | Yes |
| Aiko Kakigano et al., 2018 [27] | Interstitial Pregnancy | 1 | 33 yo Multiparous CS in anamnesis | At delivery (38) | NA | CS | 38 | 3148 NA | Placenta accreta Supracervical hysterectomy | Yes |
| Yusuke Tanaka et al., 2014 [36] | Interstitial Pregnancy | 1 | 35 yo IG 0P | At delivery (32) | 31 weeks fetal growth restriction; umbilical artery Doppler showed reversed end-diastolic velocity. | CS (Breech presentation) | 32 | 1038 (−3.0 SD) 7/9 | Placenta accreta—left in situ On day 6 postoperative: fever On day 8, placenta was spontaneously removed | No |
| Alexandria J. Hill et al., 2013 [33] | Interstitial Pregnancy | 1 | 27 yo IIG IP | At delivery (32) | 25 weeks: gestational diabetes 28 weeks: high blood pressure, no preeclampsia 30 weeks: either persistent or intermittent absent end diastolic flow; no stress tests remained reactive until delivery at 32 weeks. | CS (Breech presentation) Before the laparotomy, cystoscopy, and bilateral urethral stents were placed. | 32 W | 1430 4/4/7 | The right tube and ovary were removed with the sac and placenta | Yes |
| Anibal Scarella et al., 2012 [28] | Interstitial Pregnancy | 1 | 30 yo Multiparous | US (20) DD 6 between cornual ectopic pregnancy with placenta accreta and interstitial pregnancy. MRI 7 (26 + 1) definitive diagnosis | 20 weeks: pPROM; oligohydramnios, placenta increta 25 weeks: pulmonary hypoplasia was diagnosed. 27 + 5 weeks: abdominal discomfort; vaginal bleeding. | CS | 28 | 1000 NA/9 Severe respiratory distress syndrome; death after 12 h | Hysterectomy | Yes |
| P.H. Ng et al., 2007 [37] | Interstitial Pregnancy | 1 | 27 yo IG 0P | At delivery (38) | NA | CS (Breech presentation) | 38 | NA Healthy newborn | Adherent Placenta—left in situ. Weekly IM MTX. Placenta was delivered 17 days postoperative | No |
| Idama T.O. et al., 1998 [38] | Interstitial Pregnancy | 1 | 26 yo IG 0P | At delivery (30) | 30 weeks: intermittent abdominal pain; vomiting; Oligohydramnios; tense and tender abdomen. | CS (emergency) | 30 | 1682 1/6 | Hemoperitoneum (1200 mL) 3 blood Units | Yes |
| A.H.N. Ugwumadu et al., 1997 [26] | Interstitial Pregnancy | 1 | IG 0P | At delivery (33) | During the pregnancy: Abdominal pain 33 weeks: shock; tense and tender abdomen CTG: sinusoidal fetal heart pattern. | CS (emergency) | 33 | 2100 2/7/9 | Hemoperitoneum (2000 mL) Placenta accreta Hysterectomy | Yes |
| Laurent Petit et al., 2012 [29] | Intramural pregnancy | 1 | 36 yo | At delivery (37) | 13 weeks: diagnosis of anterior placenta previa | CS | 37 | NA Healthy newborn | Excessive hemorrhage Hysterectomy 10 blood units and 8 plasma units | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, G.; Vona, L.; Carlucci, S.; Pitsillidi, A.; Restaino, S.; Vizzielli, G.; Nappi, L. Uterine Ectopic Pregnancies and Live Births: Systematic Review of the Literature and Concepts Underlying Favorable Outcomes. Medicina 2025, 61, 1915. https://doi.org/10.3390/medicina61111915
Stabile G, Vona L, Carlucci S, Pitsillidi A, Restaino S, Vizzielli G, Nappi L. Uterine Ectopic Pregnancies and Live Births: Systematic Review of the Literature and Concepts Underlying Favorable Outcomes. Medicina. 2025; 61(11):1915. https://doi.org/10.3390/medicina61111915
Chicago/Turabian StyleStabile, Guglielmo, Laura Vona, Stefania Carlucci, Anna Pitsillidi, Stefano Restaino, Giuseppe Vizzielli, and Luigi Nappi. 2025. "Uterine Ectopic Pregnancies and Live Births: Systematic Review of the Literature and Concepts Underlying Favorable Outcomes" Medicina 61, no. 11: 1915. https://doi.org/10.3390/medicina61111915
APA StyleStabile, G., Vona, L., Carlucci, S., Pitsillidi, A., Restaino, S., Vizzielli, G., & Nappi, L. (2025). Uterine Ectopic Pregnancies and Live Births: Systematic Review of the Literature and Concepts Underlying Favorable Outcomes. Medicina, 61(11), 1915. https://doi.org/10.3390/medicina61111915








