Decoding SIGLEC12 in Bladder Cancer: In Silico Profiling of Expression, Tumor–Immune Interactions, and Prognostic Impact
Abstract
1. Introduction
2. Methods
2.1. Differential Gene Expression and Methylation Analysis
2.2. Genetic Alteration Analysis
2.3. Functional Enrichment Analysis
2.4. Survival Analysis
2.5. Tumor Immune Infiltration and Immune Checkpoints Analysis
2.6. Drug Sensitivity Analysis
3. Results
3.1. SIGLEC12 Is Significantly Upregulated in BCa and Associates with Clinicopathological Features
3.2. Genetic Alterations of SIGLEC12 in BCa
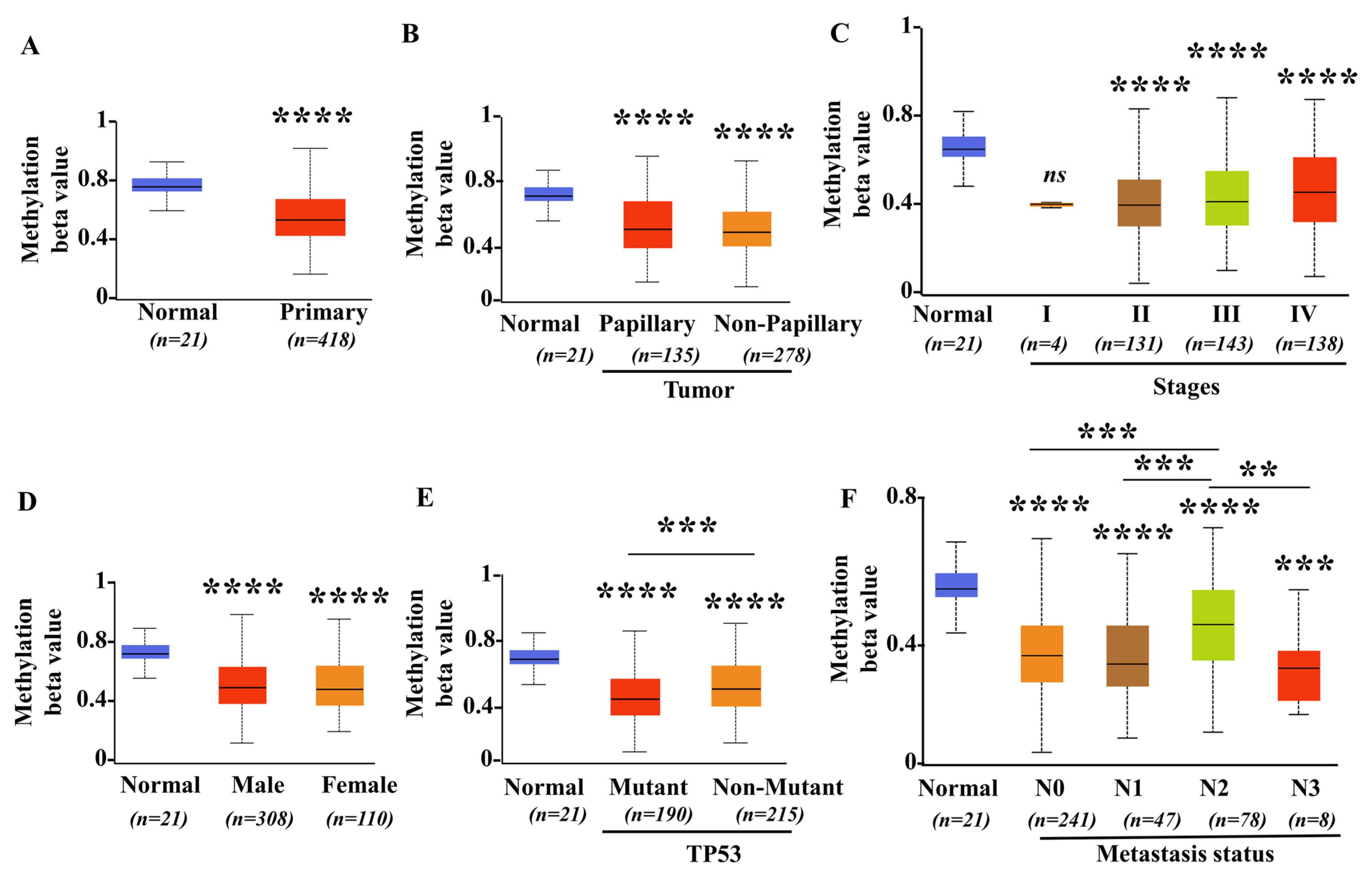
3.3. Promoter Methylation Levels of SIGLEC12 Decreased in BCa
3.4. GO and KEGG Pathway Enrichment Analysis of Genes Associated with SIGLEC12 Expression in BCa
3.5. Correlation Between SIGLEC12 Expression and Immune Cell Composition in BCa
3.6. SIGLEC12 Expression Correlates with Oncogenic Signaling but Not Survival Outcome in BCa
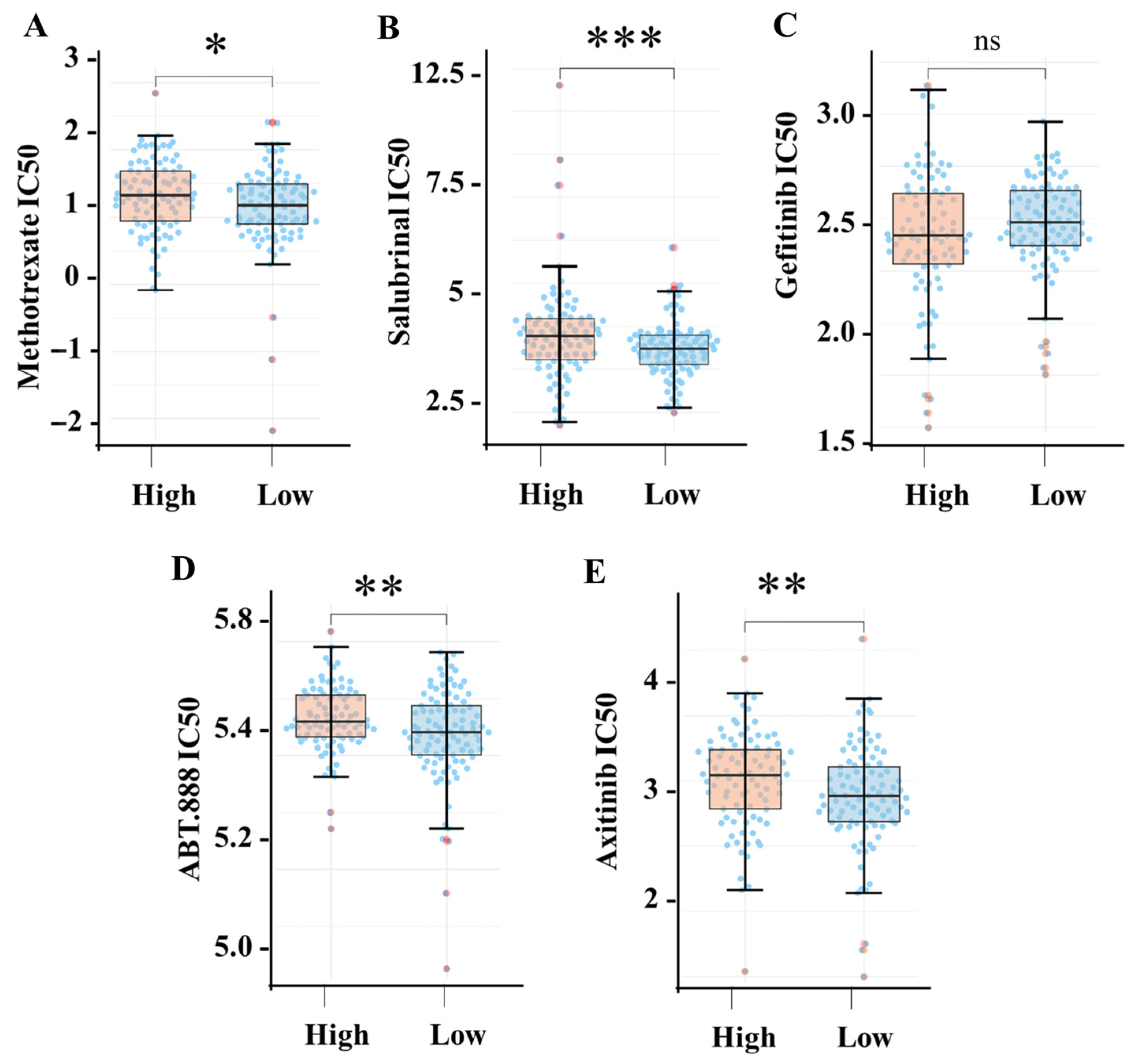
3.7. High SIGLEC12 Expression Is Associated with Drug Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Toren, P.; Wilkins, A.; Patel, K.; Burley, A.; Gris, T.; Kockelbergh, R.; Lodhi, T.; Choudhury, A.; Bryan, R.T. The Sex Gap in Bladder Cancer Survival—A Missing Link in Bladder Cancer Care? Nat. Rev. Urol. 2024, 21, 181–192. [Google Scholar] [CrossRef]
- Cassell, A.; Yunusa, B.; Jalloh, M.; Mbodji, M.M.; Diallo, A.; Ndoye, M.; Diallo, Y.; Labou, I.; Niang, L.; Gueye, S.M. Non-Muscle Invasive Bladder Cancer: A Review of the Current Trend in Africa. World J. Oncol. 2019, 10, 123–131. [Google Scholar] [CrossRef]
- Abd El-Salam, M.A.; Smith, C.E.P.; Pan, C. Insights on Recent Innovations in Bladder Cancer Immunotherapy. Cancer Cytopathol. 2022, 130, 667–683. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, B.; Dinney, C.; McConkey, D. Origins of Bladder Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 149–174. [Google Scholar] [CrossRef]
- Guo, C.C.; Czerniak, B. Bladder Cancer in the Genomic Era. Arch. Pathol. Lab. Med. 2019, 143, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.-D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The Mucin MUC1 Modulates the Tumor Immunological Microenvironment through Engagement of the Lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; Van Dinther, E.A.W.; Peters, T.; De Graaf, A.M.A.; Leusen, J.H.W.; Kreutz, M.; Figdor, C.G.; Den Brok, M.H.; Adema, G.J. Targeted Delivery of a Sialic Acid-Blocking Glycomimetic to Cancer Cells Inhibits Metastatic Spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef]
- Chiang, C.; Wang, C.; Chang, H.; More, S.V.; Li, W.; Hung, W. A Novel Sialyltransferase Inhibitor AL10 Suppresses Invasion and Metastasis of Lung Cancer Cells by Inhibiting Integrin-mediated Signaling. J. Cell. Physiol. 2010, 223, 492–499. [Google Scholar] [CrossRef]
- Jandus, C.; Simon, H.-U.; Von Gunten, S. Targeting Siglecs—A Novel Pharmacological Strategy for Immuno- and Glycotherapy. Biochem. Pharmacol. 2011, 82, 323–332. [Google Scholar] [CrossRef]
- Läubli, H.; Pearce, O.M.T.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of Myelomonocytic Siglecs by Tumor-Associated Ligands Modulates the Innate Immune Response to Cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as Therapeutic Targets in Cancer. Biology 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.; Sarkar, S.; Natoni, A.; Stark, J.C.; Riley, N.M.; Bertozzi, C.R.; Carlsten, M.; O’Dwyer, M.E. Targeting Hypersialylation in Multiple Myeloma Represents a Novel Approach to Enhance NK Cell–Mediated Tumor Responses. Blood Adv. 2022, 6, 3352–3366. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T Cell Engagers: An Emerging Therapy for Management of Hematologic Malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Bornhöfft, K.F.; Goldammer, T.; Rebl, A.; Galuska, S.P. Siglecs: A Journey through the Evolution of Sialic Acid-Binding Immunoglobulin-Type Lectins. Dev. Comp. Immunol. 2018, 86, 219–231. [Google Scholar] [CrossRef]
- Avril, T.; Freeman, S.D.; Attrill, H.; Clarke, R.G.; Crocker, P.R. Siglec-5 (CD170) Can Mediate Inhibitory Signaling in the Absence of Immunoreceptor Tyrosine-Based Inhibitory Motif Phosphorylation. J. Biol. Chem. 2005, 280, 19843–19851. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Bärenwaldt, A.; Läubli, H. The Sialoglycan-Siglec Glyco-Immune Checkpoint—A Target for Improving Innate and Adaptive Anti-Cancer Immunity. Expert. Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef]
- Khan, N.; De Manuel, M.; Peyregne, S.; Do, R.; Prufer, K.; Marques-Bonet, T.; Varki, N.; Gagneux, P.; Varki, A. Multiple Genomic Events Altering Hominin SIGLEC Biology and Innate Immunity Predated the Common Ancestor of Humans and Archaic Hominins. Genome Biol. Evol. 2020, 12, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, N.M.; Varki, A. A Second Uniquely Human Mutation Affecting Sialic Acid Biology. J. Biol. Chem. 2001, 276, 40282–40287. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.W.; Gong, Y.; Padmanabhan, S.; Burkley, B.; Langaee, T.Y.; Melander, O.; Pepine, C.J.; Dominiczak, A.F.; Cooper-DeHoff, R.M.; Johnson, J.A. Pharmacogenomic Association of Nonsynonymous SNPs in SIGLEC12, A1BG, and the Selectin Region and Cardiovascular Outcomes. Hypertension 2013, 62, 48–54. [Google Scholar] [CrossRef]
- Mitra, N.; Banda, K.; Altheide, T.K.; Schaffer, L.; Johnson-Pais, T.L.; Beuten, J.; Leach, R.J.; Angata, T.; Varki, N.; Varki, A. SIGLEC12, a Human-Specific Segregating (Pseudo) Gene, Encodes a Signaling Molecule Expressed in Prostate Carcinomas. J. Biol. Chem. 2011, 286, 23003–23011. [Google Scholar] [CrossRef]
- Yu, Z.; Lai, C.-M.; Maoui, M.; Banville, D.; Shen, S.-H. Identification and Characterization of S2V, a Novel Putative Siglec That Contains Two V Set Ig-like Domains and Recruits Protein-Tyrosine Phosphatases SHPs. J. Biol. Chem. 2001, 276, 23816–23824. [Google Scholar] [CrossRef]
- Siddiqui, S.S.; Vaill, M.; Do, R.; Khan, N.; Verhagen, A.L.; Zhang, W.; Lenz, H.-J.; Johnson-Pais, T.L.; Leach, R.J.; Fraser, G.; et al. Human-Specific Polymorphic Pseudogenization of SIGLEC12 Protects against Advanced Cancer Progression. FASEB Bioadv 2021, 3, 69–82. [Google Scholar] [CrossRef]
- Ogbodo, A.K.; Mustafov, D.; Arora, M.; Lambrou, G.I.; Braoudaki, M.; Siddiqui, S.S. Analysis of SIGLEC12 Expression, Immunomodulation and Prognostic Value in Renal Cancer Using Multiomic Databases. Heliyon 2024, 10, e24286. [Google Scholar] [CrossRef]
- Cuello, H.A.; Sinha, S.; Verhagen, A.L.; Varki, N.; Varki, A.; Ghosh, P. Human-Specific Elimination of Epithelial Siglec-XII Suppresses the Risk of Inflammation Driven Colorectal Cancers. JCI Insight 2024, 9, 181539. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Gunasekaran, P.; Leung, K.; Bindal, N.; Boutselakis, H.; Ding, M.; Bamford, S.; Cole, C.; Ward, S.; et al. COSMIC: Exploring the World’s Knowledge of Somatic Mutations in Human Cancer. Nucleic Acids Res. 2015, 43, D805–D811. [Google Scholar] [CrossRef]
- Lin, A.; Qi, C.; Wei, T.; Li, M.; Cheng, Q.; Liu, Z.; Luo, P.; Zhang, J. CAMOIP: A Web Server for Comprehensive Analysis on Multi-Omics of Immunotherapy in Pan-Cancer. Brief. Bioinform. 2022, 23, bbac129. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Sturm, G.; Finotello, F.; Petitprez, F.; Zhang, J.D.; Baumbach, J.; Fridman, W.H.; List, M.; Aneichyk, T. Comprehensive Evaluation of Transcriptome-Based Cell-Type Quantification Methods for Immuno-Oncology. Bioinformatics 2019, 35, i436–i445. [Google Scholar] [CrossRef] [PubMed]
- Plattner, C.; Finotello, F.; Rieder, D. Deconvoluting Tumor-Infiltrating Immune Cells from RNA-Seq Data Using quanTIseq. Methods Enzymol. 2020, 636, 261–285. [Google Scholar] [CrossRef]
- White, B.S.; de Reyniès, A.; Newman, A.M.; Waterfall, J.J.; Lamb, A.; Petitprez, F.; Lin, Y.; Yu, R.; Guerrero-Gimenez, M.E.; Domanskyi, S.; et al. Community Assessment of Methods to Deconvolve Cellular Composition from Bulk Gene Expression. Nat. Commun. 2024, 15, 7362. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Li, K.; Yang, H.; Lin, A.; Xie, J.; Wang, H.; Zhou, J.; Carr, S.R.; Liu, Z.; Li, X.; Zhang, J.; et al. CPADS: A Web Tool for Comprehensive Pancancer Analysis of Drug Sensitivity. Brief. Bioinform. 2024, 25, bbae237. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Amicuzi, U.; Musone, M.; Magliocchetti, M.; Di Lieto, D.; Tammaro, S.; Pastore, A.L.; Fuschi, A.; Falabella, R.; Ferro, M.; et al. Liquid Biopsy: Current Advancements in Clinical Practice for Bladder Cancer. J. Liq. Biopsy 2025, 9, 100310. [Google Scholar] [CrossRef]
- Wen, R.M.; Stark, J.C.; Marti, G.E.W.; Fan, Z.; Lyu, A.; Garcia Marques, F.J.; Zhang, X.; Riley, N.M.; Totten, S.M.; Bermudez, A.; et al. Sialylated Glycoproteins Suppress Immune Cell Killing by Binding to Siglec-7 and Siglec-9 in Prostate Cancer. J. Clin. Investig. 2024, 134, e180282. [Google Scholar] [CrossRef]
- Ma, Z.; Hao, X.; Qu, S.; Zhang, Q.; Luo, J.; Li, H.; Liu, J.; Dai, W.; Li, J.; Gu, S.; et al. Siglec-15 Antibody–GM-CSF Chimera Suppresses Tumor Progression via Reprogramming Tumor-Associated Macrophages. J. Immunother. Cancer 2025, 13, e010580. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an Immune Suppressor and Potential Target for Normalization Cancer Immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Peng, J.; Lu, C.; Zhang, M.; Qi, X.; Zhang, M.; Wang, Y. Identification of Siglec-10 as a New Dendritic Cell Checkpoint for Cervical Cancer Immunotherapy. J. Immunother. Cancer 2024, 12, e009404. [Google Scholar] [CrossRef]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’Altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.C.; Macauley, M.S.; Kawasaki, N. Siglecs as Sensors of Self in Innate and Adaptive Immune Responses. Ann. N. Y Acad. Sci. 2012, 1253, 37–48. [Google Scholar] [CrossRef]
- Varki, A.; Angata, T. Siglecs—The Major Subfamily of I-Type Lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef] [PubMed]
- Journo, G.; Ahuja, A.; Dias-Polak, D.; Eran, Y.; Bergman, R.; Shamay, M. Global CpG DNA Methylation Footprint in Kaposi’s Sarcoma. Front. Cell Infect. Microbiol. 2021, 11, 666143. [Google Scholar] [CrossRef]
- Anić, P.; Golubić Talić, J.; Božinović, K.; Dediol, E.; Mravak-Stipetić, M.; Grce, M.; Milutin Gašperov, N. Methylation of Immune Gene Promoters in Oral and Oropharyngeal Cancer. Int. J. Mol. Sci. 2023, 24, 7698. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Wang, X.; Zeng, Z.; Huang, Z.; Zhang, L.; Wei, F.; Ren, X.; Yang, L. Expression Signature, Prognosis Value, and Immune Characteristics of Siglec-15 Identified by Pan-Cancer Analysis. OncoImmunology 2020, 9, 1807291. [Google Scholar] [CrossRef]
- Ciccarese, C.; Massari, F.; Blanca, A.; Tortora, G.; Montironi, R.; Cheng, L.; Scarpelli, M.; Raspollini, M.R.; Vau, N.; Fonseca, J.; et al. Tp53 and Its Potential Therapeutic Role as a Target in Bladder Cancer. Expert. Opin. Ther. Targets 2017, 21, 401–414. [Google Scholar] [CrossRef]
- Qiu, Y.; Ke, S.; Chen, J.; Qin, Z.; Zhang, W.; Yuan, Y.; Meng, D.; Zhao, G.; Wu, K.; Li, B.; et al. FOXP3+ Regulatory T Cells and the Immune Escape in Solid Tumours. Front. Immunol. 2022, 13, 982986. [Google Scholar] [CrossRef]
- Shao, L.; Hou, W.; Scharping, N.E.; Vendetti, F.P.; Srivastava, R.; Roy, C.N.; Menk, A.V.; Wang, Y.; Chauvin, J.-M.; Karukonda, P.; et al. IRF1 Inhibits Antitumor Immunity through the Upregulation of PD-L1 in the Tumor Cell. Cancer Immunol. Res. 2019, 7, 1258–1266. [Google Scholar] [CrossRef]
- De Streel, G.; Lucas, S. Targeting Immunosuppression by TGF-Β1 for Cancer Immunotherapy. Biochem. Pharmacol. 2021, 192, 114697. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Cheng, C.-Y.; Chen, S.-P.; Lin, C.-Y.; Chang, C.-R.; Lin, W.-W. CASK Promotes Prostate Cancer Progression via Kinase-Dependent Activation of AKT. Int. J. Biol. Macromol. 2025, 311, 143965. [Google Scholar] [CrossRef]
- Szeto, G.L.; Finley, S.D. Integrative Approaches to Cancer Immunotherapy. Trends Cancer 2019, 5, 400–410. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Hrynkiewicz, R.; Grywalska, E.; Suchojad, T.; Leksowski, T.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunotherapy in Bladder Cancer: Current Methods and Future Perspectives. Cancers 2020, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Chen, J.; Fu, P. HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling. Cancers 2017, 9, 44. [Google Scholar] [CrossRef]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eckstein, M.; Eidt, S.; Denzinger, S.; Burger, M.; et al. In Stage pT1 Non-Muscle-Invasive Bladder Cancer (NMIBC), High KRT20 and Low KRT5 mRNA Expression Identify the Luminal Subtype and Predict Recurrence and Survival. Virchows Arch. 2017, 470, 267–274. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Ying, J.; Sun, Y.; Liu, J.; Yin, G. KRT6A Expedites Bladder Cancer Progression, Regulated by miR-31-5p. Cell Cycle 2022, 21, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yao, D.; Ma, X.; Hou, J.; Tian, J. A Novel Efferocytosis-Related Gene Signature for Predicting Prognosis and Therapeutic Response in Bladder Cancer. Sci. Rep. 2025, 15, 19912. [Google Scholar] [CrossRef]
- Tai, G.; Ranjzad, P.; Marriage, F.; Rehman, S.; Denley, H.; Dixon, J.; Mitchell, K.; Day, P.J.R.; Woolf, A.S. Cytokeratin 15 Marks Basal Epithelia in Developing Ureters and Is Upregulated in a Subset of Urothelial Cell Carcinomas. PLoS ONE 2013, 8, e81167. [Google Scholar] [CrossRef]
- Plimack, E.R.; Hoffman-Censits, J.H.; Viterbo, R.; Trabulsi, E.J.; Ross, E.A.; Greenberg, R.E.; Chen, D.Y.T.; Lallas, C.D.; Wong, Y.-N.; Lin, J.; et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin Is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study With Molecular Correlates of Response and Toxicity. JCO 2014, 32, 1895–1901. [Google Scholar] [CrossRef]
- Appleman, L.J.; Beumer, J.H.; Jiang, Y.; Lin, Y.; Ding, F.; Puhalla, S.; Swartz, L.; Owonikoko, T.K.; Donald Harvey, R.; Stoller, R.; et al. Phase 1 Study of Veliparib (ABT-888), a Poly (ADP-Ribose) Polymerase Inhibitor, with Carboplatin and Paclitaxel in Advanced Solid Malignancies. Cancer Chemother. Pharmacol. 2019, 84, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Saha, D.; Martuza, R.L.; Rabkin, S.D.; Wakimoto, H. Single Agent Efficacy of the VEGFR Kinase Inhibitor Axitinib in Preclinical Models of Glioblastoma. J. Neuro-Oncol. 2015, 121, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Komoike, Y. Experimental Evidence Shows Salubrinal, an eIF2α Dephosphorylation Inhibitor, Reduces Xenotoxicant-Induced Cellular Damage. Int. J. Mol. Sci. 2015, 16, 16275–16287. [Google Scholar] [CrossRef] [PubMed]



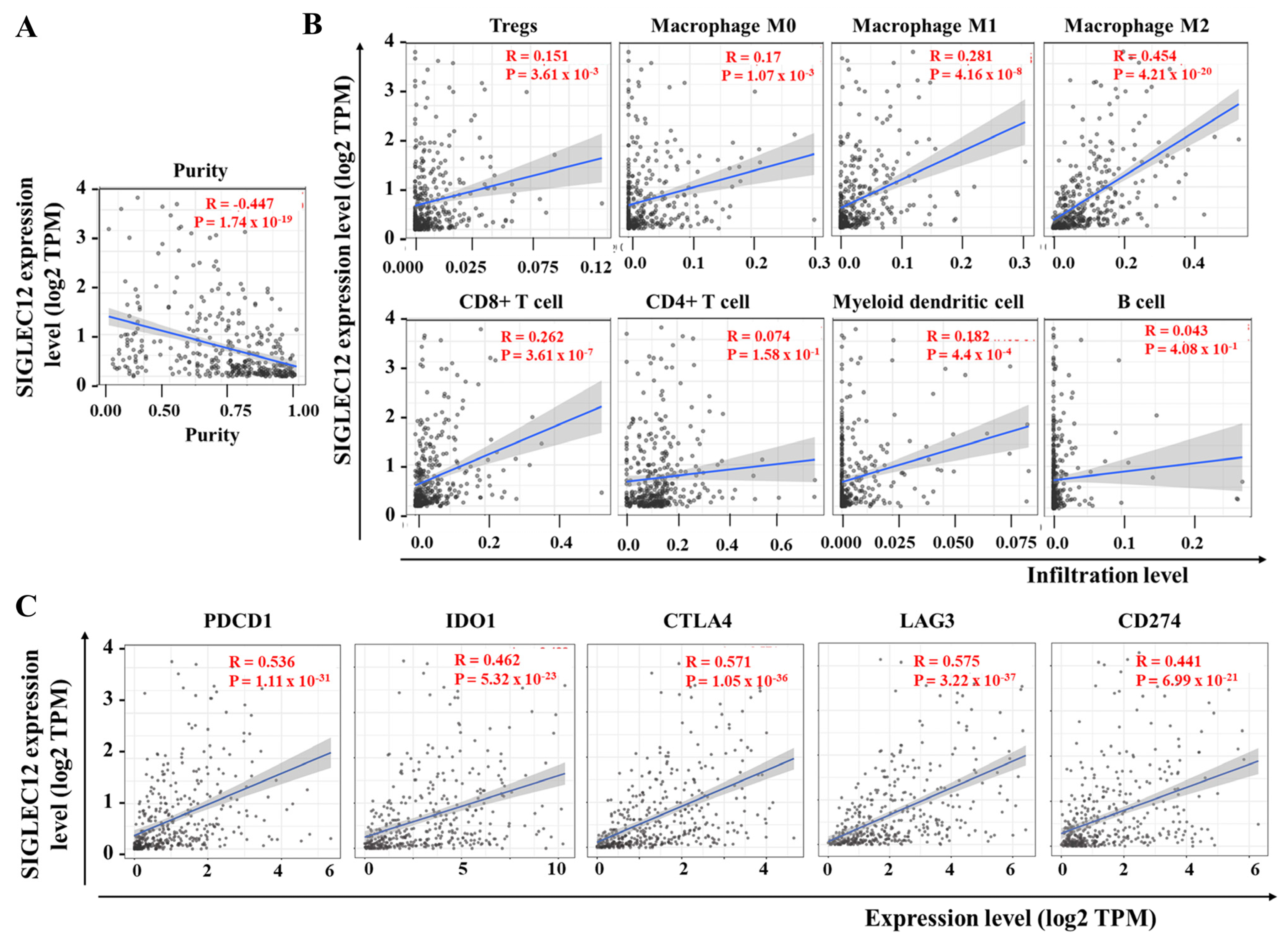

| Cell Type | Rho (R) | p-Value |
|---|---|---|
| Tregs | 0.293 | <0.001 |
| Macrophage M1 | 0.361 | <0.001 |
| Macrophage M2 | 0.256 | <0.001 |
| CD8+ T cell | 0.225 | <0.001 |
| CD4+ T cell | −0.04 | 0.4 |
| Myeloid dendritic cell | −0.175 | <0.001 |
| B cell | 0.03 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathore, V.; Lin, W.-W. Decoding SIGLEC12 in Bladder Cancer: In Silico Profiling of Expression, Tumor–Immune Interactions, and Prognostic Impact. Medicina 2025, 61, 1894. https://doi.org/10.3390/medicina61111894
Rathore V, Lin W-W. Decoding SIGLEC12 in Bladder Cancer: In Silico Profiling of Expression, Tumor–Immune Interactions, and Prognostic Impact. Medicina. 2025; 61(11):1894. https://doi.org/10.3390/medicina61111894
Chicago/Turabian StyleRathore, Varsha, and Wan-Wan Lin. 2025. "Decoding SIGLEC12 in Bladder Cancer: In Silico Profiling of Expression, Tumor–Immune Interactions, and Prognostic Impact" Medicina 61, no. 11: 1894. https://doi.org/10.3390/medicina61111894
APA StyleRathore, V., & Lin, W.-W. (2025). Decoding SIGLEC12 in Bladder Cancer: In Silico Profiling of Expression, Tumor–Immune Interactions, and Prognostic Impact. Medicina, 61(11), 1894. https://doi.org/10.3390/medicina61111894








