Lower Myeloperoxidase-ANCA Titres at Diagnosis Are Associated with End-Stage Kidney Disease Progression During Follow-Up in Rituximab-Treated Patients with Microscopic Polyangiitis
Abstract
1. Introduction
2. Materials and Methods
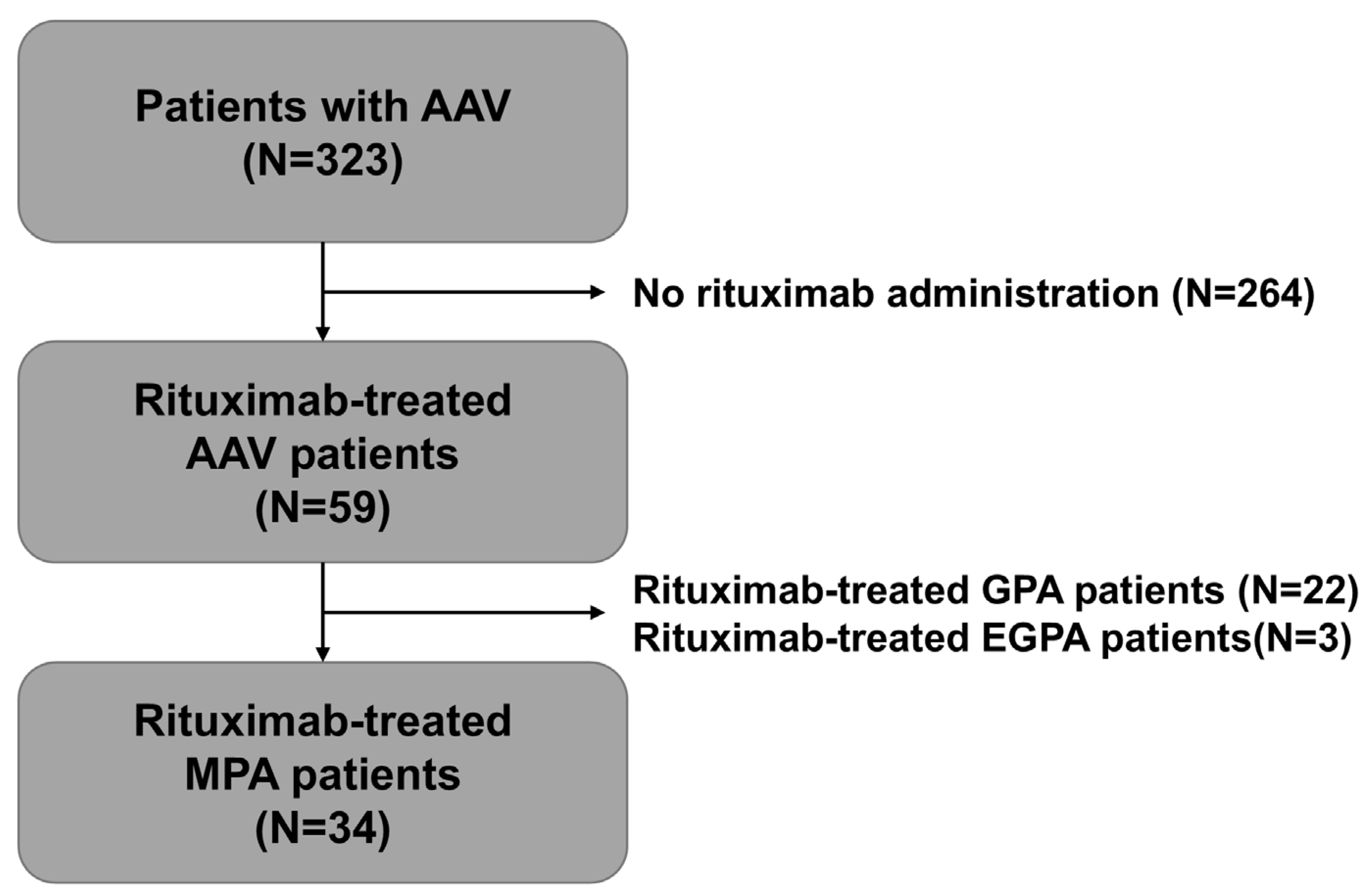
2.1. Study Patients
2.2. Ethical Statement
2.3. Clinical Data at AAV Diagnosis
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Study Subjects
3.2. Correlation Analysis
3.3. Cut-Off and Relative Risk for ESKD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | antineutrophil cytoplasmic antibody-associated vasculitis |
| ANCA | antineutrophil cytoplasmic antibody |
| AUC | area under the curve |
| BMI | body mass index |
| BVAS | the Birmingham vasculitis activity score |
| C | cytoplasmic |
| CI | confidence interval |
| ESKD | end-stage kidney disease |
| ESR | erythrocyte sedimentation rate |
| FFS | the five-factor score |
| GN | glomerulonephritis |
| HR | hazard ratio |
| IRB | institutional review board |
| MPA | microscopic polyangiitis |
| MPO | myeloperoxidase |
| P | perinuclear |
| PR3 | proteinase 3 |
| ROC | receiver operating characteristic |
| RR | relative risk |
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; A Merkel, P.; A Luqmani, R.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021, 73, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, S.; Tervaert, J.W.C.; Arimura, Y.; Bogdanos, D.P.; Csernok, E.; Damoiseaux, J.; Ferrante, M.; Flores-Suárez, L.F.; Fritzler, M.J.; Invernizzi, P.; et al. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun. Rev. 2020, 19, 102618. [Google Scholar] [CrossRef] [PubMed]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group. The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, S.; Novikov, P.; Jayne, D.; Mukhin, N. End-stage renal disease in ANCA-associated vasculitis. Nephrol. Dial. Transpl. Transplant. 2017, 32, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.C.; Zubidat, D.; Liebana, M.P.; Sethi, S.; Soler, M.J.; Zand, L.; dos Santos, F.G.; Nardelli, L.; Leon-Roman, J.; Sousa, C.; et al. Predictive Factors of Renal Recovery and Progression to End-Stage Kidney Disease in Patients With Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis With Severe Kidney Disease. Kidney Int. Rep. 2024, 9, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Nachman, P.H. ANCA Glomerulonephritis and Vasculitis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Koo, G.; Ha, J.W.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. Earliest total vascular damage index scores independently predict all-cause mortality in patients with ANCA-associated vasculitis. Clin. Exp. Rheumatol. 2024, 42, 795–802. [Google Scholar]
- Ha, J.W.; Kwon, O.C.; Park, Y.B.; Lee, S.W. A new formula consisting of the five-factor score and earliest vasculitis damage index at diagnosis for predicting poor outcomes of antineutrophil cytoplasmic antibody-associated vasculitis. Front. Med. 2025, 12, 1582892. [Google Scholar] [CrossRef] [PubMed]
- Ruth, A.-J.; Kitching, A.R.; Kwan, R.Y.; Odobasic, D.; Ooi, J.D.; Timoshanko, J.R.; Hickey, M.J.; Holdsworth, S.R. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.J.; Jennette, J.C. Rituximab in ANCA-associated disease. N. Engl. J. Med. 2010, 363, 285–286. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values |
|---|---|
| At the time of MPA diagnosis | |
| Demographic data | |
| Age (years) | 59.0 (50.8–71.3) |
| Male sex (N, (%)) | 10 (29.4) |
| Female sex (N, (%)) | 24 (70.6) |
| BMI (kg/m2) | 21.9 (20.2–25.3) |
| Ex-smoker (N, (%)) | 1 (2.9) |
| ANCA type and positivity (N, (%)) | |
| MPO-ANCA (or P-ANCA) positivity | 34 (100) |
| MPO-ANCA titre (IU/mL) | 104.0 (30.8–134.0) |
| PR3-ANCA (or C-ANCA) positivity | 1 (2.9) |
| AAV-specific indices | |
| BVAS | 15.5 (11.0–19.5) |
| FFS | 2.0 (1.0–2.0) |
| Acute-phase reactants | |
| ESR (mm/h) | 80.0 (42.0–107.0) |
| CRP (mg/L) | 9.3 (1.5–62.7) |
| Laboratory results | |
| White blood cell count (/mm3) | 7510.0 (5807.5–11,387.5) |
| Haemoglobin (g/dL) | 10.5 (8.8–12.1) |
| Platelet count (×1000/mm3) | 296.0 (234.0–372.0) |
| Fasting glucose (mg/dL) | 95.5 (89.0–131.3) |
| Blood urea nitrogen (mg/dL) | 22.2 (17.1–38.3) |
| Serum creatinine (mg/dL) | 1.4 (0.8–2.5) |
| Serum total protein (g/dL) | 6.4 (6.0–7.0) |
| Serum albumin (g/dL) | 3.4 (2.9–4.1) |
| Random urine protein/creatinine ratio | 1.1 (0.8–1.6) |
| Autoantibodies (N, (%)) | |
| Antinuclear antibody | 6 (17.6) |
| Anti-DNA | 0 (0) |
| Anti-RNP | 0 (0) |
| Anti-Sm | 0 (0) |
| Anti-Ro | 2 (5.9) |
| Anti-La | 1 (2.9) |
| Anti-Scl70 | 0 (0) |
| Anti-centromere | 0 (0) |
| Anti-GBM | 0 (0) |
| Comorbidities (N, (%)) | |
| T2DM | 5 (14.7) |
| Hypertension | 15 (44.1) |
| Dyslipidaemia | 7 (20.6) |
| During the follow-up duration | |
| ESKD (N, (%)) | 7 (20.6) |
| Follow-up duration based on ESKD (months) | 39.5 (18.3–76.3) |
| Medications administered | |
| Rituximab | 34 (100) |
| Glucocorticoids | 34 (100) |
| Mycophenolate mofetil | 19 (55.9) |
| Azathioprine | 22 (64.7) |
| Tacrolimus | 8 (23.5) |
| Methotrexate | 5 (14.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.C.; Ha, J.W.; Park, Y.-B.; Lee, S.-W. Lower Myeloperoxidase-ANCA Titres at Diagnosis Are Associated with End-Stage Kidney Disease Progression During Follow-Up in Rituximab-Treated Patients with Microscopic Polyangiitis. Medicina 2025, 61, 1892. https://doi.org/10.3390/medicina61111892
Kwon OC, Ha JW, Park Y-B, Lee S-W. Lower Myeloperoxidase-ANCA Titres at Diagnosis Are Associated with End-Stage Kidney Disease Progression During Follow-Up in Rituximab-Treated Patients with Microscopic Polyangiitis. Medicina. 2025; 61(11):1892. https://doi.org/10.3390/medicina61111892
Chicago/Turabian StyleKwon, Oh Chan, Jang Woo Ha, Yong-Beom Park, and Sang-Won Lee. 2025. "Lower Myeloperoxidase-ANCA Titres at Diagnosis Are Associated with End-Stage Kidney Disease Progression During Follow-Up in Rituximab-Treated Patients with Microscopic Polyangiitis" Medicina 61, no. 11: 1892. https://doi.org/10.3390/medicina61111892
APA StyleKwon, O. C., Ha, J. W., Park, Y.-B., & Lee, S.-W. (2025). Lower Myeloperoxidase-ANCA Titres at Diagnosis Are Associated with End-Stage Kidney Disease Progression During Follow-Up in Rituximab-Treated Patients with Microscopic Polyangiitis. Medicina, 61(11), 1892. https://doi.org/10.3390/medicina61111892






