The Role of FGFR2 as a Novel Biomarker for Treatment of Gastric Cancer—A Literature Review
Abstract
1. Introduction
2. Methods
2.1. Objectives
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Inclusion Criteria
- Studies published between January 2015 and April 2025.
- Studies published in English.
- Studies investigating FGFR2 expression, amplification, signaling pathways, therapeutic targeting, or clinical implications with relevance to gastric cancer.
- Preclinical studies (in vitro and in vivo models), clinical trials, case reports, and meta-analyses relevant to FGFR2 in gastric cancer.
- Review articles providing comprehensive insights into FGFR2’s role in gastric cancer.
2.5. Exclusion Criteria
- Studies not published in English
- Commentaries or editorials without significant primary data.
- Studies focused on FGFR2 in other malignancies without relevance to gastric cancer.
- Detection of FGFR2 amplification or mutations.
- Tumor heterogeneity of FGFR2 expression.
- Effect of FGFR2 on prognosis.
- Directed therapy targeting FGFR2.
- Resistance mechanisms and other challenges in targeted FGFR2 therapy.
3. Findings and Discussion from the Last Decade
3.1. FGFR2
3.2. FGFR2 in Cancer
4. Detection of FGFR2 Amplification or Mutations
4.1. Detection Methodology
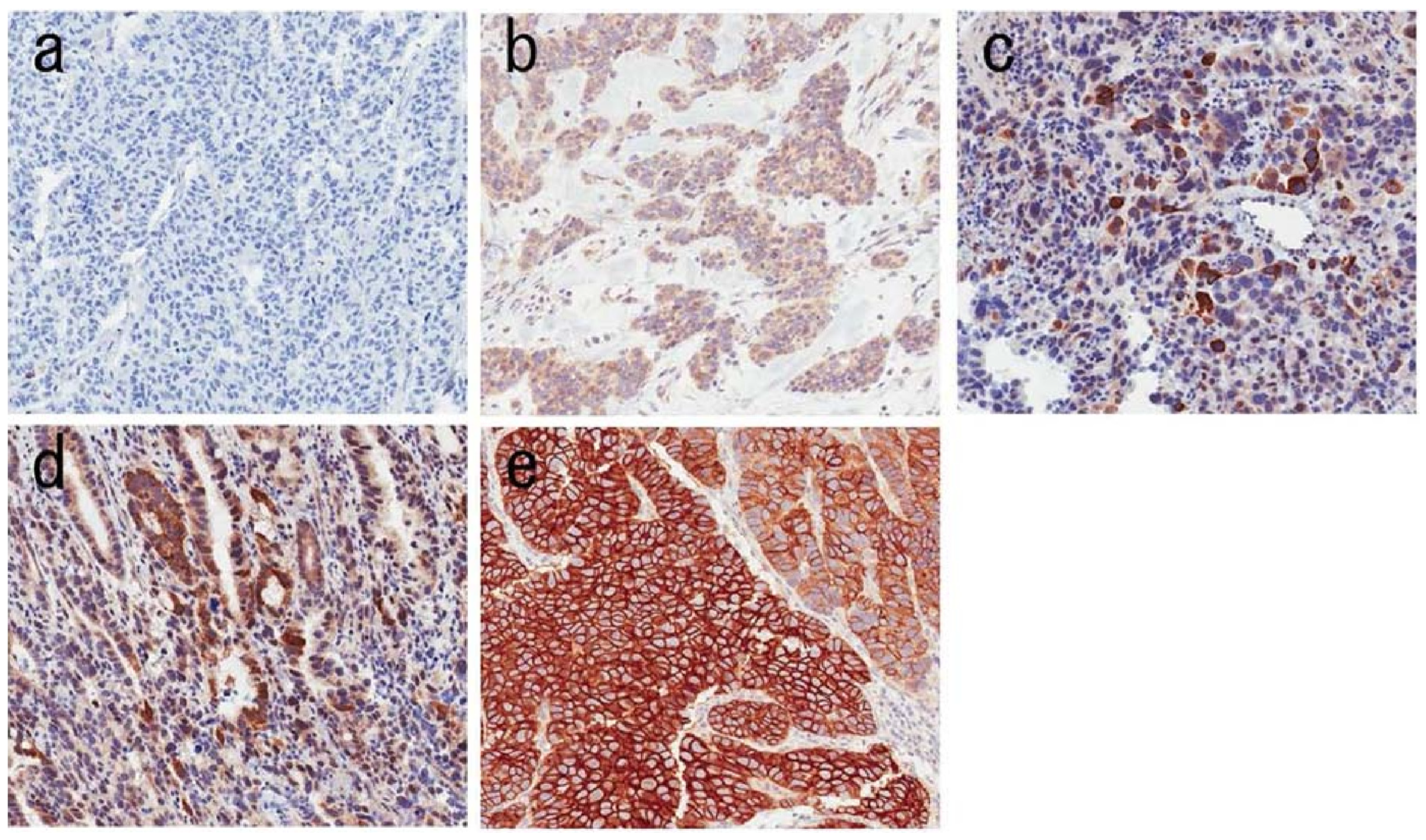
4.2. Prevalence of FGFR2 Amplification and Overexpression
4.3. Concurrent Markers and Co-Expression Studies
4.4. Molecular Mechanisms of FGFR2 Regulation
5. Tumor Heterogeneity of FGFR2b Expression
5.1. Intra-Tumoral Heterogeneity of FGFR2 Expression
5.2. Molecular and Genetic Heterogeneity
6. Prognostic Significance of FGFR2 Expression
6.1. FGFR2b Overexpression
6.2. FGFR2b Expression and Chemotherapy Response
7. Directed Therapy Targeting FGFR2
7.1. FGFR2-Targeted Tyrosine Kinase Inhibitors (TKIs)
7.1.1. AZD4547
7.1.2. Futibatinib
7.1.3. KIN-3248
7.1.4. Infigratinib
7.1.5. Pemigatinib
7.2. FGFR2-Targeting Antibodies and Antibody–Drug Conjugates
Bemarituzumab
8. Challenges in Targeted FGFR2 Therapy
Resistance to FGFR Inhibitors
9. Practical Approach
- Specimen collection
- IHC
- <10% of tumor cells staining weakly;
- ≥10% of tumor cells staining weakly;
- ≥10% but <50% of tumor cells staining strongly;
- ≥50% of cells staining strongly.
- FISH and RNA ISH
- Liquid biopsy
- Specimen type: Biopsy (six samples).
- Assay performed: Immunohistochemistry (IHC) for FGFR2b.
- Percentage of positive tumor cells: 25%.
- Staining intensity/score: 3+ (strong membranous staining in ≥10% but <50% of tumor cells or ≥50% weak–moderate staining).
- Staining distribution: Diffuse, strong.
- Nuclear/cytoplasmic staining: Predominantly cytoplasmic (membranous staining also observed).
- Interpretation/Result: Positive (3+).
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEN10 | Centromere 10 |
| CLDN18.2 | Claudin 18.2 |
| CTCs | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| DISH | Dual-color in situ hybridization |
| DLT | Dose-limiting toxicity |
| EGFR | Epidermal growth factor receptor |
| FGF | Fibroblast growth factor |
| FGFR2 | Fibroblast growth factor receptor 2 |
| FISH | Fluorescence in situ hybridization |
| GC | Gastric cancer |
| HER2 | Human epidermal growth factor receptor 2 |
| IHC | Immunohistochemistry |
| ISH | In situ hybridization |
| MAPK | Mitogen-activated protein kinase |
| mAb | Monoclonal antibody |
| mFOLFOX6 | Modified FOLFOX6 regimen (oxaliplatin, folinic acid, fluorouracil) |
| MSI | Microsatellite instability |
| NGS | Next-generation sequencing |
| ORR | Objective response rate |
| OS | Overall survival |
| PCR | Polymerase chain reaction |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RTK | Receptor tyrosine kinase |
| STAT | Signal transducer and activator of transcription |
| TMA | Tissue microarray |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Tan, X.; He, W.; Zhao, L.; Liu, H.; Li, G. Long-term relative survival of patients with gastric cancer from a large-scale cohort: A period-analysis. BMC Cancer 2024, 24, 1420. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okamoto, K.; Kawano, Y.; Kasai, A.; Kawaguchi, T.; Sagawa, T.; Sogabe, M.; Miyamoto, H.; Takayama, T. Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. J. Clin. Med. 2023, 12, 4646. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H. New therapeutic target molecules for gastric and gastroesophageal junction cancer. Int. J. Clin. Oncol. 2024, 29, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H. Emerging Targets for Systemic Treatment of Gastric Cancer: HER2 and Beyond. J. Gastric Cancer 2024, 24, 29–56. [Google Scholar] [CrossRef]
- Ilson, D.H. How to use anti-PD-1 therapy in gastric cancer: The approach in the United States. Chin. Clin. Oncol. 2024, 13, 7. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, T.Y.; Nam, S.K.; Hwang, H.J.; Han, D.; Oh, H.J.; Kong, S.H.; Park, D.J.; Oh, D.Y.; Lee, H.J.; et al. Clinicopathologic and molecular characterization of stages II–IV gastric cancer with Claudin 18.2 expression. Oncologist 2025, 30, oyae238. [Google Scholar] [CrossRef]
- Lucas, F.A.M.; Cristovam, S.N. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Hahne, J.C.; Passalacqua, R.; Valeri, N. Microsatellite instability in gastric cancer: Molecular bases, clinical perspectives, and new treatment approaches. Cell. Mol. Life Sci. 2018, 75, 4151–4162. [Google Scholar] [CrossRef]
- Cho, Y.; Ahn, S.; Kim, K.M. PD-L1 as a Biomarker in Gastric Cancer Immunotherapy. J. Gastric Cancer 2025, 25, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, Y.; Liu, H.; Wang, Y.; Zhao, S.; Xuan, Q.; Zhang, Q. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: A meta-analysis of 10 studies with 1,901 patients. Sci. Rep. 2016, 6, 37933. [Google Scholar] [CrossRef]
- Yao, J.; Sun, Q.; Wu, H.; Zhao, X.; Yang, P.; Wang, X.; Wang, X.; Gu, M.; Li, J.; Zheng, Y.; et al. Decoding the molecular landscape: HER2 and PD-L1 in advanced gastric cancer. Front. Immunol. 2025, 16, 1567308. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Aydın, E.; Tokat, Ü.M.; Özgü, E.; Adibi, A. Navigating uncharted territory: A case report and literature review on the remarkable response to personalized crizotinib containing combinational therapy in a pazopanib refractory patient with novel alterations. Ther. Adv. Med. Oncol. 2024, 16, 17588359241247023. [Google Scholar] [CrossRef]
- Plotnikov, A.N.; Hubbard, S.R.; Schlessinger, J.; Mohammadi, M. Crystal Structures of Two FGF-FGFR Complexes Reveal the Determinants of Ligand-Receptor Specificity. Cell 2000, 101, 413–424. [Google Scholar] [CrossRef]
- Katoh, M. FGFR2 (Fibroblast Growth Factor Receptor 2). Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2008. Available online: https://atlasgeneticsoncology.org/gene/40570/fgfr2-(fibroblast-growth-factor-receptor-2) (accessed on 22 March 2025).
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Zingg, D.; Bhin, J.; Yemelyanenko, J.; Kas, S.M.; Rolfs, F.; Lutz, C.; Lee, J.K.; Klarenbeek, S.; Silverman, I.M.; Annunziato, S.; et al. Truncated FGFR2 is a clinically actionable oncogene in multiple cancers. Nature 2022, 608, 609–617. [Google Scholar] [CrossRef]
- Rha, S.Y.; Zhang, Y.; Elme, A.; Pazo Cid, R.; Alacacioglu, A.; Ziogas, D.C.; Shitara, K.; Ranceva, A.; Nemecek, R.; Santoro, A.; et al. Prevalence of FGFR2b Protein Overexpression in Advanced Gastric Cancers During Prescreening for the Phase III FORTITUDE-101 Trial. JCO Precis. Oncol. 2025, 9, e2400710. [Google Scholar] [CrossRef]
- Carter, J.H.; Cottrell, C.E.; McNulty, S.N.; Vigh-Conrad, K.A.; Lamp, S.; Heusel, J.W.; Duncavage, E.J. FGFR2 amplification in colorectal adenocarcinoma. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001495. [Google Scholar] [CrossRef] [PubMed]
- Kunii, K.; Davis, L.; Gorenstein, J.; Hatch, H.; Yashiro, M.; Di Bacco, A.; Elbi, C.; Lutterbach, B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008, 68, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.; Hong, M.; Kim, S.T.; Park, S.H.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; Kim, S.; Jung, S.H.; et al. FGFR2 in gastric cancer: Protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod. Pathol. 2016, 29, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Young Kim, S.; Ahn, T.; Bang, H.; Soo Ham, J.; Kim, J.; Tae Kim, S.; Jang, J.; Shim, M.; Young Kang, S.; Hoon Park, S.; et al. Acquired resistance to LY2874455 in FGFR2-amplified gastric cancer through an emergence of novel FGFR2-ACSL5 fusion. Oncotarget 2017, 8, 15014. [Google Scholar]
- Park, Y.S.; Na, Y.S.; Ryu, M.H.; Lee, C.W.; Park, H.J.; Lee, J.K.; Park, S.R.; Ryoo, B.Y.; Kang, Y.K. FGFR2 assessment in gastric cancer using quantitative real-time polymerase chain reaction, fluorescent in Situ Hybridization, and Immunohistochemistry. Am. J. Clin. Pathol. 2015, 143, 865–872. [Google Scholar] [CrossRef]
- Minashi, K.; Yamada, T.; Hosaka, H.; Amagai, K.; Shimizu, Y.; Kiyozaki, H.; Sato, M.; Soeda, A.; Endo, S.; Ishida, H.; et al. Cancer-related FGFR2 overexpression and gene amplification in Japanese patients with gastric cancer. Jpn. J. Clin. Oncol. 2021, 51, 1523–1533. [Google Scholar] [CrossRef]
- Kuboki, Y.; Schatz, C.A.; Koechert, K.; Schubert, S.; Feng, J.; Wittemer-Rump, S.; Ziegelbauer, K.; Krahn, T.; Nagatsuma, A.K.; Ochiai, A. In situ analysis of FGFR2 mRNA and comparison with FGFR2 gene copy number by dual-color in situ hybridization in a large cohort of gastric cancer patients. Gastric Cancer 2018, 21, 401–412. [Google Scholar] [CrossRef]
- Kuroda, K.; Yashiro, M.; Miki, Y.; Sera, T.; Yamamoto, Y.; Sugimoto, A.; Nishimura, S.; Kushiyama, S.; Togano, S.; Okuno, T.; et al. Circulating tumor cells with FGFR2 expression might be useful to identify patients with existing FGFR2-overexpressing tumor. Cancer Sci. 2020, 111, 4500–4509. [Google Scholar] [CrossRef]
- Jogo, T.; Nakamura, Y.; Shitara, K.; Bando, H.; Yasui, H.; Esaki, T.; Terazawa, T.; Satoh, T.; Shinozaki, E.; Nishina, T.; et al. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin. Cancer Res. 2021, 27, 5619–5627. [Google Scholar] [CrossRef]
- Shariff, B.; Barnett, R.M.; Dayyani, F.; Maron, S.B.; McGriskin, R.; Klempner, S.; Donderici, E.Y.; Zhang, N.; Masannat, J.; Drusbosky, L.M.; et al. Circulating tumor DNA molecular analyses and real-world evidence outcomes of FGFR2 amplified gastroesophageal cancers. Oncologist 2024, 29, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, L.; Pang, Z.; Appelman, H.D.; Kuick, R.; Beer, D.G.; Li, M.; Wang, T.D. Identification and validation of FGFR2 peptide for detection of early Barrett’s neoplasia. Oncotarget 2017, 8, 87095. [Google Scholar] [CrossRef]
- Shoji, H.; Yamada, Y.; Okita, N.; Takashima, A.; Honma, Y.; Iwasa, S.; Kato, K.; Hamaguchi, T.; Shimada, Y. Amplification of FGFR2 Gene in Patients with Advanced Gastric Cancer Receiving Chemotherapy: Prevalence and Prognostic Significance. Anticancer Res. 2015, 35, 5055. [Google Scholar] [PubMed]
- Lau, D.K.; Collin, J.P.; Mariadason, J.M. Clinical Developments and Challenges in Treating FGFR2-Driven Gastric Cancer. Biomedicines 2024, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yang, J.; Wang, Y.; Xu, J.; Wang, X.; Du, F.; Hu, X.; Guo, H.; Song, C.; Tao, R.; et al. Comprehensive identification of FGFR1-4 alterations in 5 557 Chinese patients with solid tumors by next-generation sequencing. Am. J. Cancer Res. 2021, 11, 3893–3906. [Google Scholar]
- Jia, Y.X.; Li, T.F.; Zhang, D.D.; Fan, Z.M.; Fan, H.J.; Yan, J.; Chen, L.J.; Tang, H.; Qin, Y.R.; Li, X.Y. The coexpression and prognostic significance of c-MET, fibroblast growth factor receptor 2, and human epidermal growth factor receptor 2 in resected gastric cancer: A retrospective study. Onco Targets Ther. 2016, 9, 5919–5929. [Google Scholar] [CrossRef]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef]
- Byeon, S.; Jung, J.; Kim, S.T.; Kim, K.M.; Lee, J. Clinical Implication of Concurrent Amplification of MET and FGFR2 in Metastatic Gastric Cancer. Biomedicines 2023, 11, 3172. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.Y.; Kim, H.J.; Kim, K.M.; Choi, E.Y.; Kang, M.S. A reciprocal regulatory circuit between CD44 and FGFR2 via c-myc controls gastric cancer cell growth. Oncotarget 2016, 7, 28670–28683. [Google Scholar] [CrossRef]
- Tajirika, T.; Tokumaru, Y.; Taniguchi, K.; Sugito, N.; Matsuhashi, N.; Futamura, M.; Yanagihara, K.; Akao, Y.; Yoshida, K. DEAD-box protein RNA-helicase DDX6 regulates the expression of HER2 and FGFR2 at the post-transcriptional step in gastric cancer cells. Int. J. Mol. Sci. 2018, 19, 2005. [Google Scholar] [CrossRef] [PubMed]
- Aleyasin, S.A.; Moradi, A.; Abolhasani, N.; Abdollahi, M. Investigating FGFR2 gene as a blood-based epigenetic biomarker in gastric cancer. Mol. Biol. Rep. 2024, 51, 253. [Google Scholar] [CrossRef]
- Teles, S.P.; Oliveira, P.; Ferreira, M.; Carvalho, J.; Ferreira, P.; Oliveira, C. Integrated analysis of structural variation and rna expression of fgfr2 and its splicing modulator esrp1 highlight the esrp1amp-fgfr2norm-fgfr2-iiichigh axis in diffuse gastric cancer. Cancers 2020, 12, 70. [Google Scholar] [CrossRef]
- Ishiwata, T. Role of fibroblast growth factor receptor-2 splicing in normal and cancer cells. Front. Biosci. 2018, 23, 626–639. [Google Scholar] [CrossRef]
- Albin, J.; Fahrig, L.; Siemanowski, J.; Rehkaemper, J.; Gebauer, F.; Zander, T.; Buettner, R.; Bruns, C.J.; Schroeder, W.; Alakus, H.; et al. FGFR2-amplified tumor clones are markedly heterogeneously distributed in carcinomas of the upper gastrointestinal tract. J. Cancer Res. Clin. Oncol. 2023, 149, 5289–5300. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, M.H.; Lee, I.S.; Ahn, J.Y.; Lee, J.H.; Lee, H.; Kim, H.D.; Park, Y.S. Low positivity rate of fibroblast growth factor receptor 2b is associated with heterogeneous expression in gastric cancer. Gastric Cancer 2025, 28, 598–608. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, M.; Fan, S.; Zhang, T.; Fu, H.; Su, X.; Gavine, P.R.; Liu, Q.; Yin, X. Intra-tumoral heterogeneity of HER2, FGFR2, cMET and ATM in gastric cancer: Optimizing personalized healthcare through innovative pathological and statistical analysis. PLoS ONE 2015, 10, e0143207. [Google Scholar] [CrossRef]
- Okuda, T.; Taki, T.; Nishida, K.; Chinen, Y.; Nagoshi, H.; Sakakura, C.; Taniwaki, M. Molecular heterogeneity in the novel fusion gene APIP-FGFR2: Diversity of genomic breakpoints in gastric cancer with high-level amplifications at 11p13 and 10q26. Oncol. Lett. 2017, 13, 215–221. [Google Scholar] [CrossRef]
- Hyung, S.; Han, B.; Jung, J.; Kim, S.T.; Hong, J.Y.; Park, S.H.; Zang, D.Y.; Park, J.O.; Park, Y.S.; Kim, K.M.; et al. Incidence of FGFR2 Amplification and FGFR2 Fusion in Patients with Metastatic Cancer Using Clinical Sequencing. J. Oncol. 2022, 2022, 9714570. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, M.; Murase, H.; Otsuki, S.; Kawano, T.; Kojima, K. Different clinical significance of FGFR1-4 expression between diffuse-type and intestinal-type gastric cancer. World J. Surg. Oncol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiong, D.; Xiao, R.; Huang, Z. Prognostic role of fibroblast growth factor receptor 2 in human solid tumors: A systematic review and meta-analysis. Tumor Biol. 2017, 39, 1010428317707424. [Google Scholar] [CrossRef]
- Hosoda, K.; Yamashita, K.; Ushiku, H.; Ema, A.; Moriya, H.; Mieno, H.; Washio, M.; Watanabe, M. Prognostic relevance of FGFR2 expression in stage II/III gastric cancer with curative resection and S-1 chemotherapy. Oncol. Lett. 2018, 15, 1853–1860. [Google Scholar] [PubMed]
- Skupinska, M.M.; Jesiotr, M.; Chrom, P.; Mroz, A.; Cierniak, S.; Winiarek, M.; Wyrwicz, L.S.; Pieczykolan, J.; Wieczorek, M.; Stanczak, A.; et al. The role of FGFR2 amplification and expression in patients with advanced or metastatic gastric cancer receiving fluoropyrimidine-based chemotherapy. Ann. Oncol. 2018, 29, viii218. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Jang, H.J. Pathologic and prognostic impacts of FGFR2 amplification in gastric cancer: A meta-analysis and systemic review. J. Cancer 2019, 10, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, T.; Behrens, H.M.; Haag, J.; Krüger, S.; Röcken, C. FGFR2 overexpression and compromised survival in diffuse-type gastric cancer in a large central European cohort. PLoS ONE 2022, 17, e0264011. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, L.; Dian, L.; Piao, L.; Xiong, H.; Zhuang, L.; Li, S.; Yuan, X.; Hong, Q. FGF7/FGFR2 signal promotes invasion and migration in human gastric cancer through upregulation of thrombospondin-1. Int. J. Oncol. 2017, 50, 1501–1512. [Google Scholar] [CrossRef]
- Huang, T.; Liu, D.; Wang, Y.; Li, P.; Sun, L.; Xiong, H.; Dai, Y.; Zou, M.; Yuan, X.; Qiu, H. FGFR2 Promotes Gastric Cancer Progression by Inhibiting the Expression of Thrombospondin4 via PI3K-Akt-Mtor Pathway. Cell. Physiol. Biochem. 2018, 50, 1332–1345. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, T.; Wang, X.; Hu, J.; Yu, L.; Liu, Q.; Wu, N.; Liu, B.; Wei, J. FGFR2 alteration as a potential therapeutic target in poorly cohesive gastric carcinoma. J. Transl. Med. 2021, 19, 401. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.; Kang, S.Y.; Ahn, S.; Kim, K.M. Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden. Diagnostics 2022, 12, 2355. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, W.C.; Yu, C.L.; Chang, T.K.; I-Chin Wei, A.; Chang, T.M.; Liu, J.F.; Wang, S.W. FGF2 drives osteosarcoma metastasis through activating FGFR1-4 receptor pathway-mediated ICAM-1 expression. Biochem. Pharmacol. 2023, 218, 115853. [Google Scholar] [CrossRef]
- Tojjari, A.; Nagdas, S.; Saeed, A.; Saeed, A. Deciphering the FGFR2 Code: Innovative Targets in Gastric Cancer Therapy. Curr. Oncol. 2024, 31, 4305–4317. [Google Scholar] [CrossRef]
- Kim, S.T.; Ahn, S.; Lee, J.; Lee, S.J.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, K.M.; Park, J.O. Value of FGFR2 expression for advanced gastric cancer patients receiving pazopanib plus CapeOX (capecitabine and oxaliplatin). J. Cancer Res. Clin. Oncol. 2016, 142, 1231–1237. [Google Scholar] [CrossRef]
- Hur, J.Y.; Chao, J.; Kim, K.; Kim, S.T.; Kim, K.M.; Klempner, S.J.; Lee, J. High-level FGFR2 amplification is associated with poor prognosis and Lower response to chemotherapy in gastric cancers. Pathol. Res. Pract. 2020, 216, 152878. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kaida, D.; Tomita, Y.; Miyata, T.; Miyashita, T.; Fujita, H.; Kinami, S.; Ueda, N.; Takamura, H. Intra-tumoral FGFR2 Expression Predicts Prognosis and Chemotherapy Response in Advanced HER2-positive Gastric Cancer Patients. Cancer Diagn. Progn. 2022, 2, 293–299. [Google Scholar] [CrossRef]
- Seo, S.; Park, S.J.; Ryu, M.H.; Park, S.R.; Ryoo, B.Y.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, K.M.; Park, J.O. Prognostic impact of fibroblast growth factor receptor 2 gene amplification in patients receiving fluoropyrimidine and platinum chemotherapy for metastatic and locally advanced unresectable gastric cancers. Oncotarget 2017, 8, 33844. [Google Scholar] [CrossRef]
- Liu, K.; Song, X.; Zhu, M.; Ma, H. Overexpression of FGFR2 contributes to inherent resistance to MET inhibitors in MET-amplified patient-derived gastric cancer xenografts. Oncol. Lett. 2015, 10, 2003–2008. [Google Scholar] [CrossRef]
- Jang, J.; Kim, H.K.; Bang, H.; Kim, S.T.; Kim, S.Y.; Park, S.H.; Lim, H.Y.; Kang, W.K.; Lee, J.; Kim, K.M. Antitumor Effect of AZD4547 in a Fibroblast Growth Factor Receptor 2–Amplified Gastric Cancer Patient–Derived Cell Model. Transl. Oncol. 2017, 10, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Smyth, E.; Babina, I.S.; Herrera-Abreu, M.T.; Tarazona, N.; Peckitt, C.; Kilgour, E.; Smith, N.R.; Geh, C.; Rooney, C.; et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 2016, 6, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Bang, Y.J.; Mansoor, W.; Petty, R.D.; Chao, Y.; Cunningham, D.; Ferry, D.R.; Smith, N.R.; Frewer, P.; Ratnayake, J.; et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 2017, 28, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib is a novel irreversible FGFR 1-4 inhibitor that shows selective antitumor activity against FGFR-deregulated tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Kojima, T.; Kuboki, Y.; Matsubara, N.; Bando, H.; Yoh, K.; Naito, Y.; Hirai, H.; Kurokawa, Y.; et al. Phase I study of the irreversible fibroblast growth factor receptor 1–4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer Sci. 2023, 114, 574–585. [Google Scholar] [CrossRef]
- Javle, M.; King, G.; Spencer, K.; Borad, M.J. Futibatinib, an Irreversible FGFR1-4 Inhibitor for the Treatment of FGFR-Aberrant Tumors. Oncologist 2023, 28, 928–943. [Google Scholar] [CrossRef]
- Satoh, T.; Barthélémy, P.; Nogova, L.; Honda, K.; Hirano, H.; Lee, K.W.; Rha, S.Y.; Ryu, M.H.; Park, J.O.; Doi, T.; et al. Phase 2 study of futibatinib in patients with gastric or gastroesophageal junction cancer harboring FGFR2 amplifications. Eur. J. Cancer 2025, 218, 115262. [Google Scholar] [CrossRef]
- Quinzii, A.; Zecchetto, C.; Casalino, S.; Gaule, M.; Pesoni, C.; Merz, V.; Contarelli, S.; Pietrobono, S.; Benhadji, K.A.; Melisi, D. Clinical Response to Futibatinib in Patients with High-Level FGFR2-Amplified Advanced Gastric Cancer: Two Case Reports. Clin. Drug Investig. 2022, 42, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Garmezy, B.; Borad, M.J.; Bahleda, R.; Perez, C.A.; Chen, L.T.; Kato, S.; Oh, D.Y.; Severson, P.; Tam, B.Y.; Quah, C.S.; et al. A Phase I Study of KIN-3248, an Irreversible Small-molecule Pan-FGFR Inhibitor, in Patients with Advanced FGFR2/3-driven Solid Tumors. Cancer Res. Commun. 2024, 4, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shen, L.; Liu, T.; Xu, H.; Yang, J.; Wei, J.; Jiang, H.; Deng, Y.; Wang, Y.; Zhang, X.; et al. Efficacy and safety of infigratinib in locally advanced or metastatic gastric cancer or gastroesophageal junction adenocarcinoma patients with FGFR2 gene amplification. Ann. Oncol. 2023, 34, S858–S860. [Google Scholar] [CrossRef]
- Yuan, J.; Shen, L.; Liu, T.S.; Xu, H.T.; Yang, J.; Wei, J.; Jiang, H.; Deng, Y.; Pan, H.; Wang, Y.; et al. Pharmacokinetics of infigratinib and its active metabolites in Chinese patients with advanced gastric cancer harboring FGFR2 gene amplification. Clin. Transl. Sci. 2024, 17, e70091. [Google Scholar] [CrossRef]
- Shinomiya, R.; Sato, Y.; Yoshimoto, T.; Kawaguchi, T.; Hirao, A.; Okamoto, K.; Kawano, Y.; Sogabe, M.; Miyamoto, H.; Takayama, T. A case of treatment-resistant advanced gastric cancer with FGFR2 gene alteration successfully treated with pemigatinib. Int. Cancer Conf. J. 2024, 13, 240–244. [Google Scholar] [CrossRef]
- Tucker, N. FDA Grants Breakthrough Therapy Designation to Bemarituzumab for Select FGFR2b+/HER2− Advanced Gastric and GEJ Cancers. Targeted Oncology. 2021. Available online: https://www.targetedonc.com/view/fda-grants-breakthrough-therapy-designation-to-bemarituzumab-for-select-fgfr2b-her2--advanced-gastric-and-gej-cancers (accessed on 7 August 2025).
- Xiang, H.; Chan, A.G.; Ahene, A.; Bellovin, D.I.; Deng, R.; Hsu, A.W.; Jeffry, U.; Palencia, S.; Powers, J.; Zanghi, J.; et al. Preclinical characterization of bemarituzumab, an anti-FGFR2b antibody for the treatment of cancer. mAbs 2021, 13, 1981202. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Kang, Y.K.; Lee, K.W.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: Final analysis of the randomized phase 2 FIGHT trial. Gastric Cancer 2024, 27, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.K.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef]
- Gordon, A.; Johnston, E.; Lau, D.K.; Starling, N. Targeting FGFR2 Positive Gastroesophageal Cancer: Current and Clinical Developments. OncoTargets Ther. 2022, 15, 1183–1196. [Google Scholar] [CrossRef]
- Lau, W.M.; Teng, E.; Huang, K.K.; Tan, J.W.; Das, K.; Zang, Z.; Chia, T.; Teh, M.; Kono, K.; Yong, W.P.; et al. Acquired resistance to FGFR inhibitor in diffuse-type gastric cancer through an AKT-independent PKC-mediated phosphorylation of GSK3β. Mol. Cancer Ther. 2018, 17, 232–242. [Google Scholar] [CrossRef]
- Chen, J.; Bell, J.; Lau, B.T.; Whittaker, T.; Stapleton, D.; Ji, H.P. A functional CRISPR/Cas9 screen identifies kinases that modulate FGFR inhibitor response in gastric cancer. Oncogenesis 2019, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Sase, H.; Nakanishi, Y.; Aida, S.; Horiguchi-Takei, K.; Akiyama, N.; Fujii, T.; Sakata, K.; Mio, T.; Aoki, M.; Ishii, N. Acquired JHDM1D-BRAF fusion confers resistance to FGFR inhibition in FGFR2-amplified gastric cancer. Mol. Cancer Ther. 2018, 17, 2217–2225. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yashiro, M.; Fukuoka, T.; Hasegawa, T.; Morisaki, T.; Kasashima, H.; Masuda, G.; Noda, S.; Hirakawa, K. Diffuse-type gastric cancer cells switch their driver pathways from FGFR2 signaling to SDF1/CXCR4 axis in hypoxic tumor microenvironments. Carcinogenesis 2015, 36, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.S.; Le, C.; Wertheimer, A.; Escobedo, S.; So, L.; Siddegowda, B.B.; Palting, J.; Marion, S.; Stratton, S.P.; Aggeler, B. Precision Detection of FGFR2b Protein Expression in Solid Tumors: A Comprehensive Assessment of Staining Parameters via Automated Immunohistochemistry. Arch. Pathol. Lab. Med. 2025; in press. [Google Scholar] [CrossRef] [PubMed]


| Test Platform | Positivity Thresholds | Specimen | Clinical Endpoint Reported | Notes and References |
|---|---|---|---|---|
| FISH (fluorescence in situ hybridization) | Amplification defined as FGFR2/centromere 10 (CEN10) ratio > 2.10 | Biopsy, frozen, surgical specimens | Correlated with IHC and qPCR results; prognostic association with shorter OS in some cohorts | Park 2015 [25] showed biopsy equivalent/superior to surgical specimen. False negatives possible in heterogeneous tumors. |
| IHC (immunohistochemistry) | Scores defined as: 1- <10% of tumor cells staining weakly; 2- ≥10% of tumor cells staining weakly; 3- ≥10% but <50% of tumor cells staining strongly; 4- ≥50% of cells staining strongly | Biopsy, surgical specimens | Associated with amplification (validated vs. FISH); stronger positivity associated with poorer outcomes | Park 2015 [25]: strong correlation with FISH. Rha 2025 [20]: 16.2% >10% positive tumor cells → trial eligibility. Heterogeneity common (often <50% area staining). |
| qPCR | ≥8 FGFR2 copies correlated best with FISH (κ = 1.0, p < 0.001) ROC curve analysis found the optimal cutoff to be >6.71 | Frozen vs. FFPE tissue | Frozen DNA higher-quality; FFPE more degraded | Park 2015 [25]: Frozen > FFPE for sensitivity; biopsy > surgical tissue for DNA quality. |
| DISH (dual-color in situ hybridization) | FGFR2/CEN10 ≥ 2 | Tissue sections | Correlation between FGFR2 mRNA expression and gene amplification | Kuboki 2018 [27]: enabled spatial resolution, revealed intra-tumoral heterogeneity. |
| RNA in situ hybridization | Score ≥ 2 on 0–4 RNAscope scale | Tissue sections | FGFR2 mRNA correlated with gene amplification | Kuboki 2018 [27]. |
| ctDNA sequencing | Fold change ≥ 1.4 (Guardant360 panel) | Plasma samples | 7.7% positive via ctDNA (14/182) vs. 2.6–4.4% by tissue. ctDNA positivity linked to poorer survival and response to FGFR inhibitors | Jogo 2021 [29], Shariff 2024 [30]: ctDNA positive patients had poorer survival; ctDNA detected heterogeneity missed in biopsy. |
| CTCs (circulating tumor cells) | ≥5 FGFR2+ cells per 10 mL blood (FACS with Alexa 488 > 1000) | Blood samples | FGFR2+ CTCs correlated with worse recurrence-free survival; consistent with tissue FGFR2 status | Kuroda 2020 [28]. |
| NGS (next-generation sequencing) | No numeric cutoff specified; prevalence in GC = 7.9% | Panel-based (solid tumors) | Found FGFR2 alterations in 7.9% of gastric tumors (n = 5557, 9.1% amplifications, 2.8% mutations, 3.1% rearrangements) | Gu 2021 [34]; denominator given, but manuscript does not provide positivity thresholds. |
| Drug | Type | Clinical Trials/Studies (IDs) | Results | Adverse Effects | Status |
|---|---|---|---|---|---|
| AZD4547 | Selective FGFR2 TKI | Translational trial (Pearson 2016 [66]); SHINE (NCT01457846) phase II trial vs. paclitaxel | Tumors with high-level FGFR2 amplification showed partial responses (4/9 pts) No significant PFS benefit over paclitaxel despite being well-tolerated | Mostly mild; well-tolerated | Discontinued (no further dev.) |
| Futibatinib | Irreversible FGFR1–4 TKI | Phase I (NCT02052778; JapicCTI-142552); phase II (NCT04189445) | Tumor shrinkage in 58% of pts; ORR 17.9–26%; better response with higher FGFR2 copy numbers | Hyperphosphatemia as main adverse effect but no maximum tolerated dose was reached | Ongoing trials (NCT05945823, phase II trial) |
| KIN-3248 | Irreversible FGFR1–4 TKI | Phase I (NCT05242822) | 5/54 pts partial response (9.3%); terminated early for commercial reasons | Hyperphosphatemia, diarrhea, stomatitis; 1 hypersensitivity DLT | Discontinued (trial stopped early) |
| Infigratinib | FGFR1–3 TKI | Phase II (NCT05019794) | ORR 25%; DCR 80%; tumor shrinkage in 15/19 pts (max −78.5%) | Grade 3 TRAE 42.9%, most recoverable. No drug-induced death reported | Initially given accelerated approval by the FDA for cholangiocarcinoma in 2023 but withdrawn in 2024 |
| Pemigatinib | FGFR1–4 TKI | Case report (Shinomiya 2024) | Off-label use: tumor markers dropped, clinical improvement, then progression after 3 mo | Not detailed (short course) | Approved (cholangiocarcinoma); off-label in GC |
| Bemarituzumab | FGFR2b mAb | Phase I (safety, early efficacy); FIGHT (phase II, NCT03694522); FORTITUDE-101 (phase III, NCT05052801, ongoing) | FIGHT: PFS 9.5 vs. 7.4 mo; OS 19.2 vs. 13.5 mo; ORR 48.1% vs. 33% (bemarituzumab vs. control). | Manageable; improved over TKIs | Ongoing; FDA Breakthrough Therapy designation for FGFR2b positive GC (defined as >10% tumor cells staining) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lages dos Santos, J.; Caetano Oliveira, R.; Gama, J.M. The Role of FGFR2 as a Novel Biomarker for Treatment of Gastric Cancer—A Literature Review. Medicina 2025, 61, 1890. https://doi.org/10.3390/medicina61111890
Lages dos Santos J, Caetano Oliveira R, Gama JM. The Role of FGFR2 as a Novel Biomarker for Treatment of Gastric Cancer—A Literature Review. Medicina. 2025; 61(11):1890. https://doi.org/10.3390/medicina61111890
Chicago/Turabian StyleLages dos Santos, João, Rui Caetano Oliveira, and João Martins Gama. 2025. "The Role of FGFR2 as a Novel Biomarker for Treatment of Gastric Cancer—A Literature Review" Medicina 61, no. 11: 1890. https://doi.org/10.3390/medicina61111890
APA StyleLages dos Santos, J., Caetano Oliveira, R., & Gama, J. M. (2025). The Role of FGFR2 as a Novel Biomarker for Treatment of Gastric Cancer—A Literature Review. Medicina, 61(11), 1890. https://doi.org/10.3390/medicina61111890








