The Diagnostic and Prognostic Value of Reticulated Platelets in Ischemic Stroke: Is Immature Platelet Fraction a New Biomarker?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Laboratory Analysis
2.2. Radiological Imaging Method
2.3. Statistical Analysis
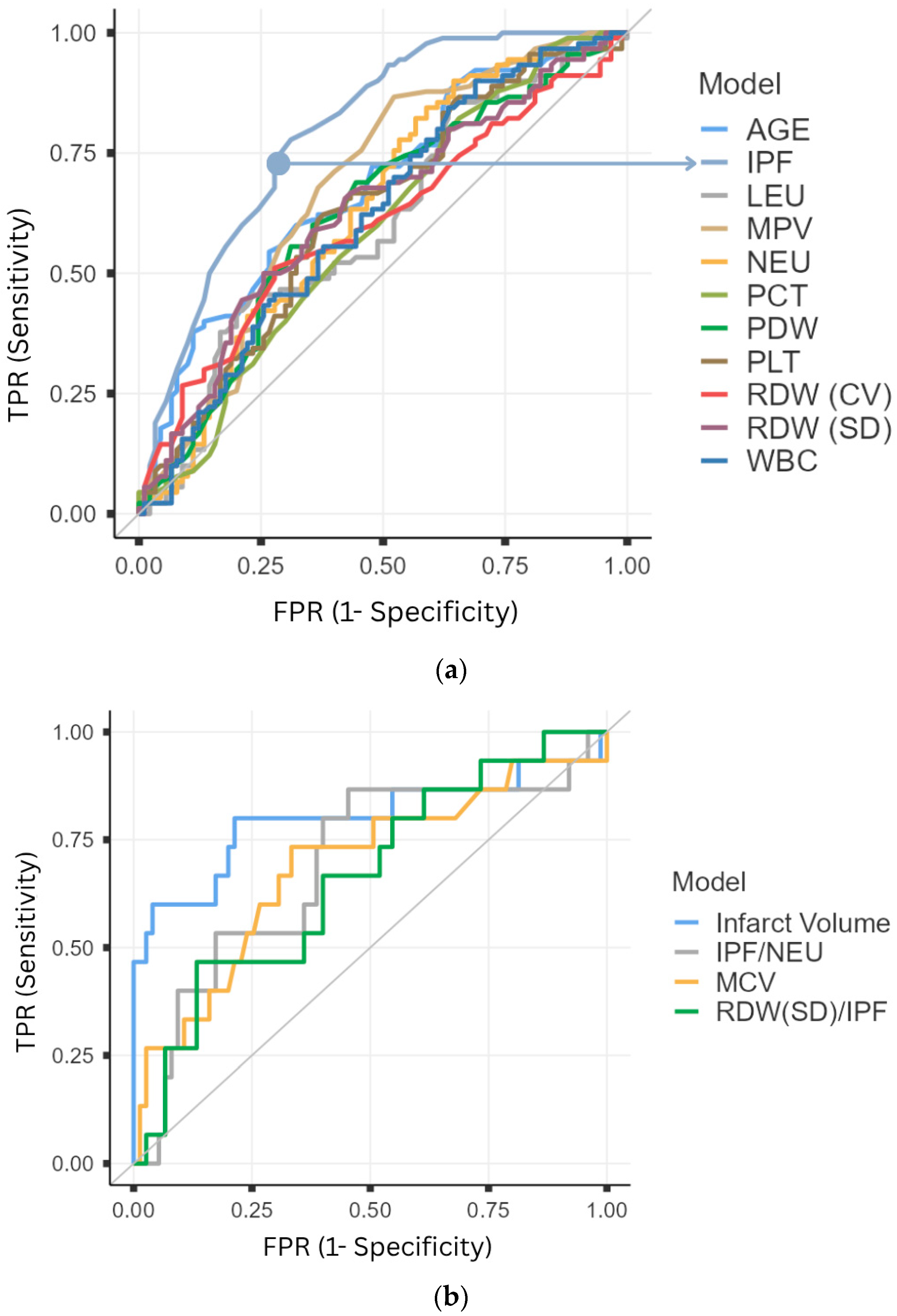
3. Results
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | Acute Ischemic Stroke |
| ED | Emergency Department |
| IPF | Immature Platelet Fraction |
| IV | Infarct volume |
| MCV | Mean Corpuscular Volume |
| NEU | Neutrophils |
| RDW | Red Cell Distribution Width |
| ROC | Receiver Operating Characteristic |
| RP | Reticulated Platelets |
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke Off. J. Int. Stroke Soc. 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- NCD Countdown 2030 collaborators. NCD Countdown 2030: Efficient pathways and strategic investments to accelerate progress towards the Sustainable Development Goal target 3.4 in low-income and middle-income countries. Lancet 2022, 399, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Ischemic Stroke. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499997 (accessed on 11 July 2025).
- Bangad, A.; Abbasi, M.; de Havenon, A. Secondary Ischemic Stroke Prevention. Neurother. J. Am. Soc. Exp. Neurother. 2023, 20, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Chen, S.; Chen, S.; Peng, Q.; Jin, H.; Hu, B. The role of leukocytes in acute ischemic stroke-related thrombosis: A notable but neglected topic. Cell. Mol. Life Sci. CMLS 2021, 78, 6251–6264. [Google Scholar] [CrossRef]
- Alhazzani, A.; Venkatachalapathy, P.; Padhilahouse, S.; Sellappan, M.; Munisamy, M.; Sekaran, M.; Sekaran, M.; Kumar, A. Biomarkers for Antiplatelet Therapies in Acute Ischemic Stroke: A Clinical Review. Front. Neurol. 2021, 12, 667234. [Google Scholar] [CrossRef]
- Scharf, R.E. Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1. Hamostaseologie 2018, 38, 203–210. [Google Scholar] [CrossRef]
- Tauseef, A.; Zafar, M.; Arshad, W.; Thirumalareddy, J.; Sood, A.; Farooque, U.; Nair, S.; Mirza, M. Role of immature platelet fraction (IPF) in sepsis patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 2148–2152. [Google Scholar] [CrossRef]
- Allan, H.E.; Vadgama, A.; Armstrong, P.C.; Warner, T.D. Platelet ageing: A review. Thromb. Res. 2023, 231, 214–222. [Google Scholar] [CrossRef]
- Gumiężna, K.; Baruś, P.; Sygitowicz, G.; Wiśniewska, A.; Ochijewicz, D.; Pasierb, K.; Klimczak-Tomaniak, D.; Kuca-Warnawin, E.; Kochman, J.; Grabowski, M.; et al. Immature platelet fraction in cardiovascular diagnostics and antiplatelet therapy monitoring. Cardiol. J. 2023, 30, 817–824. [Google Scholar] [CrossRef]
- Corpataux, N.; Franke, K.; Kille, A.; Valina, C.M.; Neumann, F.J.; Nührenberg, T.; Hochholzer, W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. J. Clin. Med. 2020, 9, 3737. [Google Scholar] [CrossRef]
- Tsankof, A.; Tsakiris, D.A.; Skoura, L.; Tsiatsiou, P.; Ztriva, E.; Ntaios, G.; Savopoulos, C.; Kaiafa, G. The Role of Immature Platelet Fraction and Reticulated Platelets in Stroke Monitoring and Outcome Prognosis: A Systematic Review. J. Clin. Med. 2025, 14, 4760. [Google Scholar] [CrossRef]
- Buttarello, M.; Mezzapelle, G.; Freguglia, F.; Plebani, M. Reticulated platelets and immature platelet fraction: Clinical applications and method limitations. Int. J. Lab. Hematol. 2020, 42, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Shahjouei, S.; Li, J.; Koza, E.; Abedi, V.; Sadr, A.V.; Chen, Q.; Mowla, A.; Griffin, P.; Ranta, A.; Zand, R. Risk of Subsequent Stroke Among Patients Receiving Outpatient vs. Inpatient Care for Transient Ischemic Attack: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2136644. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; Wang, L.D. China stroke surveillance report 2021. Mil. Med. Res. 2023, 10, 33. [Google Scholar] [CrossRef]
- Vyas, M.V.; Silver, F.L.; Austin, P.C.; Yu, A.Y.X.; Pequeno, P.; Fang, J.; Laupacis, A.; Kapral, M.K. Stroke Incidence by Sex Across the Lifespan. Stroke 2021, 52, 447–451. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]
- McCabe, D.J.; Harrison, P.; Sidhu, P.S.; Brown, M.M.; Machin, S.J. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br. J. Haematol. 2004, 126, 861–869. [Google Scholar] [CrossRef]
- Lim, S.T.; Tobin, W.O.; Murphy, S.; Kinsella, J.A.; Smith, D.R.; Lim, S.Y.; Murphy, S.M.; Coughlan, T.; Collins, D.R.; O’Neill, D.; et al. Profile of reticulated platelets in the early, subacute and late phases after transient ischemic attack or ischemic stroke. Platelets 2022, 33, 89–97. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Brazzelli, M.; Chappell, F.M.; Miranda, H.; Shuler, K.; Sandercock, P.A.G.; Dennis, M.S. ABCD2 score and secondary stroke prevention. Neurology 2015, 85, 373–380. [Google Scholar] [CrossRef]
- Bui, T.A.; Jickling, G.C.; Winship, I.R. Neutrophil dynamics and inflammaging in acute ischemic stroke: A transcriptomic review. Front. Aging Neurosci. 2022, 14, 1041333. [Google Scholar] [CrossRef]
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 888–901. [Google Scholar] [CrossRef]
- Alanazi, E.M.; Abdou, A.; Luo, J. Predicting Risk of Stroke From Lab Tests Using Machine Learning Algorithms: Development and Evaluation of Prediction Models. JMIR Form. Res. 2021, 5, e23440. [Google Scholar] [CrossRef]
- Güneş, M. The Correlation of Routine Hematological Parameters with In-hospital Mortality and Length of Hospital Stay in Patients with Large Middle Cerebral Artery Infarction. Cureus 2020, 12, e7886. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pei, L.; Liu, J.; Peng, L.; Guo, C.; Li, Y.; Duan, Z.; Du, Y.; Shang, D.; Li, S.; et al. Circulating mucosal-associated invariant T cells predict early neurological deterioration in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2025, 34, 108301. [Google Scholar] [CrossRef] [PubMed]
- Gumiężna, K.; Baruś, P.; Sygitowicz, G.; Wiśniewska, A.; Bednarek, A.; Zabłocki, J.; Piasecki, A.; Klimczak-Tomaniak, D.; Kochman, J.; Grabowski, M.; et al. Prognostic Implications of Immature Platelet Fraction at 5-Year Follow-up Among ACS Patients Treated With Dual Antiplatelet Therapy. J. Cardiovasc. Pharmacol. Ther. 2024, 29, 10742484231202864. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, D.; Schreiner, N.; Gosetti, R.; Mayer, K.; Angiolillo, D.J.; Sibbing, D.; Holdenrieder, S.; Anetsberger, A.; von Scheidt, M.; Schunkert, H.; et al. Immature Platelet Fraction Predicts Adverse Events in Patients With Acute Coronary Syndrome: The ISAR-REACT 5 Reticulated Platelet Substudy. Arter. Thromb. Vasc. Biol. 2023, 43, e83–e93. [Google Scholar] [CrossRef]
- Ospel, J.M.; Hill, M.D.; Menon, B.K.; Demchuk, A.; McTaggart, R.; Nogueira, R.; Poppe, A.; Haussen, D.; Qiu, W.; Mayank, A.; et al. Strength of Association between Infarct Volume and Clinical Outcome Depends on the Magnitude of Infarct Size: Results from the ESCAPE-NA1 Trial. AJNR Am. J. Neuroradiol. 2021, 42, 1375–1379. [Google Scholar] [CrossRef]
- Meng, X.; Ji, J. Infarct volume and outcome of cerebral ischaemia, a systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14773. [Google Scholar] [CrossRef]
- Shams Vahdati, S.; Ala, A.; Vahed, N.; Mohammadi, S.; Ameli, H. Complete Blood Count Parameters as Prognostic Factor of Stroke: A Systematic Review. Basic Clin. Neurosci. 2022, 13, 745–754. [Google Scholar] [CrossRef]
| Variables | Unit | Case | Control | ||||
| n | % | n | % | p-Value | |||
| Gender | Female | 51 | 56.7 | 41 | 45.6 | p = 0.136 χ2 = 2.223 | |
| Male | 39 | 43.3 | 49 | 54.4 | |||
| Median | Q1–Q3 | Median | Q1–Q3 | ||||
| Age | Year | 70 | 60–80 | 60 | 48–72 | p < 0.001 U = 2529.5 | |
| Laboratory Parameters | WBC | 109/L | 9.05 | 7.51–12.49 | 8.30 | 6.81–10.10 | p = 0.006 U = 3090.5 |
| RBC | 1012/L | 4.83 | 4.47–5.26 | 4.78 | 4.37–5.15 | p = 0.471 U = 3798.0 | |
| HCT | % | 43.00 | 38.78–46.13 | 41.85 | 38.40–44.68 | p = 0.164 U = 3563.5 | |
| MCV | fL | 88.10 | 85.38–93.30 | 87.80 | 83.73–91.33 | p = 0.298 U = 3686.0 | |
| MCH | pg | 28.80 | 27.78–30.05 | 28.80 | 27.23–30.20 | p = 0.792 U = 3958.0 | |
| NEU | 109/L | 6.12 | 4.72–9.75 | 5.27 | 4.02–6.51 | p = 0.020 U = 2979.5 | |
| LEU | 109/L | 1.78 | 1.11–2.38 | 1.98 | 1.44–2.86 | p = 0.019 U = 0.3232 | |
| PDW | fL | 12.25 | 10.93–13.33 | 11.15 | 10.00–12.43 | p = 0.040 U = 3036.0 | |
| RDW (SD) | % | 44.40 | 41.95–48.70 | 42.25 | 40.55–46.28 | p = 0.002 U = 2990.0 | |
| RDW (CV) | % | 14.00 | 13.20–15.13 | 13.30 | 12.60–14.60 | p = 0.016 U = 3210.0 | |
| IPF | % | 3.05 | 2.08–5.00 | 1.65 | 1.20–2.40 | p < 0.001 U = 1663.0 | |
| Mean ± SD | Mean ± SD | ||||||
| Laboratory Parameters | HGB | g/dL | 13.72 ± 2.09 | 13.46 ± 1.98 | p = 0.378 t = 0.885 | ||
| PLT | 109/L | 237.11 ± 84.09 | 270.04 ± 73.19 | p = 0.007 t = −2.705 | |||
| MPV | fL | 10.56 ± 1.00 | 9.94 ± 0.82 | p < 0.001 t = 4.556 | |||
| PCT | % | 0.25 ± 0.78 | 0.28 ± 0.66 | p = 0.025 t = −2.261 | |||
| Variable | n | % | IPF | Statistically Associated Parameters | |
|---|---|---|---|---|---|
| Localization | Lacunar and Subcortical | 41 | 45.6 | p = 0.923 H = 161.0 | Service/ICU Recommendation (p = 0.006 χ2 = 10.28), Short Term Prognosis (p < 0.001 χ2 = 27.46) |
| Watershed | 21 | 23.3 | |||
| ACA | 7 | 7.8 | |||
| MCA | 11 | 12.2 | |||
| Basilar and PCA | 6 | 6.7 | |||
| Number of Lesions | Single | 65 | 72.2 | p = 0.675 U = 766.0 | Reticulocytes (p < 0.001, U = 71.5) * |
| Multiple | 25 | 27.8 | |||
| Affected Hemisphere | Right | 39 | 43.3 | p = 0.063 H = 5.541 | Absent |
| Left | 39 | 43.3 | |||
| Bilateral | 12 | 13.4 | |||
| Main Artery Status | Occlusion Absent | 63 | 70.0 | p = 0.526 U = 775.5 | Service/ICU Recommendation (p = 0.001 χ2 = 10.50), Short Term Prognosis (p < 0.001 χ2 = 11.52 ) |
| Occlusion Present | 27 | 30.0 | |||
| Carotid Status | Normal | 23 | 25.6 | p = 0.854 H = 0.782 | Absent |
| Plaque without stenosis | 40 | 44.4 | |||
| Significant stenosis | 17 | 18.9 | |||
| Occlusion | 10 | 11.1 | |||
| Service/ICU Recommendation | Service | 40 | 44.4 | p = 0.798 U = 968.5 | MCV (p = 0.045 U = 753.0), Localization (p = 0.006 χ2 = 10.28), Main Artery Occlusion (p = 0.001 χ2 = 10.50), Infarct Volume (p < 0.001 U = 541.0) |
| ICU | 50 | 55.6 | |||
| Treatment Method | Thrombolytic | 11 | 12.2 | p = 0.211 H = 3.110 | Infarct Volume (p = 0.004 H = 10.95) |
| Interventional | 20 | 22.2 | |||
| Non-Specific | 59 | 65.6 | |||
| Short Term Prognosis (Initial 30 days) | Alive | 75 | 83.3 | p = 0.132 U = 423.5 | MCV (p = 0.023 U = 352.0), NEU/IPF (p = 0.021, U = 350.0), RDW (SD)/IPF (p = 0.048 U = 380.0), Reticulocytes (p = 0.015 U = 39.0) *, Main Artery Occlusion (p < 0.001 χ2 = 27.46), Localization, Infarct Volume (p < 0.001 U = 225.0) |
| Deceased | 15 | 16.7 | |||
| Variables | Area | SE | p | 95% CI | Cutoff | Sensivity | Specifity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | (%) | (%) | (%) | (%) | |||||
| Diagnostic Significance in Acute Ischemic Stroke | ||||||||||
| IPF (%) | 0.795 | 0.033 | <0.001 | 0.730 | 0.860 | ≥2.45 | 68.89 | 77.78 | 75.61 | 71.43 |
| AGE (Year) | 0.679 | 0.040 | <0.001 | 0.601 | 0.757 | ≥62 | 73.33 | 54.44 | 61.68 | 67.12 |
| MPV (fL) | 0.681 | 0.040 | <0.001 | 0.602 | 0.760 | ≥10.65 | 47.78 | 86.67 | 78.18 | 62.40 |
| NEU (109/L) | 0.632 | 0.042 | 0.002 | 0.550 | 0.714 | ≥6.94 | 43.33 | 82.22 | 70.91 | 59.20 |
| PDW (%) | 0.625 | 0.042 | 0.004 | 0.543 | 0.707 | ≥11.35 | 68.89 | 55.56 | 60.78 | 64.10 |
| RDW-SD (%) | 0.631 | 0.042 | 0.002 | 0.549 | 0.712 | ≥42.15 | 74.44 | 50.00 | 59.82 | 66.18 |
| WBC (109/L) | 0.618 | 0.042 | 0.006 | 0.537 | 0.700 | ≥11.83 | 31.11 | 90.00 | 75.68 | 56.64 |
| RDW-CV (%) | 0.604 | 0.042 | 0.016 | 0.521 | 0.687 | ≥13.35 | 72.22 | 51.11 | 59.63 | 64.79 |
| PLT (109/L) | 0.374 | 0.042 | 0.003 | 0.292 | 0.455 | ≤254 | 63.33 | 61.11 | 61.96 | 62.50 |
| PCT (&) | 0.409 | 0.042 | 0.036 | 0.326 | 0.493 | ≤0.22 | 34.44 | 82.22 | 65.96 | 55.64 |
| LEU (109/L) | 0.399 | 0.042 | 0.019 | 0.316 | 0.482 | ≤2.53 | 80.00 | 42.22 | 58.06 | 67.86 |
| Short-Term Prognostic Significance in Acute Ischemic Stroke | ||||||||||
| Infarct Volume (cm3) | 0.800 | 0.082 | <0.001 | 0.640 | 0.960 | ≥34.11 | 80.00 | 78.70 | 42.80 | 95.70 |
| MCV (fL) | 0.687 | 0.082 | 0.023 | 0.527 | 0.847 | ≥90.5 | 73.30 | 66.70 | 30.60 | 92.60 |
| IPF/NEU | 0.311 | 0.080 | 0.021 | 0.155 | 0.467 | ≤0.51 | 86.65 | 54.67 | 27.66 | 95.35 |
| RDW(SD)/IPF | 0.662 | 0.076 | 0.048 | 0.514 | 0.810 | ≥1.95 | 86.68 | 54.67 | 27.66 | 95.35 |
| Variables | B | SE | Wald | df | p | Exp(B) | 95% CI for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Diagnostic Model 1 | ||||||||
| Age (Year) | 0.037 | 0.014 | 7.069 | 1 | 0.008 | 1.038 | 1.010 | 1.067 |
| IPF (%) | 0.955 | 0.185 | 26.587 | 1 | <0.001 | 2.599 | 1.808 | 3.737 |
| NEU (109/L) | 0.015 | 0.068 | 4.735 | 1 | 0.030 | 1.161 | 1.015 | 1.327 |
| RDW-SD (%) | 0.070 | 0.038 | 3.333 | 1 | 0.068 | 1.073 | 0.995 | 1.157 |
| LEU (109/L) | −0.045 | 0.175 | 0.067 | 1 | 0.796 | 0.956 | 0.678 | 1.347 |
| Short-Term Prognostic Model 2 | ||||||||
| Infarct Volume (cm3) | 0.008 | 0.002 | 11.362 | 1 | 0.001 | 1.008 | 1.003 | 1.013 |
| MCV (fL) | 0.148 | 0.061 | 5.945 | 1 | 0.015 | 1.159 | 1.029 | 1.305 |
| IPF (%) | 0.026 | 0.327 | 0.006 | 1 | 0.936 | 1.027 | 0.541 | 1.947 |
| IPF/NEU | −0.737 | 1.184 | 0.387 | 1 | 0.534 | 0.479 | 0.047 | 4.875 |
| IPF/LEU | −0.415 | 0.260 | 2.542 | 1 | 0.111 | 0.660 | 0.397 | 1.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, F.C.; Demirci, O.L.; Vişneci, E.F.; Ataş, A.E.; Kır, H.H.; Yıldırım, H.B.; Deniz, Ç.D.; Acar, D.; Erdem, S.S.; Gül, M. The Diagnostic and Prognostic Value of Reticulated Platelets in Ischemic Stroke: Is Immature Platelet Fraction a New Biomarker? Medicina 2025, 61, 1887. https://doi.org/10.3390/medicina61101887
Tekin FC, Demirci OL, Vişneci EF, Ataş AE, Kır HH, Yıldırım HB, Deniz ÇD, Acar D, Erdem SS, Gül M. The Diagnostic and Prognostic Value of Reticulated Platelets in Ischemic Stroke: Is Immature Platelet Fraction a New Biomarker? Medicina. 2025; 61(10):1887. https://doi.org/10.3390/medicina61101887
Chicago/Turabian StyleTekin, Fatih Cemal, Osman Lütfi Demirci, Emin Fatih Vişneci, Abdullah Enes Ataş, Hasan Hüseyin Kır, Hasan Basri Yıldırım, Çiğdem Damla Deniz, Demet Acar, Said Sami Erdem, and Mehmet Gül. 2025. "The Diagnostic and Prognostic Value of Reticulated Platelets in Ischemic Stroke: Is Immature Platelet Fraction a New Biomarker?" Medicina 61, no. 10: 1887. https://doi.org/10.3390/medicina61101887
APA StyleTekin, F. C., Demirci, O. L., Vişneci, E. F., Ataş, A. E., Kır, H. H., Yıldırım, H. B., Deniz, Ç. D., Acar, D., Erdem, S. S., & Gül, M. (2025). The Diagnostic and Prognostic Value of Reticulated Platelets in Ischemic Stroke: Is Immature Platelet Fraction a New Biomarker? Medicina, 61(10), 1887. https://doi.org/10.3390/medicina61101887






