Do SGLT2 Inhibitors Improve Cardiovascular Outcomes After Acute Coronary Syndrome Regardless of Diabetes? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Data Sources
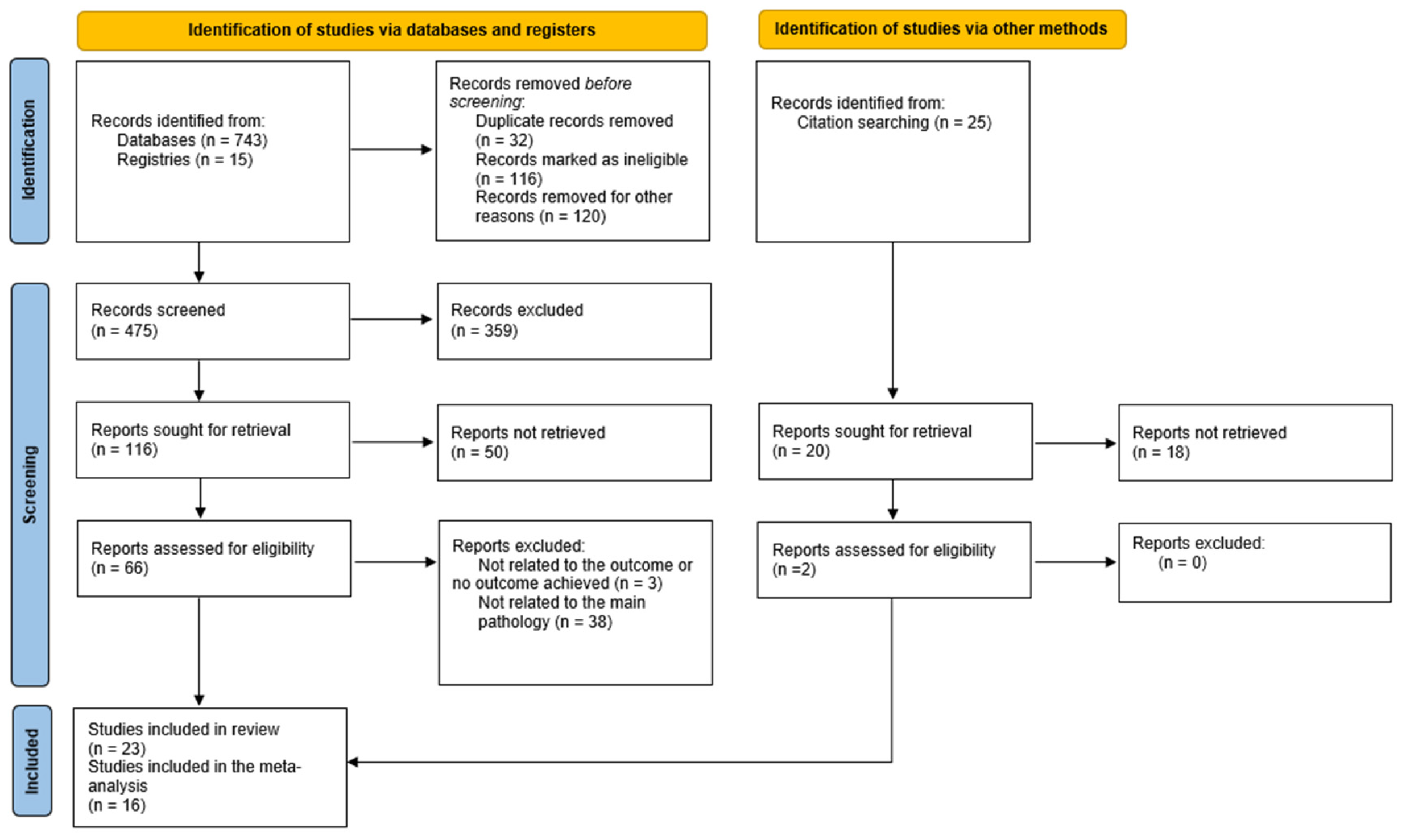
2.3. Selection Process and Data Collection
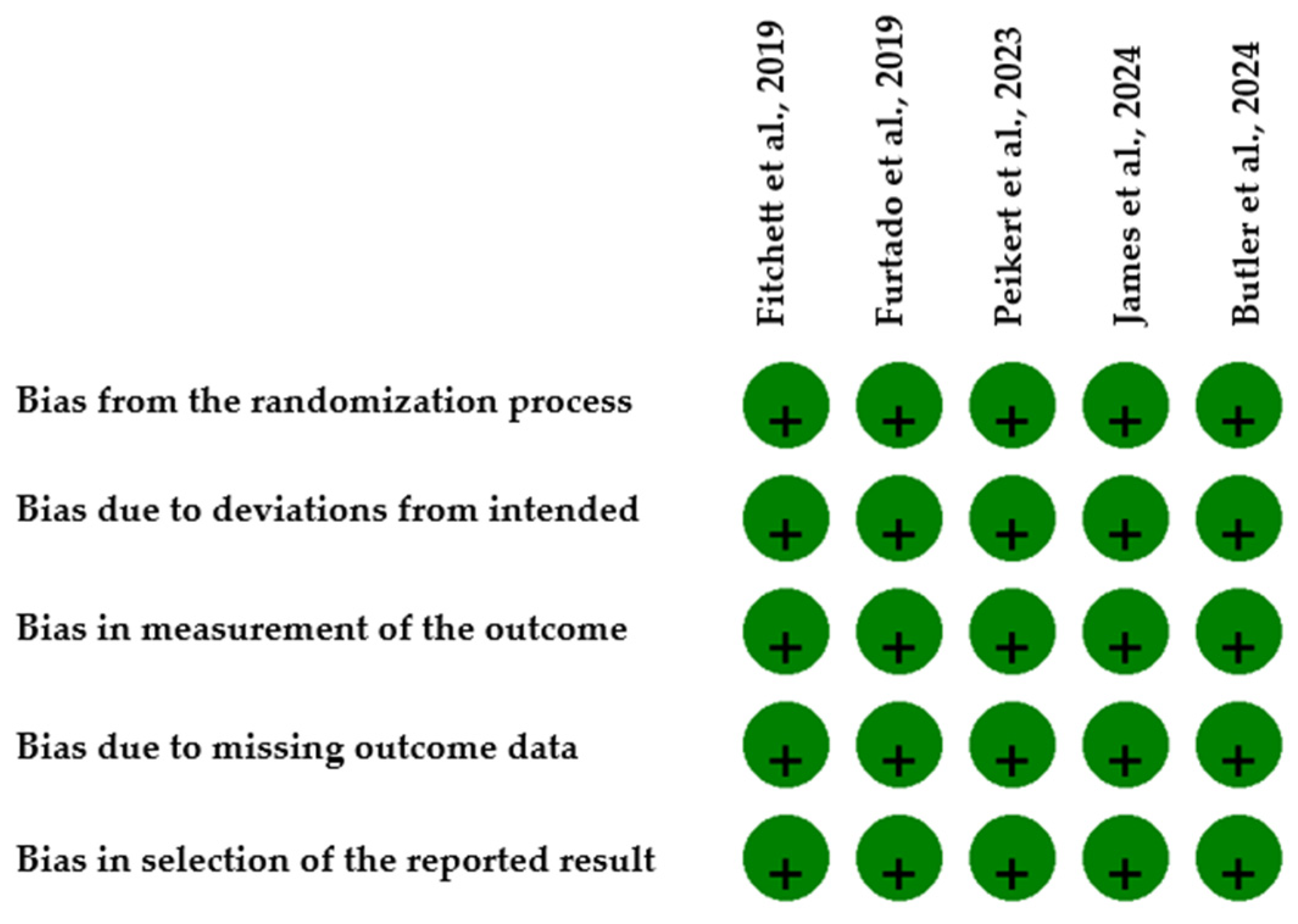
2.4. Reporting Bias Assessment and Certainty of Evidence
2.5. Statistical Analysis
3. Results
3.1. Study Search and Characteristics of Included Studies
3.2. Primary Outcome
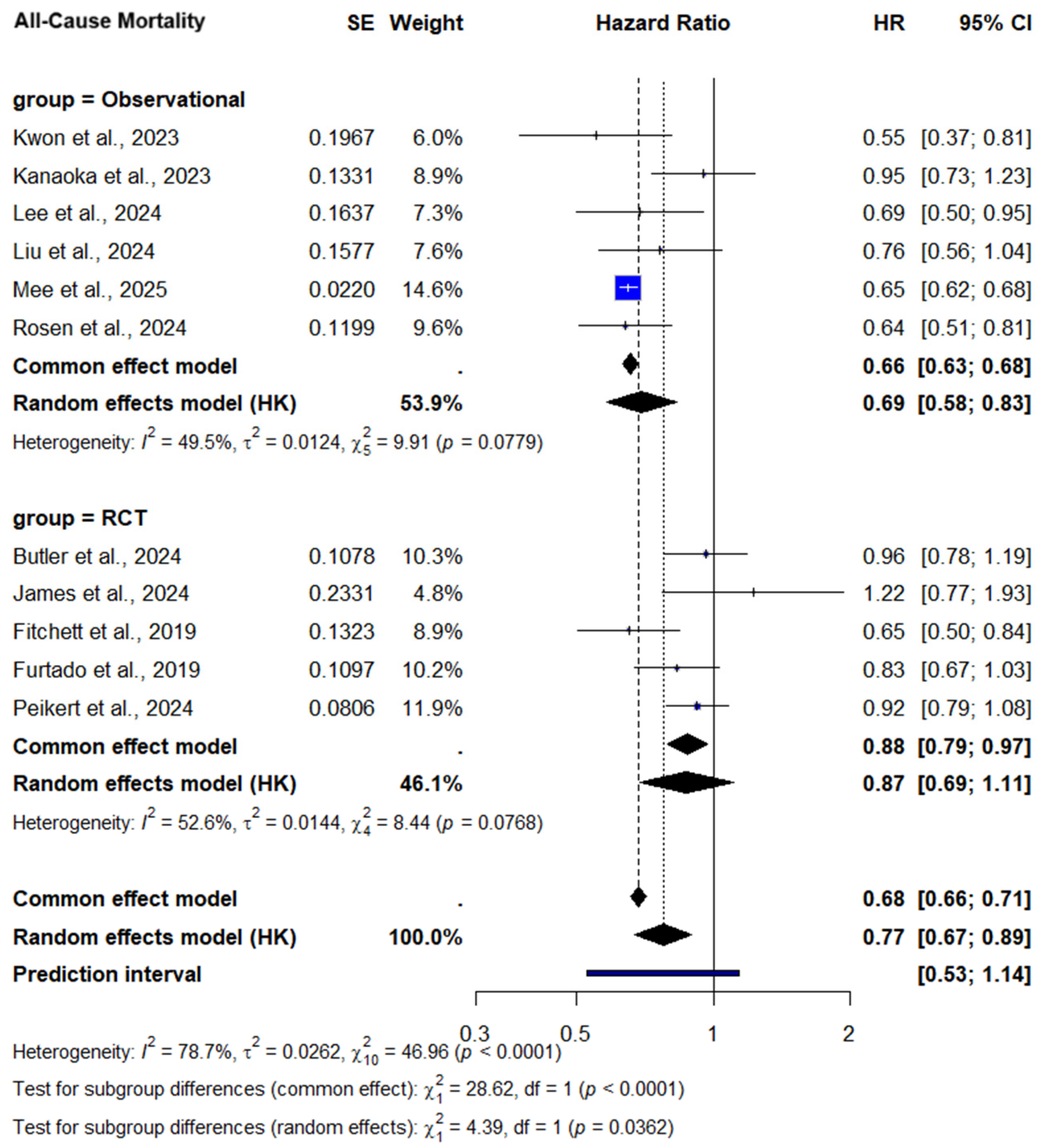
3.2.1. All-Cause Mortality
All-Cause Mortality—Subgroup Analysis by Diabetes Status
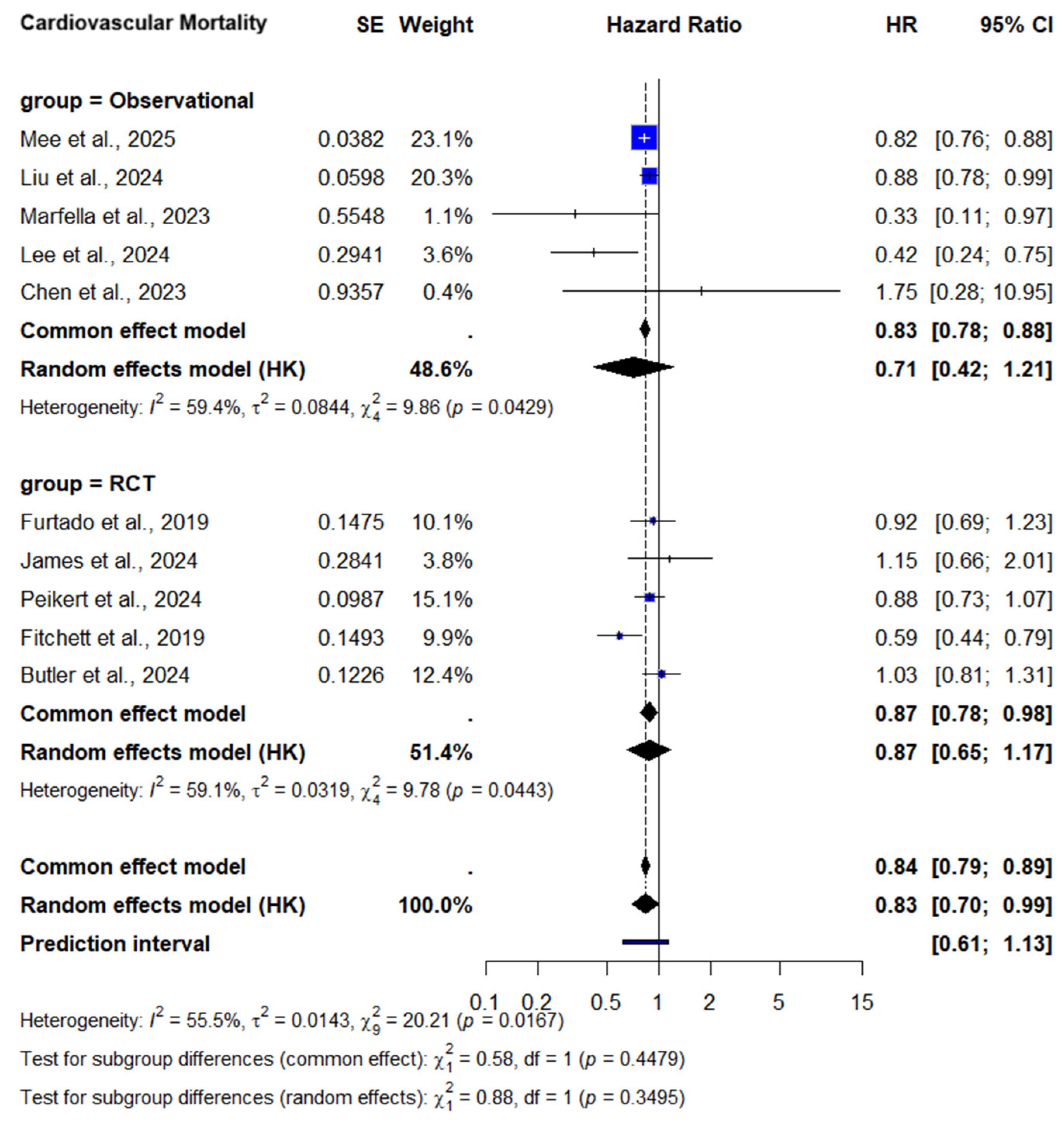
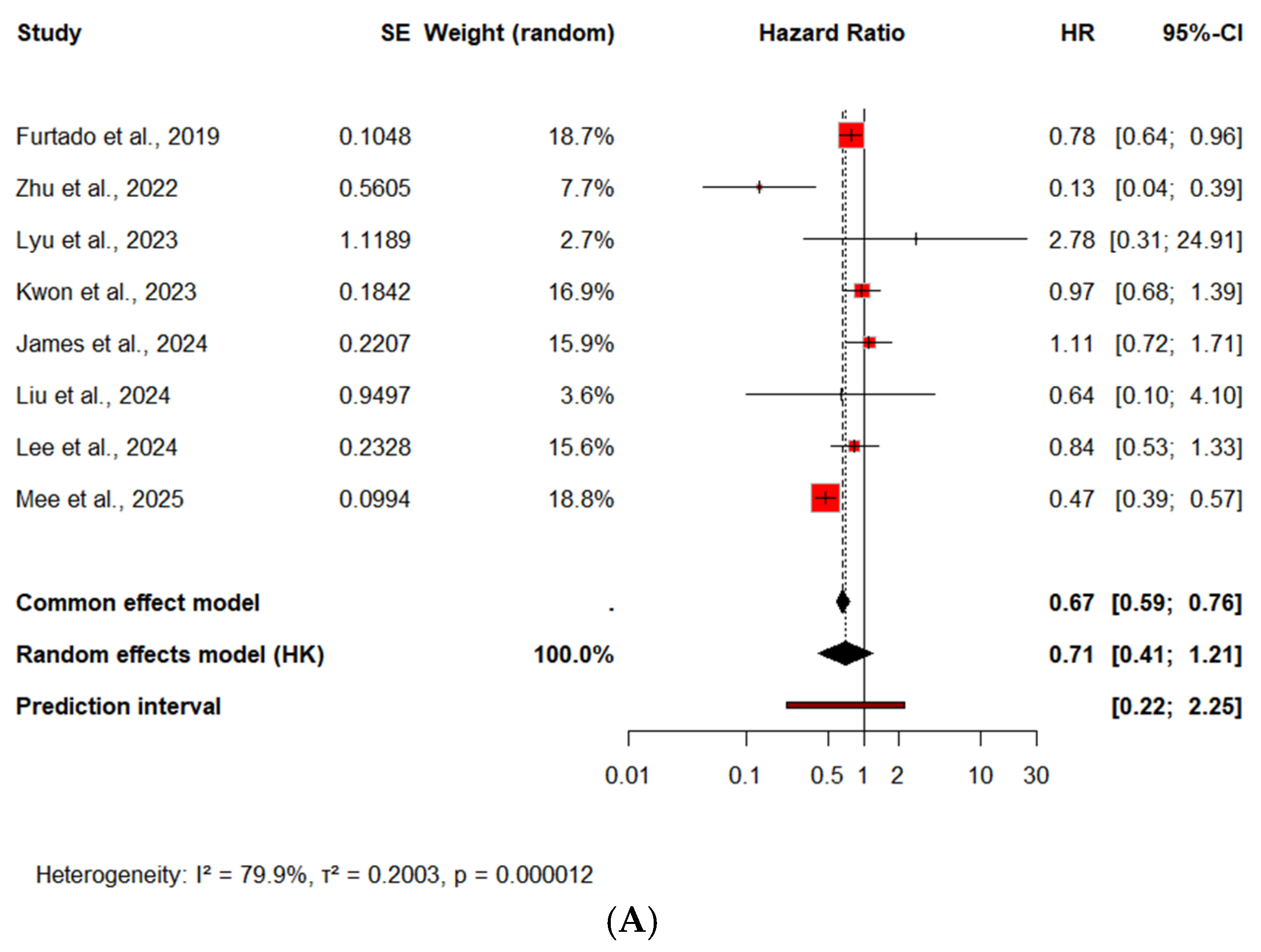
3.2.2. Cardiovascular Mortality
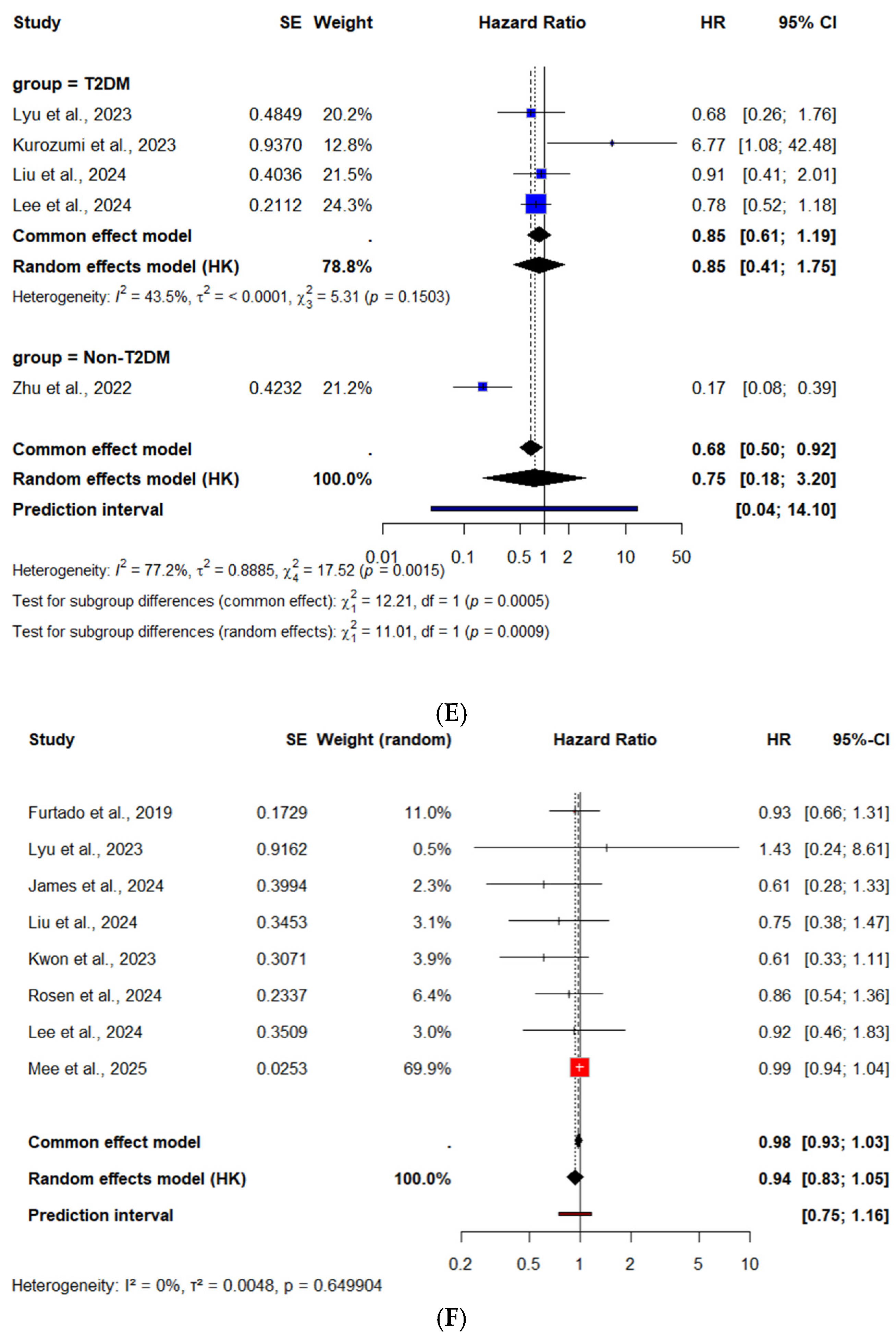
CV Mortality—Subgroup Analysis by Diabetes Status
3.2.3. Subgroup Analysis by Study Design
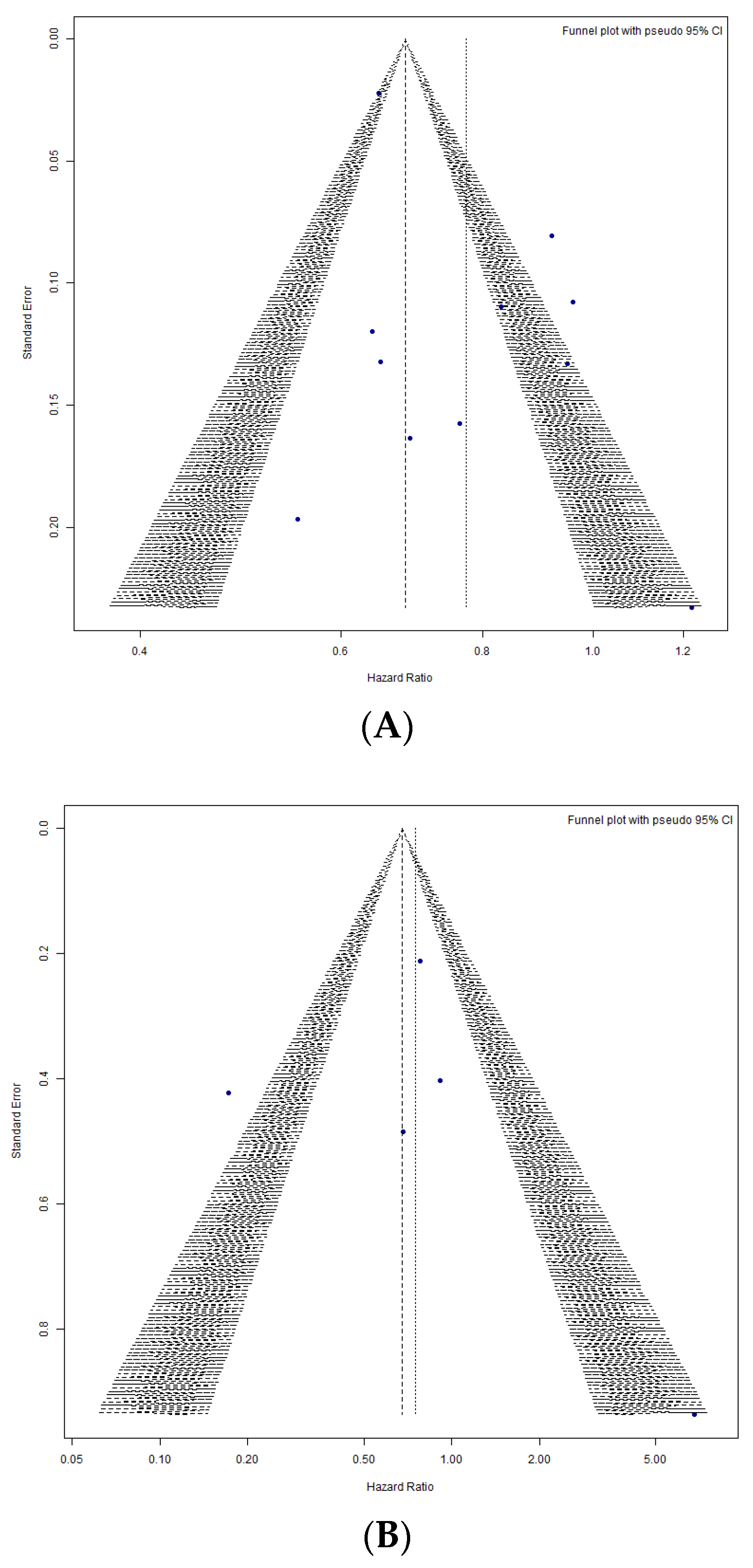
3.2.4. Publication Bias Analysis
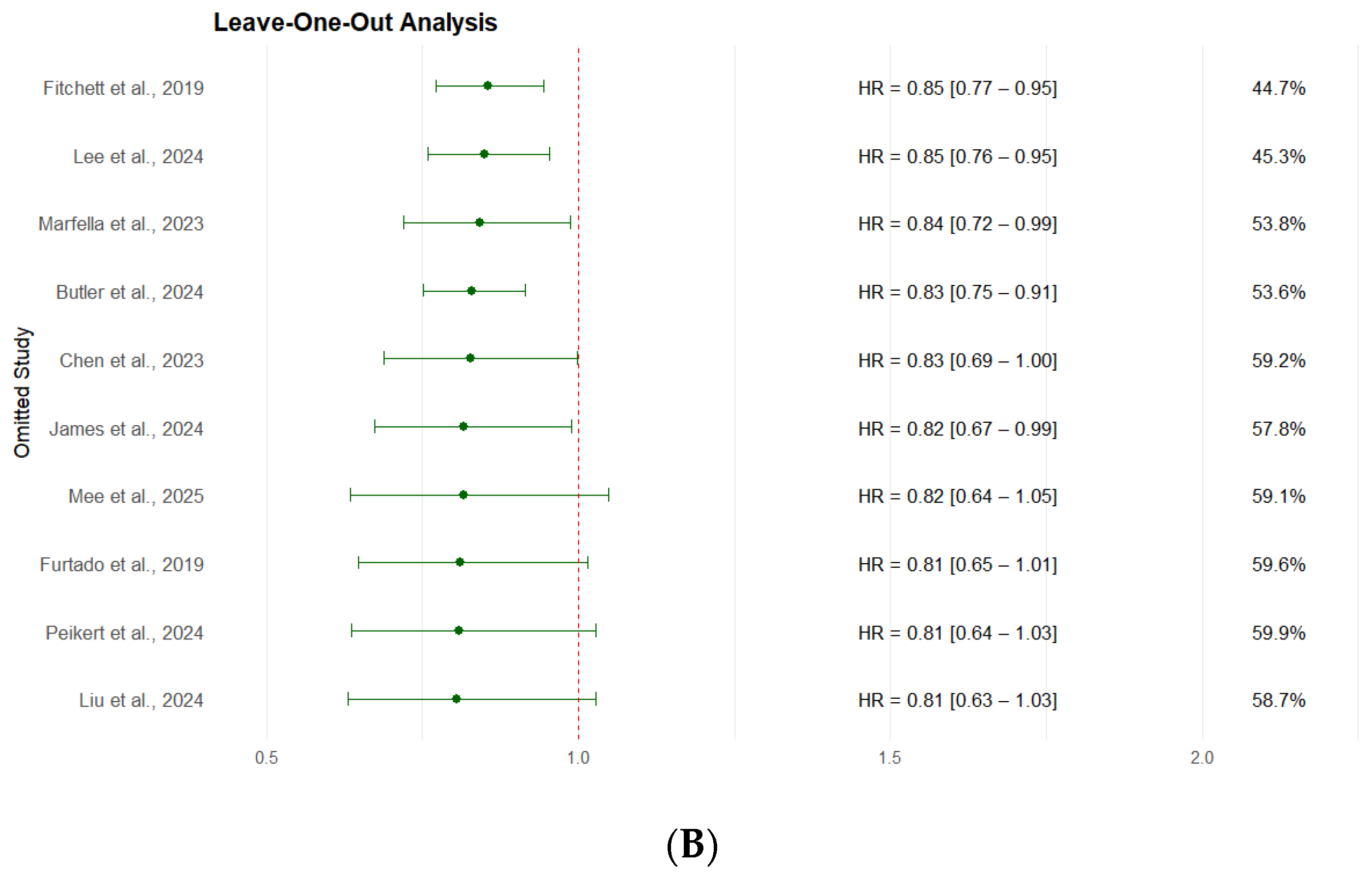
3.2.5. Leave-One-Out Sensitivity Analysis
3.3. Secondary Outcomes
3.3.1. Recurrent MI
Recurrent MI—Subgroup Analysis by Diabetes Status
3.3.2. Rehospitalization for ACS
3.3.3. Revascularization Rates
3.3.4. Stroke After ACS
Stroke—Subgroup Analysis by Diabetes Status
4. Discussion
4.1. Context of Other Evidence
4.2. Limitations
4.3. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGLT2 inhibitors | Sodium–glucose co-transporter-2 (SGLT2) inhibitors |
| CKD | Chronic Kidney Disease |
| ACS | Acute Coronary Syndrome |
| STEMI | ST Elevation Myocardial Infarction |
| NSTEMI | Non-ST Elevation Myocardial Infarction |
| UA | Unstable Angina |
| HF | Heart Failure |
| T2DM | Type 2 Diabetes Mellitus |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| PSM | Propensity Score Matching |
| IPTW | Inverse Probability of Treatment Weighting |
References
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capdanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.C.; Stefansson, B.V.; Batiushin, M.; Bilchenko, O.; Cherney, D.Z.I.; Chertow, G.M.; Douthat, W.; Dwyer, J.P.; Escudero, E.; Pecoits-Filho, R.; et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: Baseline characteristics. Nephrol. Dial. Transplant. 2020, 35, 1700–1711. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef]
- Chang, T.Y.; Lu, C.T.; Huang, H.L.; Chou, R.H.; Chang, C.C.; Liu, C.T.; Huang, P.H.; Lin, S.J. Association of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Use With Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus Patients With Stabilized Acute Myocardial Infarction: A Propensity Score Matching Study. Front. Cardiovasc. Med. 2022, 9, 882181. [Google Scholar] [CrossRef]
- Chen, J.; Chang, J.; Shi, Q.; Li, X.; Wang, L.; Zhao, H. Cardiovascular protective effect of sodium-glucose cotransporter 2 inhibitors on patients with acute coronary syndrome and type 2 diabetes mellitus: A retrospective study. BMC Cardiovasc. Disord. 2023, 23, 495. [Google Scholar] [CrossRef]
- Adel, S.M.H.; Jorfi, F.; Mombeini, H.; Rashidi, H.; Fazeli, S. Effect of a low dose of empagliflozin on short-term outcomes in type 2 diabetics with acute coronary syndrome after percutaneous coronary intervention. Saudi. Med. J. 2022, 43, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Mghaieth Zghal, F.; Abbassi, M.; Silini, A.; Ben Halima, M.; Jebberi, Z.; Daly, F.; Ouali, S.; Farhati, A.; Ben Mansour, N.; Boudiche, S.; et al. Impact of sodium-glucose cotransporter inhibitors in acute coronary syndrome patients on endothelial function and atherosclerosis related-biomarkers ATH-SGLT2i pilot study. Medicine 2024, 103, e40536. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, Z.; Li, T.; Chen, K.; Zeng, Z. SGLT2 inhibitor improves the prognosis of patients with coronary heart disease and prevents in-stent restenosis. Front. Cardiovasc. Med. 2024, 10, 1280547. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef]
- Furtado, R.H.M.; Bonaca, M.P.; Raz, I.; Zelniker, T.A.; Mosenzon, O.; Cahn, A.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Previous Myocardial Infarction: Subanalysis From the DECLARE-TIMI 58 Trial. Circulation 2019, 139, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Foà, A.; Desai, A.S.; Jhund, P.S.; Carberry, J.; Lam, C.S.P.; Kosiborod, M.N.; et al. Dapagliflozin in patients with heart failure and previous myocardial infarction: A participant-level pooled analysis of DAPA-HF and DELIVER. Eur. J. Heart Fail. 2024, 26, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, K.; Iwanaga, Y.; Nakai, M.; Nishioka, Y.; Myojin, T.; Kubo, S.; Okada, K.; Noda, T.; Sakata, Y.; Miyamoto, T.; et al. Sodium-glucose cotransporter 2 inhibitor use in early-phase acute coronary syndrome with severe heart failure. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, A.; Shishido, K.; Yamashita, T.; Sato, D.; Uchida, S.; Koyama, E.; Tamaki, Y.; Hayashi, T.; Miyashita, H.; Yokoyama, H.; et al. Sodium-Glucose Cotransporter-2 Inhibitors Stabilize Coronary Plaques in Acute Coronary Syndrome With Diabetes Mellitus. Am. J. Cardiol. 2024, 214, 47–54. [Google Scholar] [CrossRef]
- Lyu, Y.S.; Oh, S.; Kim, J.H.; Kim, S.Y.; Jeong, M.H. Comparison of SGLT2 inhibitors with DPP-4 inhibitors combined with metformin in patients with acute myocardial infarction and diabetes mellitus. Cardiovasc. Diabetol. 2023, 22, 185. [Google Scholar] [CrossRef]
- Rosén, H.C.; Mohammad, M.A.; Jernberg, T.; James, S.; Oldgren, J.; Erlinge, D. SGLT2 Inhibitors for Patients with Type 2 Diabetes Mellitus After Myocardial Infarction: A Nationwide Observation Registry Study from SWEDEHEART. 2024. Available online: www.thelancet.com (accessed on 1 July 2025).
- Zhu, Y.; Zhang, J.L.; Yan, X.J.; Sun, L.; Ji, Y.; Wang, F.F. Effect of dapagliflozin on the prognosis of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Cardiovasc. Diabetol. 2022, 21, 186. [Google Scholar] [CrossRef]
- Marfella, R.; Sardu, C.; D’Onofrio, N.; Fumagalli, C.; Scisciola, L.; Sasso, F.C.; Siniscalchi, M.; Marfella, L.V.; D’Andrea, D.; Minicucci, F.; et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: An observational study in patients with type 2 diabetes. BMC Med. 2023, 21, 71. [Google Scholar] [CrossRef]
- Mee, X.; Kheng Lim, G.; Ibrahim, R.; Nhat Pham, H.; Abdelnabi, M.; Allam, M.; Bcharah, G.; Tan, M.C.; Barry, T.; Farina, J.; et al. SGLT2 inhibitors and cardiovascular outcomes in patients with acute myocardial infarction: A retrospective cohort analysis. Eur. Heart. J. Cardiovasc. Pharmacother. 2025, 11, 334–342. [Google Scholar] [CrossRef]
- Liu, T.; Fan, Z.; Xiao, B.; He, C.; Wang, S. Association of sodium-glucose cotransporter 2 inhibitors with risk of major adverse cardiovascular events in type 2 diabetes patients with acute coronary syndrome: A propensity score matched analysis. Cardiovasc. Diabetol. 2024, 23, 106. [Google Scholar] [CrossRef]
- Lee, H.F.; Chan, Y.H.; Hsu, T.J.; Chuang, C.; Li, P.R.; Yeh, Y.H.; Su, H.C.; Hsiao, F.C.; See, L.C. Clinical Outcomes in Type 2 Diabetes Patients After Acute Myocardial Infarction: A Comparison of Sodium–Glucose Cotransporter 2 Inhibitors vs. Non-Users. Clin. Pharmacol. Ther. 2024, 116, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Myong, J.P.; Lee, Y.; Choi, Y.J.; Yi, J.E.; Seo, S.M.; Jang, S.W.; Kim, P.J.; Lee, J.M. Sodium-Glucose Cotransporter-2 Inhibitors After Acute Myocardial Infarction in Patients with Type 2 Diabetes: A Population-Based Investigation. J. Am. Heart Assoc. 2023, 12, e027824. [Google Scholar] [CrossRef]
- Dayem, K.A.; Younis, O.; Zarif, B.; Attia, S.; AbdelSalam, A. Impact of dapagliflozin on cardiac function following anterior myocardial infarction in non-diabetic patients—DACAMI (a randomized controlled clinical trial). Int. J. Cardiol. 2023, 379, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, W.; Kubota, Y.; Hoshika, Y.; Mozawa, K.; Tara, S.; Tokita, Y.; Yodogawa, K.; Iwasaki, Y.K.; Yamamoto, T.; Takano, H.; et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: The EMBODY trial. Cardiovasc. Diabetol. 2020, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Sharma, A.; Zinman, B.; Ofstad, A.P.; Fitchett, D.; Brueckmann, M.; Wanner, C.; Zwiener, I.; George, J.T.; Inzucchi, S.E.; et al. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes categories: Post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes. Metab. 2020, 22, 1141–1150. [Google Scholar] [CrossRef]
- Verma, S.; Mazer, C.D.; Fitchett, D.; Inzucchi, S.E.; Pfarr, E.; George, J.T.; Zinman, B. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: Subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 2018, 61, 1712–1723. [Google Scholar] [CrossRef]
- Chipayo-Gonzales, D.; Shabbir, A.; Vergara-Uzcategui, C.; Nombela-Franco, L.; Jimenez-Quevedo, P.; Gonzalo, N.; Nuñez-Gil, I.; Mejia-Renteria, H.; Macaya-Ten, F.; Tirado-Conte, G.; et al. Treatment with SGLT2 Inhibitors in Patients with Diabetes Mellitus and Extensive Coronary Artery Disease: Mortality and Cardiovascular Outcomes. Diabetes Ther. 2023, 14, 1853–1865. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Myftari, V.; Prosperi, S.; Mariani, M.V.; Colombo, L.; Germano, R.; Marek-Iannucci, S.; Cestie, C.; Ferranti, F.; et al. Early in-hospital use of SGLT2i in heart failure patients with ischemic etiology. Cardiovasc. Drugs Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Rahhal, A.; Hamamyh, T.; Chapra, A.; Zaza, K.J.; Najim, M.; Hemadneh, M.; Faraj, H.; Kanjo, W.; Yasin, A.; Toba, H.; et al. Sodium–glucose cotransporter-2 inhibitors improve cardiovascular outcomes post-acute coronary syndrome complicated by acute heart failure. Front. Cardiovasc. Med. 2024, 11, 1383669. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Gragnano, F.; Gallinoro, E.; Cesaro, A.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; Sansonetti, A.; et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol. Res. 2023, 187, 106597. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chen, Q.; Mao, L.; Xiao, T.; Wang, Y.; Gu, Q.; Wang, Q.; Ji, Y.; Sun, L. Association of SGLT2 inhibitor dapagliflozin with risks of acute kidney injury and all-cause mortality in acute myocardial infarction patients. Eur. J. Clin. Pharmacol. 2024, 80, 613–620. [Google Scholar] [CrossRef]
- Lee, H.F.; Chan, Y.H.; Chuang, C.; Li, P.R.; Yeh, Y.H.; Hsiao, F.C.; Peng, J.R.; See, L.C. Cardiovascular, renal, and lower limb outcomes in patients with type 2 diabetes after percutaneous coronary intervention and treated with sodium–glucose cotransporter 2 inhibitors vs. dipeptidyl peptidase-4 inhibitors. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 301–310. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Rambaldi, P.F.; Fumagalli, C.; Marfella, L.V.; La Grotta, R.; Frigé, C.; Pellegrini, V.; D’Andrea, D.; et al. GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: An observational study in patients with type 2 diabetes. Cardiovasc. Diabetol. 2024, 23, 10. [Google Scholar] [CrossRef]
- Liu, T.; Fan, Z.; Li, Y.; Xiao, B.; He, C. Combination treatment of SGLT2i and GLP-1RA associated with improved cardiovascular outcomes in type 2 diabetes patients with acute coronary syndrome: A propensity score-matched cohort study. Int. J. Cardiol. 2025, 431, 133229. [Google Scholar] [CrossRef] [PubMed]
- Longato, E.; Di Camillo, B.; Sparacino, G.; Gubian, L.; Avogaro, A.; Fadini, G.P. Cardiovascular outcomes of type 2 diabetic patients treated with SGLT-2 inhibitors versus GLP-1 receptor agonists in real-life. BMJ Open Diabetes Res. Care 2020, 8, e001451. [Google Scholar] [CrossRef] [PubMed]
- Quisi, A.; Quisi, N.S.N.; Alıcı, G.; Donma, İ.; Yıldırım, A.; Genç, Ö. Effect of dapagliflozin on the no-reflow phenomenon in patients with acute myocardial infarction and type II diabetes mellitus. Acta Cardiol. 2025, 80, 394–402. [Google Scholar] [CrossRef]
- Ponzoni, M.; Rea, F.; Corrao, G.; Barron, D.J.; Cantarutti, A.; Morabito, G. Long-term effectiveness of SGLT2 inhibitors in type 2 diabetes patients with myocardial injury: A population-based study. Heart Lung 2025, 73, 236–242. [Google Scholar] [CrossRef] [PubMed]






| Study | Bias Due to Confounding | Bias in Selection of Participants | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [13] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 PN; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Kanaoka et al. [21] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 PN; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Kurozumi et al. [22] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 PN; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Lyu et al. [23] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 Y; 1.6 Y; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Rosen et al. [24] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 Y; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Zhu et al. [25] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 Y; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Marfella et al. [26] | 1.1 PY; 1.2 PY; 1.4 PY; 1.5 PN; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Moderate Risk |
| Mee et al. [27] | 1.1 Y; 1.2 Y; 1.4 Y; 1.5 Y; 1.6 Y; 1.7 NA Low risk | 2.1 Y; 2.2 Y; 2.4 Y Low risk | 3.1 Y; 3.2 Y; 3.3 Y Low risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 Y; 5.2 Y; 5.3 Y Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Moderate risk | Low to Moderate Risk |
| Liu et al. [28] | 1.1 Y; 1.2 PN; 1.4 Y; 1.5 PY; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 N; 2.4 Y Moderate risk | 3.1 Y; 3.2 PN; 3.3 PN Moderate risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 PY; 5.2 PN; 5.3 PN Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Low risk | Moderate Risk |
| Lee et al. [29] | 1.1 Y; 1.2 PN; 1.4 Y; 1.5 PY; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 N; 2.4 Y Moderate risk | 3.1 Y; 3.2 PN; 3.3 PN Moderate risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 PY; 5.2 PN; 5.3 PN Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Low risk | Moderate Risk |
| Kwon et al. [30] | 1.1 Y; 1.2 PN; 1.4 Y; 1.5 PY; 1.6 PN; 1.7 NA Moderate risk | 2.1 Y; 2.2 N; 2.4 Y Moderate risk | 3.1 Y; 3.2 PN; 3.3 PN Moderate risk | 4.1 PN; 4.3 PY; 4.4 PY; 4.5 Y Low risk | 5.1 PY; 5.2 PN; 5.3 PN Low risk | 6.1 N; 6.2 PY; 6.3 PY; 6.4 PN Low risk | 7.1 PN; 7.2 PN; 7.3 PN Low risk | Moderate Risk |
| Author, Year & Country | Study Design and Type | Follow-Up (Months) | Group | Number | Age [Years ± SD; (IQR)] | Male (n. %) | T2DM (n. %) | Type of SGLT2 Inhibitor (n. %) | STEMI (n. %) NSTEMI (n. %) |
|---|---|---|---|---|---|---|---|---|---|
| Fitchett et al. [18] 2019 International (42 countries) | RCT | 37.2 | SGLT2 | 3048 | 62.8 ± 8.6 | 2189 (71.8%) | 3048 (100%) | Empagliflozin 10 or 25 mg | NR |
| Control | 1518 | 63.0 ± 8.8 | 1098 (72.3%) | 1518 (100%) | Placebo | NR | |||
| Furtado et al. [19] 2019 International (33 countries) | RCT | 50.4 | SGLT2 | 1777 | 62.0 (57.68) | 1364 (76.75%) | 1777 (100%) | Dapagliflozin 10 mg | NR |
| Control | 1807 | 62.0 (57.68) | 1375 (76.09%) | 1807 (100%) | Placebo | NR | |||
| Butler et al. [11] 2024 International (22 countries) | RCT EMPACT-MI | 17.9 | SGLT2 | 3260 | 63.6 ± 11.0 | 2448 (75.09%) | 1035 (31.7%) | Empagliflozin 10 mg | 2444 (75%) 814 (25%) |
| Control | 3262 | 63.7 ± 10.8 | 2449 (75.07%) | 1046 (32.1%) | Placebo | 2401 (73.6%) 861 (26.4%) | |||
| James et al. [10] 2024 Sweden & UK | RCT DAPA-MI | 11.6 | SGLT2 | 2019 | 63.0 ± 11.06 | 1631 (80.8%) | 0% | Dapagliflozin 10 mg | 1465 (72.6%) 544 (26.9%) |
| Control | 1998 | 62.8 ± 10.64 | 1579 (79%) | 0% | Placebo | 1428 (71.5%) 562 (28.1%) | |||
| Peikert et al. [20] 2024 USA | RCT | 22.9 | SGLT2 | 1830 | 68.7 ± 9.7 | 2825 (75.7%) | 1835 (49.2%) | Dapagliflozin 10 mg | NR |
| Control | 1901 | Placebo | NR | ||||||
| Mee et al. [27] 2025 USA | Observational Retrospective PSM 1:1 | 12 | SGLT2 | 44,777 | 68.5 ± 11.9 | 30,699 (68.55%) | 28,610 (63.9%) | NR | NR |
| Control | 44,777 | 68.8 ± 13.3 | 30,566 (68.26%) | 29,407 (65.7%) | NR | NR | |||
| Zhu et al. [25] 2022 China | Observational Retrospective | 23 | SGLT2 | 141 | 60.6 ± 13.6 | 105 (74.5%) | 96 (68.1%) | Dapagliflozin 10 mg | 99 (70.2%) NR |
| Control | 645 | 62.5 ± 13.5 | 497 (77.1%) | 96 (14.9%) | Never used | 396 (61.4%) NR | |||
| Chen et al. [13] 2023 China | Observational Retrospective | 10 | SGLT2 | 128 | 64 (56.7) | 96 (75%) | 128 (100%) | Empagliflozin 10 mg 81 (63.3%) Dapagliflozin 10 mg 47 (36.7%) | 51 (39.8%) 33 (25.8%) |
| Control | 104 | 67 (57.71) | 79 (76.7%) | 104 (100%) | Never used | 54 (51.9%) 23 (22.1%) | |||
| Kanaoka et al. [21] 2023 Japan | Observational Retrospective PSM 1:1 | 24 | SGLT2 | 1591 | NR | 1221 (76.74%) | 1591 (100%) | NR | NR |
| Control | 1591 | NR | 1222 (76.8%) | 1591 (100%) | Never used | NR | |||
| Kurozumi et al. [22] 2023 Japan | Observational | 6 | SGLT2 | 40 | 65.48 ± 13.46 | 32 (80%) | 40 (100%) | Empagliflozin 10 mg 9 (22.5%) Dapagliflozin 10 mg 31 (77.5%) | 29 (72.5%) 6 (15%) |
| Control | 69 | 73.81 ± 11.76 | 50 (72.46%) | 69 (100%) | Never used | 41 (59.4%) 17 (24.6%) | |||
| Kwon et al. [30] 2023 Korea | Observational PSM 1:2 | 25.2 | SGLT2 | 938 | 56.4 ± 11.3 | 769 (82%) | 938 (100%) | Empagliflozin 10 mg 302 (32.2%) Dapagliflozin 10 mg 605 (64.5%) Ipragliflozin 10 mg 31 (3.3%) | 550 (58.6%) 388 (41.4%) |
| Control | 1876 | 57.6 ± 11.3 | 1482 (79%) | 1876 (100%) | Non Users | 1137 (60.6) 739 (39.4%) | |||
| Lyu et al. [23] 2023 Korea | Observational IPTW | 12 | SGLT2 | 186 | 59.11 ± 11.52 | 150 (80.7%) | 186 (100%) | NR | 100 (53.8%) NR |
| Control | 593 | 66.12 ± 10.86 | 422 (71.2%) | 593 (100%) | Never used | 227 (38.3%) NR | |||
| Marfella et al. [26] 2023 Italy | Observational Prospective | 12 | SGLT2 | 177 | 66.2 ± 6.3 | 115 (65%) | 177 (100%) | NR | 99 (55.9%) NR |
| Control | 200 | 65.4 ± 6.1 | 128 (64%) | 200 (100%) | Never used | 111 (55.5%) NR | |||
| Lee et al. [29] 2024 Taiwan | Observational PSM 1:1 | from index date to the independent occurrence of the study outcomes, discontinuation of medication, or end of the study period | SGLT2 | 944 | NR | NR | 944 (100%) | Empagliflozin 10 mg (57%) Dapagliflozin 10 mg (38%) Canagliflozin 100 mg (5%) | NR |
| Control | 944 | NR | NR | 944 (100%) | Non Users | NR | |||
| Liu et al. [28] 2024 China | Observational, retrospective PSM 1:1 | 12 | SGLT2 | 226 | 62.9 ± 10.8 | 143 (63.3%) | 226 (100%) | NR | 104 (46%) 68 (30.1%) |
| Control | 226 | 62.1 ± 11.7 | 129 (57.1%) | 226 (100%) | Never used | 110 (48.7%) 69 (30.5%) | |||
| Rosen et al. [24] 2024 Sweden | Observational registry study from SWEDEHEART | 12 | SGLT2 | 2498 | 69 (61–75) | 1944 (77.8%) | 2498 (100%) | Dapagliflozin 480 (19.2%) Empaglifozin 1951 (78.1%) Canagliflozin 8 (0.3%) | 969 (38.8%) 1529 (61.2%) |
| Control | 8773 | 73 (65–79) | 6084 (69.3%) | 8773 (100%) | Never used | 2671 (30.5%) 6101 (69.6%) |
| Author & Year | Study Design and Type | Main Pathology | Moment of Initiation (Days or Weeks After ACS) |
|---|---|---|---|
| Fitchett et al. [18] 2019 | Pre-specified sub-group analysis EMPA-REG OUTCOME | Previous MI | After 8 weeks |
| Furtado et al. [19] 2019 | Prespecified sub-group analysis of DECLARE-TIMI 58 patients with previous MI and T2DM | Previous MI | After 8 weeks |
| Butler et al. [11] 2024 | EMPACT-MI | AMI at risk for HF | Within 14 days |
| James et al. [10] 2024 | DAPA-MI | AMI without T2DM or HF | Within 7–10 days |
| Peikert et al. [20] 2024 | Planned participant-level pooled analysis of patients with previous MI and HF, DAPA-HF and DELIVER RCT sub-analysis | Previous MI | After 12 weeks |
| Mee et al. [27] 2025 | Observational Retrospective PSM 1:1 | AMI | Within the first 14 days after initial AMI |
| Zhu et al. [25] 2022 | Observational Retrospective | AMI | At discharge |
| Chen et al. [13] 2023 | Observational Retrospective | ACS | Before/during hospitalization |
| Kanaoka et al. [21] 2023 | Observational Retrospective PSM 1:1 | ACS | Within 2 weeks, used before 194 (12%) |
| Kurozumi et al. [22] 2023 | Observational | ACS | During hospitalization |
| Kwon et al. [30] 2023 | Observational PSM 1:2 | AMI | Within 14 days AMI + T2DM treated with PCI |
| Lyu et al. [23] 2023 | Observational IPTW | AMI | Discharge |
| Marfella et al. [26] 2023 | Observational, Prospective | AMI | At least 6 months before |
| Lee et al. [29] 2024 | Observational PSM 1:1 | AMI | Within 12 weeks discharge |
| Liu et al. [28] 2024 | Observational, retrospective PSM 1:1 | ACS | During hospitalization |
| Rosen et al. [24] 2024 | Observational registry study from SWEDEHEART | AMI | Within 120 days before hospital discharge, prescribed during hospitalisation or redeemed prescription within 3 days. |
| Outcome | No. of Studies | Certainty Rating Domains | HR [95% CI] | Overall Certainty of Evidence | ||||
|---|---|---|---|---|---|---|---|---|
| Risk Of Bias (RoB 2 and ROBINS I) | Inconsistency | Indirectness | Imprecision | Publication Bias | ||||
| All-cause mortality | 5 RCTs + 6 observational (n = 130,288) | No concern | Serious a | No concern | No concern | Serious b | 0.77 [0.67–0.89] | ⬤⬤◯◯ Low |
| CV mortality | 5 RCTs + 5 observational (n = 121,627) | No concern | Some concern c | No concern | No concern | No concern | 0.83 [0.70–0.99] | ⬤⬤⬤◯ Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, I.M.; Luca, S.A.; Crișan, S.; Cozlac, A.-R.; Stoica, S.; Luca, C.T.; Timar, B.; Gaita, D. Do SGLT2 Inhibitors Improve Cardiovascular Outcomes After Acute Coronary Syndrome Regardless of Diabetes? A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1866. https://doi.org/10.3390/medicina61101866
Suciu IM, Luca SA, Crișan S, Cozlac A-R, Stoica S, Luca CT, Timar B, Gaita D. Do SGLT2 Inhibitors Improve Cardiovascular Outcomes After Acute Coronary Syndrome Regardless of Diabetes? A Systematic Review and Meta-Analysis. Medicina. 2025; 61(10):1866. https://doi.org/10.3390/medicina61101866
Chicago/Turabian StyleSuciu, Ioana Maria, Silvia Ana Luca, Simina Crișan, Alina-Ramona Cozlac, Svetlana Stoica, Constantin Tudor Luca, Bogdan Timar, and Dan Gaita. 2025. "Do SGLT2 Inhibitors Improve Cardiovascular Outcomes After Acute Coronary Syndrome Regardless of Diabetes? A Systematic Review and Meta-Analysis" Medicina 61, no. 10: 1866. https://doi.org/10.3390/medicina61101866
APA StyleSuciu, I. M., Luca, S. A., Crișan, S., Cozlac, A.-R., Stoica, S., Luca, C. T., Timar, B., & Gaita, D. (2025). Do SGLT2 Inhibitors Improve Cardiovascular Outcomes After Acute Coronary Syndrome Regardless of Diabetes? A Systematic Review and Meta-Analysis. Medicina, 61(10), 1866. https://doi.org/10.3390/medicina61101866