Tumor Growth Rate in Neuroendocrine Neoplasms: An Additional Tool for Treatment Strategies?
Abstract
1. Introduction
2. Tumor Growth Rate: The State of the Art
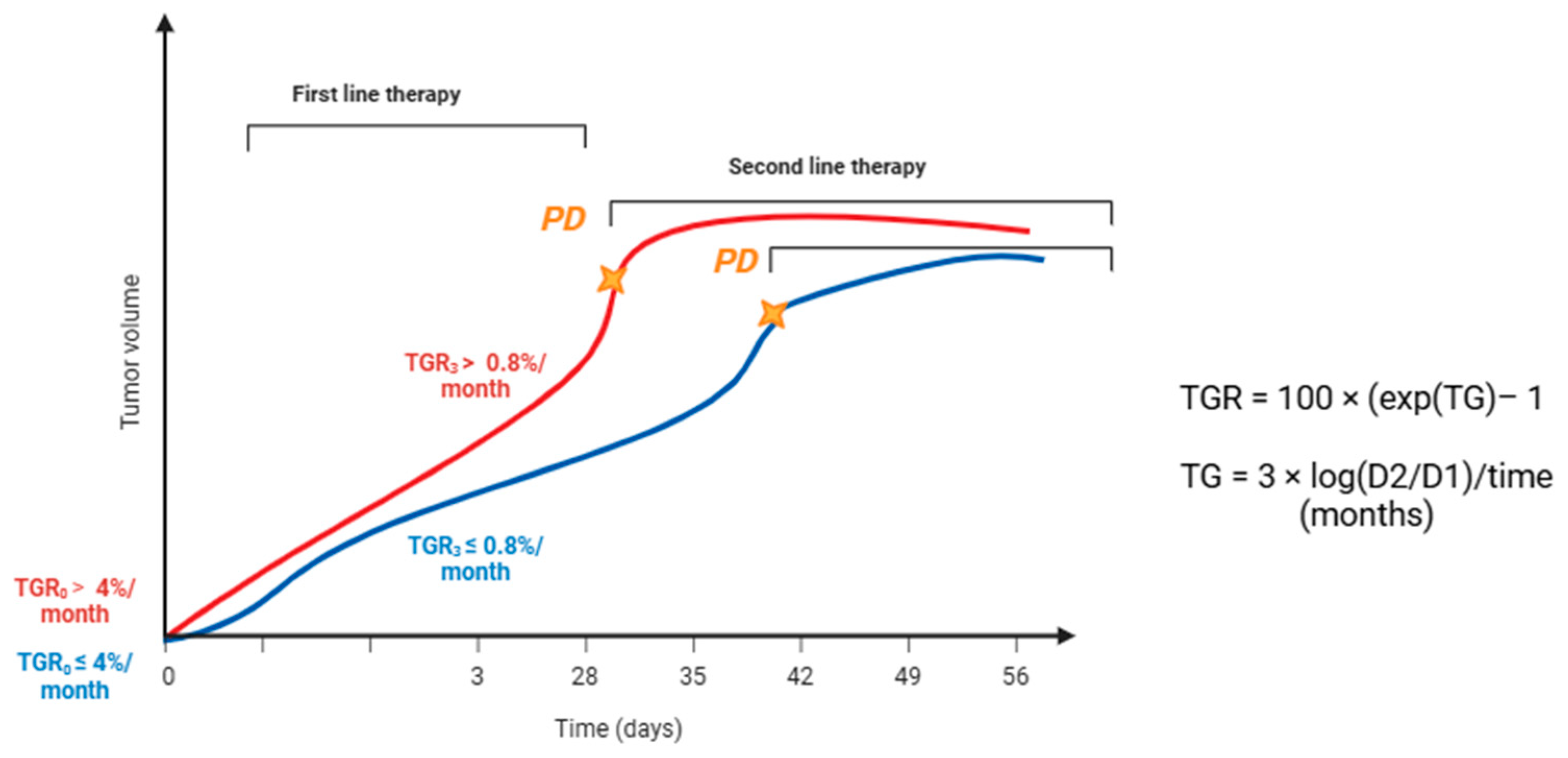
2.1. Definition
2.2. Treatment Strategies in Neuroendocrine Neoplasms and Challenges in Response Evaluation
2.3. Tumor Growth Rate in Neuroendocrine Neoplasms: Evidence and Application
2.3.1. Post Hoc Analysis of the CLARINET
2.3.2. The GREPONET Studies
2.3.3. TGR in PRRT and Novel Approaches
2.3.4. Additional Retrospective Evidence
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym | Definition |
|---|---|
| AUC | Area under the curve |
| CR | Complete Response |
| CT | Computed Tomography |
| cTGR | Cylindrical Tumor Growth Rate |
| DT | Doubling Time |
| L-NEN | Lung-Neuroendocrine neoplasm |
| MEN1 | Multiple Endocrine Neoplasia type 1 |
| MRI | Magnetic Resonance Imaging |
| mTOR | Mammalian target of rapamycin |
| NEN | Neuroendocrine neoplasm |
| ORR | Overall Response Rate |
| panNET | Pancreatic NET |
| PD | Progression Disease |
| PR | Partial Response |
| PRRT | Peptide receptor radionuclide therapy |
| RECIST 1.1 | Response Evaluation Criteria in Solid Tumors version 1.1 |
| RLT | Radioligand therapy |
| SD | Stable Disease |
| SGR | Specific Growth Rate |
| SSA | Somatostatin Analogs |
| TGR | Tumor Growth Rate |
| TKI | Tyrosine Kinase Inhibitor |
| WHO | World Health Organization |
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Current understanding of pathogenetic mechanisms in neuroendocrine neoplasms. Expert Rev. Endocrinol. Metab. 2024, 19, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.; Ezzat, S.; De Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Hendifar, A.E.; Ramirez, R.A.; Anthony, L.B.; Liu, E. Current Practices and Novel Techniques in the Diagnosis and Management of Neuroendocrine Tumors of Unknown Primary. Pancreas 2019, 48, 1111–1118. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Therapeutic strategies for patients with neuroendocrine neoplasms: Current perspectives. Expert Rev. Endocrinol. Metab. 2022, 17, 389–403. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Prasad, V.; Koumarianou, A.; Denecke, T.; Sundin, A.; Deroose, C.M.; Pavel, M.; Christ, E.; Lamarca, A.; Caplin, M.; Castaño, J.P.; et al. Challenges in developing response evaluation criteria for peptide receptor radionuclide therapy: A consensus report from the European Neuroendocrine Tumor Society Advisory Board Meeting 2022 and the ENETS Theranostics Task Force. J. Neuroendocrinol. 2025, 37, e13479. [Google Scholar] [CrossRef]
- van Treijen, M.J.; Schoevers, J.M.H.; Heeres, B.C.; van der Zee, D.; Maas, M.; Valk, G.D.; Tesselaar, M.E. Defining disease status in gastroenteropancreatic neuroendocrine tumors: Choi-criteria or RECIST? Abdom. Radiol. 2022, 47, 1071–1081. [Google Scholar] [CrossRef]
- Ronot, M.; Burgio, M.D.; Gregory, J.; Hentic, O.; Vullierme, M.-P.; Ruszniewski, P.; Zappa, M.; de Mestier, L. Appropriate use of morphological imaging for assessing treatment response and disease progression of neuroendocrine tumors. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101827. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Dromain, C.; de Herder, W.W.; Costa, F.P.; Deroose, C.M.; Frilling, A.; Koumarianou, A.; Krenning, E.P.; Raymond, E.; Bodei, L.; et al. ENETS standardized (synoptic) reporting for molecular imaging studies in neuroendocrine tumours. J. Neuroendocrinol. 2022, 34, e13040. [Google Scholar] [CrossRef]
- Collins, V.P.; Loeffler, R.K.; Tivey, H. Observations on growth rates of human tumors. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1956, 76, 988–1000. [Google Scholar] [PubMed]
- Kuroishi, T.; Tominaga, S.; Morimoto, T.; Tashiro, H.; Itoh, S.; Watanabe, H.; Fukuda, M.; Ota, J.; Horino, T.; Ishida, T.; et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn. J. Cancer Res. 1990, 81, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Ferté, C.; Fernandez, M.; Hollebecque, A.; Koscielny, S.; Levy, A.; Massard, C.; Balheda, R.; Bot, B.; Gomez-Roca, C.; Dromain, C.; et al. Tumor growth rate is an early indicator of antitumor drug activity in phase i clinical trials AC. Clin. Cancer Res. 2014, 20, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Crona, J.; Ronot, M.; Opalinska, M.; Lopez, C.L.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Bezerra, R.O.F.; Borg, P.; et al. Value of Tumor Growth Rate (TGR) as an Early Biomarker Predictor of Patients’ Outcome in Neuroendocrine Tumors (NET)—The GREPONET Study. Oncologist 2019, 24, e1082–e1090. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R.; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long-term survival. Neuroendocrinology 2016, 104, 26–32. [Google Scholar] [CrossRef]
- Faggiano, A. Long-acting somatostatin analogs and well differentiated neuroendocrine tumors: A 20-year-old story. J. Endocrinol. Investig. 2024, 47, 35–46. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.M.T.; Wolin, E.M.; Ruszniewski, P.; Clarinet Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef]
- Faggiano, A.; Modica, R.; Calzo, F.L.; Camera, L.; Napolitano, V.; Altieri, B.; de Cicco, F.; Bottiglieri, F.; Sesti, F.; Badalamenti, G.; et al. Lanreotide Therapy vs Active Surveillance in MEN1-Related Pancreatic Neuroendocrine Tumors < 2 Centimeters. J. Clin. Endocrinol. Metab. 2019, 105, 78–84. [Google Scholar] [CrossRef]
- Albertelli, M.; Dotto, A.; Di Dato, C.; Malandrino, P.; Modica, R.; Versari, A.; Colao, A.; Ferone, D.; Faggiano, A.; Nike, O.B.O. PRRT: Identikit of the perfect patient. Rev. Endocr. Metab. Disord. 2021, 22, 563–579. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.; Frilling, A.; de Herder, W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [PubMed]
- Capdevila, J.; Teulé, A.; Barriuso, J.; Castellano, D.; Lopez, C.; Manzano, J.L.; Alonso, V.; García-Carbonero, R.; Dotor, E.; Matos, I.; et al. Phase II Study of Everolimus and Octreotide LAR in Patients with Nonfunctioning Gastrointestinal Neuroendocrine Tumors: The GETNE1003_EVERLAR Study. Oncologist 2019, 24, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; Russo, G.L.; Procopio, G. Everolimus treatment for neuroendocrine tumors: Latest results and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.-J.; Lee, S.-H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef]
- Faivre, S.; Ronot, M.; Dreyer, C.; Serrate, C.; Hentic, O.; Bouattour, M.; Bruno, O.; Couvelard, A.; Vilgrain, V.; Raymond, E. Imaging response in neuroendocrine tumors treated with targeted therapies: The experience of sunitinib. Target. Oncol. 2012, 7, 127–133. [Google Scholar] [CrossRef]
- Solis-Hernandez, M.P.; del Valle, A.F.; Carmona-Bayonas, A.; Garcia-Carbonero, R.; Custodio, A.; Benavent, M.; Gordoa, T.A.; Nuñez-Valdovino, B.; Canovas, M.S.; Matos, I.; et al. Evaluating radiological response in pancreatic neuroendocrine tumours treated with sunitinib: Comparison of Choi versus RECIST criteria (CRIPNET_ GETNE1504 study). Br. J. Cancer 2019, 121, 537–544. [Google Scholar] [CrossRef]
- Zappi, A.; Persano, I.; Galvani, L.; Parlagreco, E.; Andrini, E.; Campana, D.; Brizzi, M.P.; Lamberti, G.; La Salvia, A. Chemotherapy in Well Differentiated Neuroendocrine Tumors (NET) G1, G2, and G3: A Narrative Review. J. Clin. Med. 2023, 12, 717. [Google Scholar] [CrossRef]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.N.; Lin, M.; Geschwind, J.-F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017, 283, 883–894. [Google Scholar] [CrossRef]
- Dromain, C.; Pavel, M.E.; Ruszniewski, P.; Langley, A.; Massien, C.; Baudin, E.; Caplin, M.E. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 2019, 19, 66. [Google Scholar] [CrossRef]
- Scalorbi, F.; Garanzini, E.M.; Calareso, G.; Marzi, C.; Di Rocco, G.; Argiroffi, G.; Baccini, M.; Pusceddu, S.; Marchianò, A.; Maccauro, M. Cylindrical TGR as early radiological predictor of RLT progression in GEPNETs: A proof of concept. Sci. Rep. 2024, 14, 15782. [Google Scholar] [CrossRef]
- Pettersson, O.J.; Fröss-Baron, K.; Crona, J.; Sundin, A. Tumor growth rate in pancreatic neuroendocrine tumor patients undergoing PRRT with 177Lu-DOTATATE. Endocr. Connect. 2021, 10, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Whitman, J.; Paciorek, A.; Le, B.K.; Nakakura, E.K.; Behr, S.C.; Joseph, N.; Zhang, L.; Hope, T.A.; Bergsland, E.K. Baseline tumor growth rate highlights the heterogeneity of well differentiated gastroenteropancreatic neuroendocrine tumors and predicts for increases in Ki67 index over time. J. Neuroendocrinol. 2023, 35, e13260. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ronot, M.; Moalla, S.; Crona, J.; Opalinska, M.; Lopez Lopez, C.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Bezerra, R.O.F.; et al. Tumor Growth Rate as a Validated Early Radiological Biomarker Able to Reflect Treatment-Induced Changes in Neuroendocrine Tumors: The GREPONET-2 Study. Clin. Cancer Res. 2019, 25, 6692–6699. [Google Scholar] [CrossRef]
- Dromain, C.; Loaiza-Bonilla, A.; Mirakhur, B.; Beveridge, T.J.; Fojo, A.T. Novel Tumor Growth Rate Analysis in the Randomized CLARINET Study Establishes the Efficacy of Lanreotide Depot/Autogel 120 mg with Prolonged Administration in Indolent Neuroendocrine Tumors. Oncologist 2021, 26, e632–e638. [Google Scholar] [CrossRef]
- Bs, J.J.B.; Smith, P.M.; Tan, M.; Solórzano, C.C.; Lopez-Aguiar, A.G.; Dillhoff, M.; Beal, E.W.; Poultsides, G.; Makris, E.; Rocha, F.G.; et al. Specific Growth Rate as a Predictor of Survival in Pancreatic Neuroendocrine Tumors: A Multi-institutional Study from the United States Neuroendocrine Study Group. Ann. Surg. Oncol. 2020, 27, 3915–3923. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; La Salvia, A.; Mazzilli, R.; Pandozzi, C.; Pecora, G.; Barrea, L.; Colao, A.; Faggiano, A. Impact of treatment on quality of life in neuroendocrine neoplasm survivors. Endocr.-Relat. Cancer 2025, 32. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
| Therapy | Evidence/Key Trials | Main Findings | Response Assessment Challenges |
|---|---|---|---|
| Somatostatin analogs (SSA) (Octreotide, Lanreotide) | PROMID, CLARINET | Significant PFS benefit vs. placebo; efficacy also in lung NET and MEN1-related duodenopancreatic NET | Rare CR/PR by RECIST 1.1; mainly cytostatic effect (disease stabilization); careful radiological monitoring required |
| Radioligand therapy (PRRT/RLT) (^177Lu-DOTATATE) | NETTER-1, NETTER-2 | Prolonged PFS and improved symptom control; 18% ORR vs. 3% with high-dose SSA; efficacy in both midgut and G2-G3 GEP-NET | Efficacy assessed only by RECIST 1.1; density or growth-rate changes not captured; TGR could provide earlier response detection |
| Targeted therapies (Everolimus, Sunitinib) | RADIANT trials (everolimus); Phase III sunitinib (panNET) | Everolimus: improved PFS in GEP and lung NET; Sunitinib: improved PFS (11.4 vs. 5.5 mo) and OS (38.6 vs. 29.1 mo) | RECIST may underestimate benefit; Choi criteria are more sensitive for sunitinib (PR 47.4% vs. 12.8% by RECIST); necrosis/density changes are not reflected in RECIST |
| Chemotherapy | Limited studies have been used in high-grade/progressive NET | More effective in aggressive disease; volumetric changes are more evident | Role of TGR exploratory; rapid TGR decrease may predict response, persistent TGR may indicate resistance |
| Locoregional therapies (embolization, chemoembolization, radioembolization, ablation) | Observational studies | Can induce necrosis, cavitation, density changes | RECIST is often inadequate; TGR may provide quantitative kinetics, but validation is limited due to multifocal disease and technical issues |
| References | Study Type | Population | Main Findings on TGR | Reported TGR Thresholds/Values |
|---|---|---|---|---|
| [32] | Post hoc analysis of a randomized trial (CLARINET) | 204 NET | TGR0 ≤ 4%/month, irrespective of treatment allocation, achieved longer PFS than those with TGR0 > 4%/month. | Even among patients with TGR0 > 4%/month, lanreotide prolonged PFS compared with placebo (96.3 vs. 37.7 weeks). |
| [33] | Proof-of-concept study | 58 GEP-NET | Cylindrical TGR (cTGR) outperformed conventional TGR in predicting progression (ROC AUC 1.00 vs. 0.92). | No absolute cut-off reported; cTGR showed higher predictive accuracy. |
| [34] | Retrospective study | 151 PanNET | TGR markedly decreased during PRRT; high pre-treatment TGR identified non-responders and correlated with shorter PFS. | Pre-PRRT TGR median ~ +2.3%/mo; during PRRT, reduced to ~–0.3%/mo (stabilization). |
| [35] | Observational retrospective study | 48 GEP-NET (G1-G3) | High baseline TGR is associated with shorter time to treatment, worse OS, and increased Ki-67 during follow-up. | Cut-off: >11.7%/month defined “high TGR”. |
| [15] | Multicenter retrospective study | 127 NET (G1-G2) | TGR validated as early biomarker reflecting treatment-induced changes and predicting PFS/response. | No fixed threshold; dynamic changes in TGR are used for prediction. |
| [36] | Multicenter retrospective study | 22 GEP-NET (G1-G2) | TGR at 3 months (TGR3m) is predictive of PFS and identifies high-risk patients needing closer follow-up. | TGR3m increase associated with poor outcomes; no single universal cut-off, but rising TGR = adverse. |
| [37] | Retrospective cohort study | 198 GEP-NET | Approach combining tumor growth and regression parameters captured worse PFS. | A reduction of 26% in TGR was related to significantly higher tumor volume doubling time. |
| [38] | Multicenter retrospective cohort study | 288 PanNET | SGR, mathematically related to TGR, was independently associated with OS. Patients with higher SGR had significantly worse outcomes, supporting its role as a prognostic biomarker. | High SGR (positive growth) correlated with poorer OS; exact cut-offs varied, but SGR > 0 defined progressive disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modica, R.; Liccardi, A.; Benevento, E.; Minotta, R.; Di Iasi, G.; Di Nola, M.; Coletta, M.; Colao, A. Tumor Growth Rate in Neuroendocrine Neoplasms: An Additional Tool for Treatment Strategies? Medicina 2025, 61, 1852. https://doi.org/10.3390/medicina61101852
Modica R, Liccardi A, Benevento E, Minotta R, Di Iasi G, Di Nola M, Coletta M, Colao A. Tumor Growth Rate in Neuroendocrine Neoplasms: An Additional Tool for Treatment Strategies? Medicina. 2025; 61(10):1852. https://doi.org/10.3390/medicina61101852
Chicago/Turabian StyleModica, Roberta, Alessia Liccardi, Elio Benevento, Roberto Minotta, Gianfranco Di Iasi, Massimo Di Nola, Michele Coletta, and Annamaria Colao. 2025. "Tumor Growth Rate in Neuroendocrine Neoplasms: An Additional Tool for Treatment Strategies?" Medicina 61, no. 10: 1852. https://doi.org/10.3390/medicina61101852
APA StyleModica, R., Liccardi, A., Benevento, E., Minotta, R., Di Iasi, G., Di Nola, M., Coletta, M., & Colao, A. (2025). Tumor Growth Rate in Neuroendocrine Neoplasms: An Additional Tool for Treatment Strategies? Medicina, 61(10), 1852. https://doi.org/10.3390/medicina61101852







