Efficacy and Safety of Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
2.5. Risk of Bias and Certainty of Evidence
2.6. Statistical Analysis
3. Results
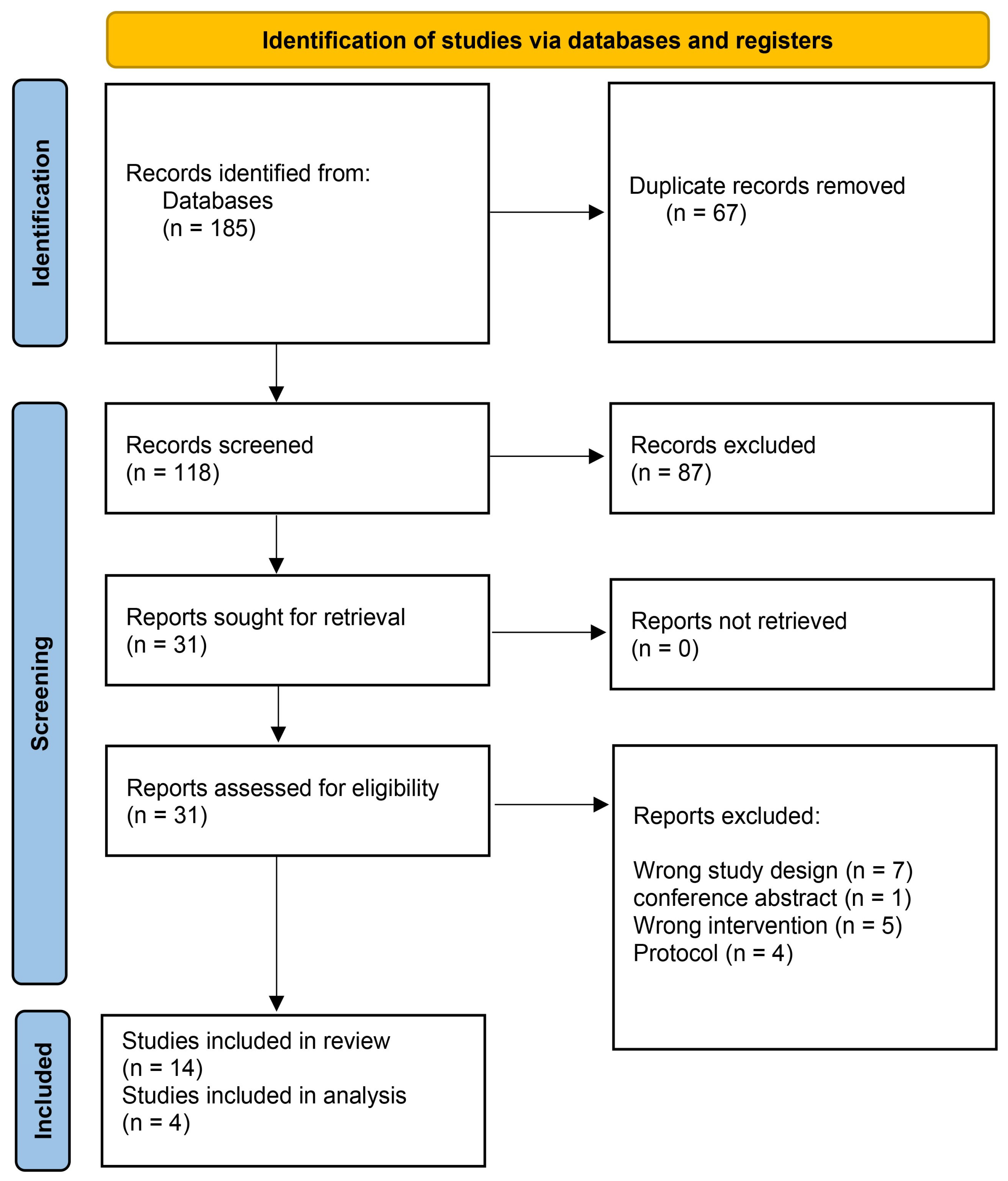
3.1. Search Results and Study Selection
3.2. Study Characteristics
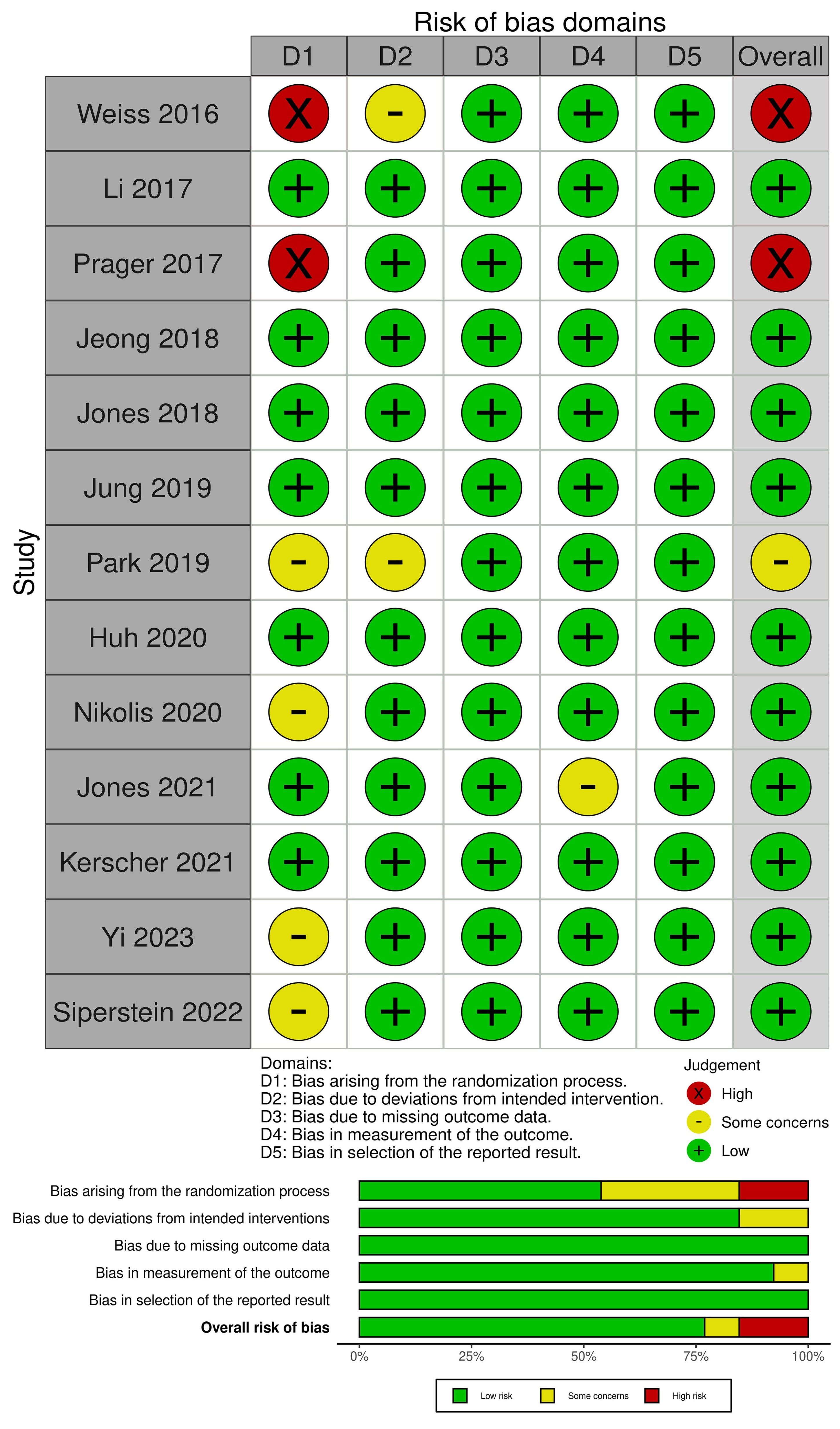
3.3. Quality Assessment
3.4. Meta-Analysis
3.4.1. Global Aesthetic Improvement Scale (GAIS) Responder Rate
3.4.2. Mean Change in Global Aesthetic Improvement Scale (GAIS) over Time
3.4.3. Moderate to Severe Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendelson, B.; Wong, C.-H. Changes in the Facial Skeleton with Aging: Implications and Clinical Applications in Facial Rejuvenation. Aesthetic Plast. Surg. 2012, 36, 753–760. [Google Scholar] [CrossRef]
- Statistics, P. Plastic Surgery Statistics Report; American Society of Plastic Surgeons: Arlington Heights, IL, USA, 2017; p. 25. [Google Scholar]
- Chirico, F.; Colella, G.; Cortese, A.; Bove, P.; Fragola, R.; Rugge, L.; Audino, G.; Sgaramella, N.; Tartaro, G. Non-surgical touch-up with hyaluronic acid fillers following facial reconstructive surgery. Appl. Sci. 2021, 11, 7507. [Google Scholar] [CrossRef]
- Coleman, S.R.; Grover, R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthetic Surg. J. 2006, 26, S4–S9. [Google Scholar] [CrossRef]
- Farolch-Prats, L.; Nome-Chamorro, C. Facial contouring by using dermal fillers and botulinum toxin A: A practical approach. Aesthetic Plast. Surg. 2019, 43, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Linkov, G.; Mally, P.; Czyz, C.N.; Wulc, A.E. Quantification of the Aesthetically Desirable Female Midface Position. Aesthetic Surg. J. 2018, 38, 231–240. [Google Scholar]
- Hwang, K.; Park, C.Y. The divine proportion: Origins and usage in plastic surgery. Plast. Reconstr. Surg.–Glob. Open 2021, 9, e3419. [Google Scholar]
- Pantermehl, S.; Foth, A.; Meyer, E.; Barbeck, M.; Jung, O. In Vitro Cytocompatibility Analysis and Comparison of Different Hyaluronic Acid Fillers for Minimally Invasive Esthetics. In Vivo 2024, 38, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- de la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and Physicochemical Characteristics of Hyaluronic Acid Fillers: Overview and Relationship to Product Performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.C.; Fogaca, A.; Palermo, E.; Fontes, M.; Barud, H.S.; Dametto, A.C. A New Cohesive High-Concentrated Hyaluronic Acid Gel Filler: Correlation between Rheologic Properties and Clinical Indications. J. Biomed. Res. Environ. Sci. 2023, 4, 614–618. [Google Scholar] [CrossRef]
- Doerfler, L.; Hanke, C.W. Arterial Occlusion and Necrosis Following Hyaluronic Acid Injection and a Review of the Literature. J. Drugs Dermatol. 2019, 18, 587–591. [Google Scholar]
- Vrabel, M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Oncol. Nurs. Forum 2015, 42, 552–554. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Eldridge, S.; Campbell, M.; Campbell, M.; Dahota, A.; Giraudeau, B.; Higgins, J.; Reeves, B.; Siegfried, N. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): Additional considerations for cluster-randomized trials. Cochrane Methods Cochrane Database Syst. Rev. 2016, 10 (Suppl. S1), 1–17. [Google Scholar]
- Huh, C.H.; Eom, Y.; Yang, S.D.; Shin, J.W.; Seo, K.K. A Randomized, Active-Controlled, 52-Week Study of Hyaluronic Acid Fillers for Anteromedial Malar Region Augmentation. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2648. [Google Scholar] [CrossRef]
- Jeong, K.H.; Gwak, M.J.; Moon, S.K.; Lee, S.J.; Shin, M.K. Efficacy and durability of hyaluronic acid fillers for malar enhancement: A prospective, randomized, split-face clinical controlled trial. J. Cosmet. Laser Ther. 2018, 20, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Baumann, L.; Moradi, A.; Shridharani, S.; Palm, M.; Teller, C.; Taylor, M.; Kontis, T.C.; Chapas, A.; Kaminer, M.S.; et al. A Randomized, Comparator-Controlled Study of HARC for Cheek Augmentation and Correction of Midface Contour Deficiencies. J. Drugs Dermatol. 2021, 20, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.T.; Vanaman Wilson, M.J.; Bolton, J.; Zaleski-Larsen, L.; Wu, D.C.; Goldman, M.P. A Single Center, Prospective, Randomized, Sham-Controlled, Double-Blinded, Split-Face Trial Using Microinjections of Transparent Hyaluronic Acid Gel for Cheek Rejuvenation. Dermatol. Surg. 2018, 44, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Lee, W.S.; Kim, H.T.; Moon, Y.S.; Paik, S.H.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Won, C.H.; Kim, B.J. A Multi-center, randomized, double blinded, comparative study of two hyaluronic acid fillers for temporary restoration of mid-face volume in Asians. J. Cosmet. Dermatol. 2020, 19, 1619–1626. [Google Scholar] [CrossRef]
- Kerscher, M.; Agsten, K.; Kravtsov, M.; Prager, W. Effectiveness evaluation of two volumizing hyaluronic acid dermal fillers in a controlled, randomized, double-blind, split-face clinical study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 239–247. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Wu, Y.; Sun, J.; Li, Q.; Guo, S.; Jia, Y.; Murphy, D.K. A Randomized, Controlled, Multicenter Study of Juvederm Voluma for Enhancement of Malar Volume in Chinese Subjects. Plast. Reconstr. Surg. 2017, 139, 1250e–1259e. [Google Scholar] [CrossRef]
- Nikolis, A.; Enright, K.M.; Lazarova, D.; Sampalis, J. The Role of Clinical Examination in Midface Volume Correction Using Hyaluronic Acid Fillers: Should Patients Be Stratified by Skin Thickness? Aesthetic Surg. J. Open Forum 2020, 2, ojaa005. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, J.M.; Seok, J.; Seo, S.J.; Kim, M.N.; Youn, C.S. Comparative split-face study of durational changes in hyaluronic acid fillers for mid-face volume augmentation. Dermatol. Ther. 2019, 32, e12950. [Google Scholar] [CrossRef] [PubMed]
- Prager, W.; Agsten, K.; Kerscher, M. Patient-Reported Outcomes following Split-Face Injection of 2 Volumizing Fillers in the Upper Cheeks. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1412. [Google Scholar] [CrossRef]
- Ren, R.; Xue, H.; Gao, Z.; Wang, H.; Xia, X.; Qu, Y.; Bromée, T.; Almegård, B.; Zhao, H. Restoring long-lasting midface volume in the Asian face with a hyaluronic acid filler: A randomized controlled multicenter study. J. Cosmet. Dermatol. 2024, 23, 1985–1991. [Google Scholar] [CrossRef]
- Effectiveness Safety of Large Gel Particle Hyaluronic Acid with Lidocaine for Correction of Midface Volume Deficit or Contour Deficiency: ERRATUM. Dermatol. Surg. 2016, 42, 1233. [CrossRef]
- Yi, C.C.; Hahn, H.M.; Lim, H.; Kim, Y.J.; Choi, Y.W.; Kim, J.H. A multicenter, randomized, double-blind, comparative study of a multiphasic hyaluronic acid filler and existing hyaluronic acid fillers for temporary restoration of the midface volume of Asian individuals. J. Plast. Reconstr. Aesthetic Surg. 2023, 82, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Siperstein, R.; Nestor, E.; Meran, S.; Grunebaum, L. A split-face, blind, randomized placebo-controlled clinical trial investigating the efficacy and safety of hyaluronic acid filler for the correction of atrophic facial scars. J. Cosmet. Dermatol. 2022, 21, 3768–3778. [Google Scholar] [CrossRef]
- Few, J.; Cox, S.E.; Paradkar-Mitragotri, D.; Murphy, D.K. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: Patient-reported outcomes at 2 years. Aesthetic Surg. J. 2015, 35, 589–599. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Fei, X.; Fan, Q.; Mao, J. Monophasic and biphasic hyaluronic acid fillers for esthetic correction of nasolabial folds: A meta-analysis of randomized controlled trials. Aesthetic Plast. Surg. 2022, 46, 1407–1422. [Google Scholar] [CrossRef]
- Ting, W.; Chong, Y.; Long, X.; Shu, M.; Wang, H.; Huang, J.; Zeng, A.; Bai, Z.; Wang, R.; Zhang, X.; et al. A Randomized, Evaluator-Blinded, Multicenter Study to Compare Injectable Poly-D, L-Lactic Acid vs Hyaluronic Acid for Nasolabial Fold Augmentation. Aesthetic Surg. J. 2024, 44, NP898–NP905. [Google Scholar] [CrossRef]
- Hong, J.K.; Park, S.J.; Seo, S.J.; Park, K.Y.; Youn, C.S. Quantitative Evaluation of Volume Augmentation and Durational Changes in the Anteromedial Cheek with Hyaluronic Acid Fillers Using Three-Dimensional Measurement: 2-Year Results from a Comparative Split-Face Study. Plast. Reconstr. Surg. 2022, 150, 87e–91e. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.F.; Hicks, J.A.; Nguyen, T.; Meckfessel, M. Split-Face Comparison of Two Hyaluronic Acid Fillers: Intersection of Rheology and Tissue Behavior in Midface Rejuvenation. Aesthetic Surg. J. Open Forum 2025, 7, ojaf006. [Google Scholar] [CrossRef]
- Cohen, J.L.; Patel, M.; Hicks, J. Ten-year global postmarket safety surveillance of delayed complications with a flexible cheek filler. Dermatol. Surg. 2022, 48, 1126–1127. [Google Scholar] [CrossRef]
- Beleznay, K.; Carruthers, J.D.; Humphrey, S.; Carruthers, A.; Jones, D. Update on avoiding and treating blindness from fillers: A recent review of the world literature. Aesthetic Surg. J. 2019, 39, 662–674. [Google Scholar] [CrossRef]
- Doyon, V.C.; Liu, C.; Fitzgerald, R.; Humphrey, S.; Jones, D.; Carruthers, J.D.A.; Beleznay, K. Update on Blindness from Filler: Review of Prognostic Factors, Management Approaches, and a Century of Published Cases. Aesthetic Surg. J. 2024, 44, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Mandy, S.H. Satisfying patient expectations with soft-tissue augmentation. Dermatol. Online J. 2009, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Moers-Carpi, M.; Vogt, S.; Santos, B.M.; Planas, J.; Vallve, S.R.; Howell, D.J. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol. Surg. 2007, 33 (Suppl. S2), S144–S151. [Google Scholar]
| Study ID | Place of Study | Study Design | Trial Registration Number | Sample Size | Age (Years) | Gender (M/F) | Group 1 (Intervention) | Volume of Injection | Site of Injection | Outcomes Assessed | Conclusion | Study Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weiss 2016 [27] | USA | RCT | NA | 200 | 52.9 ± 7.6 mean | 17/183 | LGP-HAL | 3.8 mL | Midface (supraperiosteal to subcutaneous layer) | GAIS score, FACE-Q Score, MMVS | The treatment was safe and effective for correcting midface volume loss using LGP-HA-L | 2, 14 weeks, 12 months |
| Li 2017 [22] | China | RCT | NA | 116 | 20–67 median | 6/140 | Volume | 1 mL | Deep subcutaneous/sub-periosteal (medial malar region) | Midface volume, GAIS score, patient satisfaction | Juvéderm Voluma is effective and well tolerated for midface (malar) augmentation in Chinese subjects. Significant volume increase (1.83 mL vs. 0.11 mL at 6 months, p < 0.001); high responder rates (98.2% by investigators, 93.8% by subjects); mild, localize | 1, 6 months |
| Prager 2017 [25] | Germany | RCT | NA | 45 | 38–66 median | 2 Male 43 Female | CPM-26 (25 mg/mL HA) | 2 mL | Subdermal/periosteal | GAIS score | Both CPM-26 and VYC-20 were effective and well-received, with a consistent trend favoring CPM-26 based on patient-reported outcomes. | 3, 12, 18 months |
| Jeong 2018 [17] | Korea | RCT | NA | 10 | 32–60 median | 0/10 | Mono-HA filler | 0.6 mL | Deep dermis | GAIS score, Moire topography, adverse events | Both novel biphasic and monophasic hyaluronic acid fillers showed similar effectiveness and safety for malar enhancement over 24 weeks, with only mild and transient injection site reactions; MRI proved useful for assessing filler distribution and volume changes | 2, 4, 8, 12, 24 weeks |
| Jones 2018 [19] | USA | RCT | NA | 20 | 58.4 ± 10.4 mean | 0/20 | SP-HAL | 1 mL | Intradermal microdroplet | Cheek volume, patient satisfaction | One session of intradermal microdroplet injections of SP-HAL to the mid to lower cheek failed to show superiority over normal saline in improving skin wrinkling and elastosis; procedure was safe, with only mild, transient side effects | 7, 14, 28, 90, 180 days |
| Jung 2020 [20] | Korea | RCT | NCT02721368 | 83 | 35–65 median | 19/64 | Neuvamis | 1 mL (max per side) | Not mentioned | GAIS score | Volume Lidocaine was non-inferior to VYC-20L for temporary midface volume restoration at 24 weeks; both fillers were effective, safe, and well tolerated with minimal safety concerns | 0, 4, 12, 24 weeks |
| Park 2019 [24] | South Korea | RCT | NA | 9 | 30–80 median | NA | Belotero HA filler | 0.5 mL | Midface region | GAIS score, MFV scale | Monophasic fillers (B, J) demonstrated longer-lasting midface volumization than biphasic fillers (R, Y). Filler B showed excellent performance and injectability, especially in high-pressure areas, supported by objective 3D imaging data. | 2, 4, 12, 24 weeks |
| Huh 2020 [16] | Korea | RCT | NCT02119780 | 68 | 20–65 median | NA | YVOC (22 mg/mL HA) | 4.0 mL (each side) | Anteromedial malar | GAIS score, MFV score | The HA fillers injected for the anteromedial malar augmentation maintained the volume well for up to 52 weeks. Additionally, both YVOC and RESS show similar effectiveness and safety profiles | 2, 14, 28, 52 weeks |
| Nikolis 2020 [23] | Canada | RCT | NA | 30 | 30–75 median | 0/30 | Restylane Lyft (HAL) | 2× per session | Not mentioned | MFVDS score, GAIS score, patient satisfaction | The use of a treatment algorithm may improve outcomes for patients seeking injectable treatments for midfacial volume loss and contour deficiencies | 2, 8, 16 weeks |
| Jones 2021 [18] | USA (New York) | RCT | NA | 210 | 24–80 median | 24/176 | HARC | 6.0 mL (each side) | Subperiosteal/subcutaneous (midface) | GAIS score, FACE-Q Score | HARC was well tolerated and non-inferior to Control for correction of midface fullness at 12 weeks after last injection. Aesthetic improvement and subject satisfaction were high and lasted through week 48 | 12, 24, 36 weeks |
| Kerscher 2017 [21] | Germany | RCT | NA | 45 | 18–63 median | 1 Male 44 Female | CPM-26 | 2 mL | Not mentioned | Photographic assessment, GAIS scores | CPM-26 was non-inferior to VYC-20 based on MAS ratings at M3 and demonstrated a favorable safety and effectiveness profile for midfacial volume enhancement with results lasting up to M18. | 1, 8, 12, 18 months |
| Yi 2023 [28] | Korea | RCT | NA | 92 | 46.5 ± 8.72 mean | 4 Male 98 Female | Giselleligne (multilayered HA) | NA | Midface Region | MFVDS score, GAIS score, operator satisfaction | This study showed that HAVOL is effective and well tolerated for midface treatment in a Chinese population | 1, 4, 8, 12, 24 weeks |
| Siperstein 2022 [29] | US | RCT | NA | 15 | 42.1 mean | 4 Male 11 Female | VYC-17.5 L (a hyaluronic acid filler) | 1 mL per side | Midface region | GAIS score, MMVS score, subject satisfaction, effectiveness and safety of HAVOL in the treatment of midface volume deficit and/or midface contour deficiency | Giselleligne is a safer, more user-friendly, and more effective alternative to existing products for improving the midfacial volume. | 1, 3, 6, 9, and 12 months post-injection |
| Ren 2024 [26] | China | RCT | NCT03289052 | 148 | 41.3 ± 10.1 mean | 12/136 | HA_VOL (Restylane® Volyme) group | 8 mL (Total) | Midface region | GAIS score, MMVS score, subject satisfaction, effectiveness and safety of HAVOL in the treatment of midface volume deficit and/or midface contour deficiency | Giselleligne is a safer, more user-friendly, and more effective alternative to existing products for improving the midfacial volume. | 1, 3, 6, 9, and 12 months post-injection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safia, A.; Abd Elhadi, U.; Merchavy, S.; Batheesh, R.; Bathish, N. Efficacy and Safety of Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1823. https://doi.org/10.3390/medicina61101823
Safia A, Abd Elhadi U, Merchavy S, Batheesh R, Bathish N. Efficacy and Safety of Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(10):1823. https://doi.org/10.3390/medicina61101823
Chicago/Turabian StyleSafia, Alaa, Uday Abd Elhadi, Shlomo Merchavy, Ramzy Batheesh, and Naji Bathish. 2025. "Efficacy and Safety of Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review and Meta-Analysis" Medicina 61, no. 10: 1823. https://doi.org/10.3390/medicina61101823
APA StyleSafia, A., Abd Elhadi, U., Merchavy, S., Batheesh, R., & Bathish, N. (2025). Efficacy and Safety of Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review and Meta-Analysis. Medicina, 61(10), 1823. https://doi.org/10.3390/medicina61101823