Outcomes of Endoscopic Sleeve Gastroplasty: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Risk of Bias Assessment
2.3. Endoscopic Sleeve Gastroplasty Definition and Technique
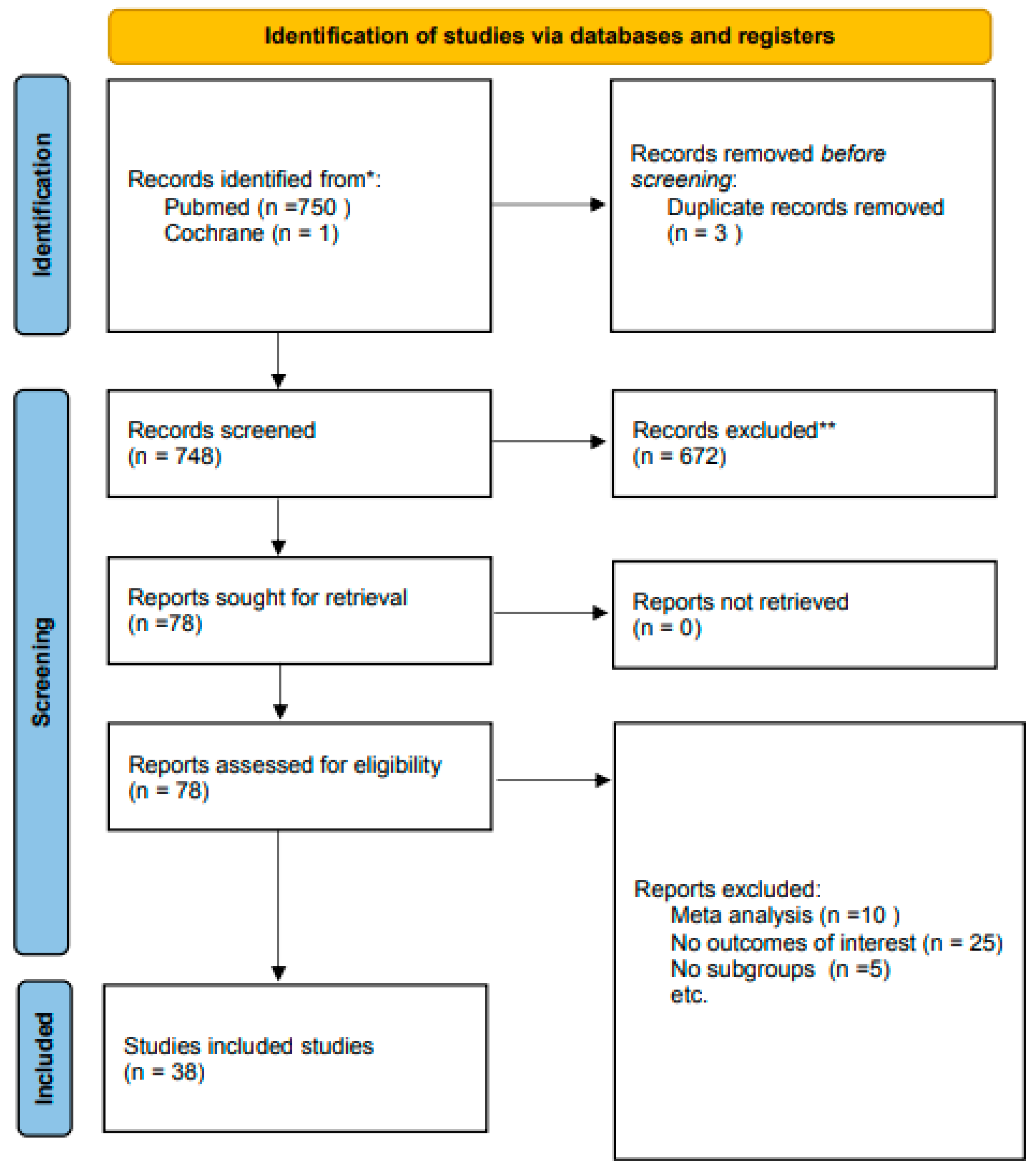
3. Results
Weight Loss and Comorbidity Resolution
4. Discussion
5. Limitations
6. Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 28 July 2025).
- Ng, M.; Fleming, T.; Robinson, M. Global, regional, and national prevalence of overweight and obesity in children and adults. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 1492. [Google Scholar] [CrossRef]
- de Moura, D.T.H.; Barrichello, S., Jr.; de Moura, E.G.H.; de Souza, T.F.; Neto, M.D.P.G.; Grecco, E.; Sander, B.; Hoff, A.C.; Matz, F.; Ramos, F.; et al. Endoscopic sleeve gastroplasty in the management of weight regain after sleeve gastrectomy. Endoscopy 2020, 52, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Matteo, M.V.; Bove, V.; Ciasca, G.; Carlino, G.; Di Santo, R.; Vinti, L.; Boškoski, I. Success Predictors of Endoscopic Sleeve Gastroplasty. Obes. Surg. 2024, 34, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Yanovski, S.Z.; Yanovski, J.A. Long-term drug treatment for obesity: A systematic and clinical review. JAMA 2014, 311, 74–86. [Google Scholar] [CrossRef]
- Mauro, A.; Lusetti, F.; Scalvini, D.; Bardone, M.; De Grazia, F.; Mazza, S.; Pozzi, L.; Ravetta, V.; Rovedatti, L.; Sgarlata, C.; et al. A Comprehensive Review on Bariatric Endoscopy: Where We Are Now and Where We Are Going. Medicina 2023, 59, 636. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Acosta, A.; Camilleri, M.; Mundi, M.S.; Rajan, E.; Topazian, M.D.; Gostout, C.J. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin. Gastroenterol. Hepatol. 2017, 15, 37–43.e1. [Google Scholar] [CrossRef]
- Galvão-Neto, M.D.; Grecco, E.; Souza, T.F.; Quadros, L.G.; Silva, L.B.; Campos, J.M. ENDOSCOPIC SLEEVE GASTROPLASTY-MINIMALLY INVASIVE THERAPY FOR PRIMARY OBESITY TREATMENT. Arq. Bras. Cir. Dig. 2016, 29 (Suppl. S1), 95–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ASGE/ASMBS Task Force on Endoscopic Bariatric Therapy. A pathway to endoscopic bariatric therapies. Surg. Obes. Relat. Dis. 2011, 7, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Galvão, M.P.; Bautista-Castaño, I.; Fernandez-Corbelle, J.P.; Trell, M.; Lopez, N. Endoscopic Sleeve Gastroplasty for Obesity Treatment: Two Years of Experience. Arq. Bras. Cir. Dig. 2017, 30, 18–20. [Google Scholar] [CrossRef]
- Mohan, B.P.; Asokkumar, R.; Khan, S.R.; Kotagiri, R.; Sridharan, G.K.; Chandan, S.; Adler, D.G. Outcomes of endoscopic sleeve gastroplasty, how does it compare to laparoscopic sleeve gastrectomy? A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E558–E565. [Google Scholar] [CrossRef]
- Hedjoudje, A.; Dayyeh, B.K.A.; Cheskin, L.J.; Adam, A.; Neto, M.G.; Badurdeen, D.; Kumbhari, V. Efficacy and Safety of Endoscopic Sleeve Gastroplasty, A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 1043–1053.e4. [Google Scholar] [CrossRef]
- Fogel, R.; De Fogel, J.; Bonilla, Y.; De La Fuente, R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest. Endosc. 2008, 68, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Dials, J.; Demirel, D.; Halic, T.; De, S.; Ryason, A.; Kundumadam, S.; Al-Haddad, M.; Gromski, M.A. Hierarchical task analysis of endoscopic sleeve gastroplasty. Surg. Endosc. 2022, 36, 5167–5182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Chen, C.-Y. Current status of endoscopic sleeve gastroplasty: An opinion review. World J. Gastroenterol. 2020, 26, 1107–1112. [Google Scholar] [CrossRef]
- Morales, J.G.; Pérez, L.C.; Marques, A.; Arribas, B.M.; Arribas, R.B.; Ramo, E.; Escalada, C.; Arribas, C.; Himpens, J. Modified endoscopic gastroplasty for the treatment of obesity. Surg. Endosc. 2018, 32, 3936–3942. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.B.; Hoff, A.C.; Kucera, A.; Weaver, E.; Sebring, L.; Gooch, L.; Walton, K.; Lee, D.; Cratty, T.; Beal, S.; et al. Endoscopic sleeve gastroplasty in class III obesity: Efficacy, safety, and durability outcomes in 404 consecutive patients. World J. Gastrointest. Endosc. 2023, 15, 469–479. [Google Scholar] [CrossRef]
- Dayyeh, B.K.A.; Bazerbachi, F.; Vargas, E.J.; Sharaiha, R.Z.; Thompson, C.C.; Thaemert, B.C.; Wilson, E.B. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. Lancet 2022, 400, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.J.; Kim, B.W.; Kim, B.G.; Kim, J.H.; Kim, J.S.; Kim, J.I.; Kim, W. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: A retrospective multicenter study on immediate and long-term outcome over 5 years. Surg. Endosc. 2016, 30, 5283–5289. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Elahmedi, M.; Alqahtani, Y.A.; Al-Darwish, A. Endoscopic sleeve gastroplasty in 109 consecutive children and adolescents with obesity: Two-year outcomes of a new modality. Am. J. Gastroenterol. 2019, 114, 1857–1862. [Google Scholar] [CrossRef]
- Alqahtani, A.; Al-Darwish, A.; Mahmoud, A.E.; Alqahtani, Y.A.; Elahmedi, M. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest. Endosc. 2019, 89, 1132–1138. [Google Scholar] [CrossRef]
- Asokkumar, R.; Lim, C.H.; Tan, A.S.; Lee, P.C.; Eng, A.; Tan, J.; Lopez-Nava, G.; Ganguly, S.; Chang, J.; Khor, C. Safety and early efficacy of endoscopic sleeve gastroplasty (ESG) for obesity in a multi-ethnic Asian population in Singapore. JGH Open 2021, 5, 1351–1356. [Google Scholar] [CrossRef]
- Badurdeen, D.; Hoff, A.C.; Hedjoudje, A.; Adam, A.; Itani, M.I.; Farha, J.; Abbarh, S.; Kalloo, A.N.; Khashab, M.A.; Singh, V.K.; et al. Endoscopic sleeve gastroplasty plus liraglutide versus endoscopic sleeve gastroplasty alone for weight loss. Gastrointest. Endosc. 2021, 93, 1316–1324.e1. [Google Scholar] [CrossRef]
- Barrichello, S.; de Moura, D.T.H.; de Moura, E.G.H.; Jirapinyo, P.; Hoff, A.C.; Fittipaldi-Fernandez, R.J.; Thompson, C.C. Endoscopic sleeve gastroplasty in the management of overweight and obesity: An international multicenter study. Gastrointest. Endosc. 2019, 90, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.; Keighley, T.; Petocz, P.; Blumfield, M.; Rich, G.G.; Cohen, F.; Soni, A.; Maimone, I.R.; Fayet-Moore, F.; Isenring, E.; et al. Efficacy and safety of endoscopic sleeve gastroplasty and laparoscopic sleeve gastrectomy with 12+ months of adjuvant multidisciplinary support. BMC Prim. Care 2022, 23, 26. [Google Scholar] [CrossRef]
- Cheskin, L.J.; Hill, C.; Adam, A.; Fayad, L.; Dunlap, M.; Badurdeen, D.; Koller, K.; Bunyard, L.; Frutchey, R.; Al-Grain, H.; et al. Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: A case-matched study. Gastrointest. Endosc. 2020, 91, 342–349.e1. [Google Scholar] [CrossRef]
- Espinet-Coll, E.; Díaz-Galán, P.; Nebreda-Durán, J.; Gómez-Valero, J.A.; Vila-Lolo, C.; Bautista-Altamirano, C.; Bargalló-García, A.; Galvao-Neto, M.; Muñoz-Navas, M.; Bargalló-Carulla, D. Persistence of sutures and gastric reduction after endoscopic sleeve gastroplasty: Radiological and endoscopic assessment. Obes. Surg. 2022, 32, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Espinet-Coll, E.; Nebreda-Durán, J.; Galvao-Neto, M.; Bautista-Altamirano, C.; Diaz-Galán, P.; Gómez-Valero, J.A.; Vila-Lolo, C.; Guirola-Puche, M.A.; Fernández-Huélamo, A.; Bargalló-Carulla, D.; et al. Suture pattern does not influence outcomes of endoscopic sleeve gastroplasty in obese patients. Endosc. Int. Open 2020, 8, E1349–E1358. [Google Scholar] [CrossRef] [PubMed]
- Farha, J.; McGowan, C.; Hedjoudje, A.; Itani, M.I.; Abbarh, S.; Simsek, C.; Ichkhanian, Y.; Vulpis, T.; James, T.W.; Fayad, L.; et al. Endoscopic sleeve gastroplasty: Suturing the gastric fundus does not confer benefit. Endoscopy 2020, 53, 727–731. [Google Scholar] [CrossRef]
- Fayad, L.; Adam, A.; Schweitzer, M.; Cheskin, L.J.; Ajayi, T.; Dunlap, M.; Badurdeen, D.S.; Hill, C.; Paranji, N.; Lalezari, S.; et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: A case-matched study. Gastrointest. Endosc. 2019, 89, 782–788. [Google Scholar] [CrossRef]
- Fayad, L.; Cheskin, L.J.; Adam, A.; Badurdeen, D.S.; Hill, C.; Agnihotri, A.; Dunlap, M.; Simsek, C.; Khashab, M.A.; Kalloo, A.N.; et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: Efficacy, durability, and safety. Endoscopy 2019, 51, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Quero, G.; Vix, M.; Guerriero, L.; Pizzicannella, M.; Lapergola, A.; D’uRso, A.; Swanstrom, L.; Mutter, D.; Dallemagne, B.; et al. 6-month gastrointestinal quality of life (QoL) results after endoscopic sleeve gastroplasty and laparoscopic sleeve gastrectomy: A propensity score analysis. Obes. Surg. 2020, 30, 1944–1951. [Google Scholar] [CrossRef]
- Glaysher, M.A.; Moekotte, A.L.; Kelly, J. Endoscopic sleeve gastroplasty: A modified technique with greater curvature compression sutures. Endosc. Int. Open 2019, 7, E1303–E1309. [Google Scholar] [CrossRef]
- Gudur, A.R.; Geng, C.X.; Kshatri, S.; Martin, D.; Haug, R.; Radlinski, M.; Lei, Y.; Buerlein, R.C.; Strand, D.S.; Sauer, B.G.; et al. Comparison of endoscopic sleeve gastroplasty versus surgical sleeve gastrectomy: A metabolic and bariatric surgery accreditation and quality improvement program database analysis. Gastrointest. Endosc. 2022, 97, 11–21.e4. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.L.; Saumoy, M.; Dawod, Q.; Dawod, E.; Shukla, A.; Aronne, L.; et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest. Endosc. 2021, 93, 1110–1118. [Google Scholar] [CrossRef]
- Hill, C.; El Zein, M.; Agnihotri, A.; Dunlap, M.; Chang, A.; Agrawal, A.; Kumbhari, V. Endoscopic sleeve gastroplasty: The learning curve. Endosc. Int. Open 2017, 5, E900–E904. [Google Scholar] [CrossRef]
- Jagtap, N.; Kalapala, R.; Katakwar, A.; Sharma, M.; Aslam, M.; Gupta, R.; Rao, P.N.; Goud, R.; Tandan, M.; Kanakagiri, H.; et al. Endoscopic sleeve gastroplasty—Minimally invasive treatment for non-alcoholic fatty liver disease and obesity. Indian J. Gastroenterol. 2021, 40, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Brunaldi, V.O.; Neto, M.G. Endoscopic sleeve gastroplasty: A narrative review on historical evolution, physiology, outcomes, and future standpoints. Chin. Med. J. 2022, 135, 774–778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, N.; Abu Dayyeh, B.K.; Breviere, G.L.-N.; Neto, M.P.G.; Sahdala, N.P.; Shaikh, S.N.; Hawes, R.H.; Gostout, C.J.; Goenka, M.K.; Orillac, J.R.; et al. Endoscopic sutured gastroplasty: Procedure evolution from first-in-man cases through current technique. Surg. Endosc. 2017, 32, 2159–2164. [Google Scholar] [CrossRef]
- Li, R.; Veltzke-Schlieker, W.; Adler, A.; Specht, M.; Eskander, W.; Ismail, M.; Badakhshi, H.; Galvao, M.P.; Zorron, R. Endoscopic sleeve gastroplasty (ESG) for high-risk patients, high body mass index (>50 kg/m2) patients, and contraindication to abdominal surgery. Obes. Surg. 2021, 31, 3400–3409. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Laster, J.; Negi, A.; Fook-Chong, S.; Bautista-Castaño, I.; Asokkumar, R. Endoscopic sleeve gastroplasty (ESG) for morbid obesity: How effective is it? Surg. Endosc. 2021, 36, 352–360. [Google Scholar] [CrossRef]
- Lopez-Nava, G.; Sharaiha, R.Z.; Vargas, E.J.; Bazerbachi, F.; Manoel, G.N.; Bautista-Castaño, I.; Acosta, A.; Topazian, M.D.; Mundi, M.S.; Kumta, N.; et al. Endoscopic sleeve gastroplasty for obesity: A multicenter study of 248 patients with 24 months follow-up. Obes. Surg. 2017, 27, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Neto, M.G.; Silva, L.B.; de Quadros, L.G.; Grecco, E.; Filho, A.C.; de Amorim, A.M.B.; de Santana, M.F.; dos Santos, N.T.; de Lima, J.H.F.; de Souza, T.F.; et al. Brazilian consensus on endoscopic sleeve gastroplasty. Obes. Surg. 2020, 31, 70–78. [Google Scholar] [CrossRef]
- Neto, M.G.; Moon, R.C.; de Quadros, L.G.; Grecco, E.; Filho, A.C.; de Souza, T.F.; Mattar, L.A.; de Sousa, J.A.G.; Abu Dayyeh, B.K.; Morais, H.; et al. Safety and short-term effectiveness of endoscopic sleeve gastroplasty using overstitch: Preliminary report from a multicenter study. Surg. Endosc. 2020, 34, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, M.; Lapergola, A.; Fiorillo, C.; Spota, A.; Mascagni, P.; Vix, M.; Mutter, D.; Costamagna, G.; Marescaux, J.; Swanström, L.; et al. Does endoscopic sleeve gastroplasty stand the test of time? Objective ESG appearance and weight loss in a large group of patients. Surg. Endosc. 2020, 34, 3696–3705. [Google Scholar] [CrossRef]
- Sarkar, A.; Tawadros, A.; Andalib, I.; Shahid, H.M.; Tyberg, A.; Alkhiari, R.; Gaidhane, M.; Kedia, P.; John, E.S.; Bushe, B.; et al. Safety and efficacy of endoscopic sleeve gastroplasty for obesity management in new bariatric endoscopy programs: A multicenter international study. Ther. Adv. Gastrointest. Endosc. 2022, 15, 26317745221093883. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, A.; Sui, Z.; Hill, C.; Dunlap, M.; Rivera, A.R.; Khashab, M.A.; Kalloo, A.N.; Fayad, L.; Cheskin, L.J.; Marinos, G.; et al. Endoscopic sleeve gastroplasty is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: A large, international multicenter study. Obes. Surg. 2018, 28, 1812–1821. [Google Scholar] [CrossRef]
- Saumoy, M.; Schneider, Y.; Zhou, X.K.; Shukla, A.; Kahaleh, M.; Aronne, L.; Sharaiha, R.Z. A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest. Endosc. 2018, 87, 442–447. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Hajifathalian, K.; Kumar, R.; Saunders, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.; Herr, A.; Igel, L.; et al. Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Clin. Gastroenterol. Hepatol. 2021, 19, 1051–1057.e2. [Google Scholar] [CrossRef]
- PRISMA. PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses [Internet]. Available online: https://www.prisma-statement.org/ (accessed on 17 December 2024).
- Vargas, E.J.; Rizk, M.; Gomez-Villa, J.; Edwards, P.K.; Jaruvongvanich, V.; Storm, A.C.; Acosta, A.; Lake, D.; Fidler, J.; Bharucha, A.E.; et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: A comparative prospective study. Gut 2022, 72, 1073–1080. [Google Scholar] [CrossRef]
- James, T.W.; Reddy, S.; Vulpis, T.; McGowan, C.E. Endoscopic Sleeve Gastroplasty Is Feasible, Safe, and Effective in a Non-academic Setting: Short-Term Outcomes from a Community Gastroenterology Practice. Obes. Surg. 2019, 30, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Edmundowicz, S. Early experience with endoscopic sleeve gastroplasty and hints at mechanisms of action. Clin. Gastroenterol. Hepatol. 2017, 15, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Kumta, N.A.; Saumoy, M.; Desai, A.P.; Sarkisian, A.M.; Benevenuto, A.; Tyberg, A.; Kumar, R.; Igel, L.; Verna, E.C.; et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin. Gastroenterol. Hepatol. 2017, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Campos, J.M.; Fayad, L.; Familiar, P.; Kumbhari, V.; Novikov, A.A.; Besharati, S.; Ghandour, B.; Maselli, D.B.; Vargas, E.J.; et al. Revisional endoscopic gastroplasty (re-ESG) after weight recurrence post-endoscopic sleeve gastroplasty or laparoscopic sleeve gastrectomy: A systematic review. Endosc. Int. Open 2023, 11, E678–E685. [Google Scholar]
- Abu Dayyeh, B.K.; Stier, C.; Alqahtani, A.; Sharaiha, R.; Bandhari, M.; Perretta, S.; Jirapinyo, S.P.; Prager, G.; Cohen, R.V. IFSO Bariatric Endoscopy Committee evidence-based review and position statement on endoscopic sleeve gastroplasty for obesity management. Obes. Surg. 2024, 34, 4349, Erratum in Obes Surg. 2024, 34, 4318–4348. [Google Scholar] [CrossRef]

| Author (Year) | Study Design | Participants (n) | BMI (Mean, SD, Range) | Weight Loss (Mean, SD, Range) | Follow Up Period | Results (Mean, SD, Range) |
|---|---|---|---|---|---|---|
| De Moura et al. (2020) [4] | Multicenter prospective study | 233 | N/A | 19.7% TBWL | 12 months | Sustained weight loss, improved comorbidities |
| Matteo et al. (2024) [5] | Retrospective cohort | 315 | Median BMI: 36.1 (34.2, 39.4) females; 39.2 (36.0, 43.7) males | 17–20% TBWL at 2 years, 16% at 5 years | 24 months | Median %TBWL: 12.8% (6.41–19.4) at 24 months |
| Ryuet al. (2016) [20] | Multicenter retrospective study | 34 | Mean BMI: 34.8 ± 4.4 | %TWL: 13.2 ± 3.9 at 6 months, 18.3 ± 5.5 at 1 year | 12 months | %EWL: 51.9 ± 19.1 (6 months), 69.9 ± 29.9 (1 year) |
| Wang et al. (2020) [16] | Multicenter retrospective study | 34 | Mean BMI: 34.8 ± 4.8 | %TWL: 13.2% ± 3.9 at 6 months, 18.3% ± 5.5 at 1 year | 12 months | %EWL: 51.9% ± 19.1 (6 months), 69.9% ± 29.9 (1 year) |
| Lopez et al. (2017) [11] | Multicenter randomized trial | 146 | BMI: class III obesity | 20.5% TBWL at 12 months | 12 months | %EWL: ~20.5% |
| Abu Dayyeh BK et al. (2017) [8] | Case series | 25 | Mean BMI Mean 35.5 ± 2.6 kg/m2 | EWL (mean): 53% ± 17% (6 months), 56% ± 23% (9 months), 54% ± 40% (12 months), 45% ± 41% (20 months) | 9 months (range 5–20 months) | Sustained weight loss with a median of 45% EWL at 20 months, improved satiation, delayed gastric emptying, and increased insulin sensitivity |
| Graus Morales et al. (2018) [17] | Retrospective single-center study | 148 (72 monitored 18 months) | 35.11 ± 5.5 kg/m2 (all) | 17.62 ± 9.22 kg (12 months, 17.53% WL) | 12–18 months | 79.25 ± 43% EWL (18 months) |
| De Moura et al. (2019) [4] | Retrospective review | N/A | N/A | Up to 20.9% total body weight loss | Up to 2 years | Favorable outcomes with 60.4% excess weight loss |
| Alqahtani et al. (2019) [21] | Prospective cohort study | 1000 | 33.3 ± 4.5 kg/m2 | 6 months: 13.7% ± 6.8% TBWL 12 months: 15.0% ± 7.7% TBWL 18 months: 14.8% ± 8.5% TBWL | 18 months | Significant weight loss within the first 18 months 13 of 17 diabetes cases in complete remission All 28 hypertension cases in remission |
| Alqahtani, A. et al. (2019) [22] | Prospective observational study | 109 | 33 (mean) | 6 months: 14.4 12 months: 16.2% ± 8.3% total weight loss 24 months: 13.7% ± 8.0% total weight loss | 2 years | Weight loss of approximately 12 kg by 18 months |
| Asokkumar, R. et al. (2021) [23] | Case series | 35 | 34 ± 4.9 kg/m2 (range not explicitly stated) | 3 months: 14.5% ± 4.8% total body weight loss (TBWL) 6 months: 16.2% ± 4.9% TBWL | 6 months | Weight loss at 3 months: 13.2 ± 4.8 kg Weight loss at 6 months: 14.1 ± 5.9 kg BMI reduction at 6 months: 5.7 ± 1.5 kg/m2 |
| Badurdeen et al. (2021) [24] | Retrospective study | 52 | 35.7 (mean) ± 2.02 kg/m2 | ESG—only group: 7 months: 20.95 ± 3.21 kg lost, 20.51% ± 1.68% TBWL | 12 months | Body fat reduction at 12 months: ESG-only: 10.54% ± 1.88% |
| Barrichello et al. (2019) [25] | Case series | 193 | 34.11 ± 2.97 kg/m2 | 6 months: 14.25% ± 5.26% total weight loss (TWL) 1 year: 15.06% ± 5.22% TWL | 6 months and a year | BMI reduction: initial: 34.11 ± 2.97 kg/m2 6 months: 29.21 ± 2.64 kg/m2 1 year: 28.91 ± 2.99 kg/m2 excess weight loss (EWL): 6 months: 56.15% ± 22.93% 1 year: 59.41% ± 25.69% |
| Carr et al. (2022) [26] | Cohort study | 61 | 34.11 ± 2.97 kg/m2 | BMI reduction: initial: 34.11 ± 2.97 kg/m2, 6 months: 29.21 ± 2.64 kg/m2 | 1 year | EWL: 6 months: 56.15% ± 22.93%; 1 year: 59.41% ± 25.69% |
| Cheskin et al. (2020) [27] | Prospective study | 386 | ESG: 37.8 (SD: 4.8) kg/m2 HIDLT: 37.3 (SD: 4.8) kg/m2 | ESG: %TWL: 13.6% (SD: 7.6%) at 12 m HIDLT: %TWL: 0.8% (SD: 5.0%) | 12 months | ESG resulted in significantly greater weight loss compared to HIDLT; no serious adverse events reported |
| Espinet-Coll, E. et al. (2022) [28] | Prospective study | 38 | Mean: 39.5 kg/m2 | %TWL: 14.9% (SD: 6.8%) at 12 m | 12 months | Suture patterns did not significantly affect weight loss outcomes |
| Espinet-Coll et al. (2020) [29] | Prospective study | 88 | Mean: 40.1(SD: 5.8) kg/m2 | %TWL: 17.1% (SD: 7.2%) at 12 m | 12 months | Sustained gastric volume reduction observed at 12 m post-ESG; no major adverse events reported |
| Farha et al. (2020) [30] | Retrospective | 247 | Mean: 38.2 kg/m2 | %TWL: 16.4% at 12 m | 12 months | Suturing the gastric fundus does not provide additional weight loss benefit |
| Fayad et al. (2019) [31] | Retrospective | 137 | ESG: 37.8 (SD: 4.8) kg/m2 | ESG: 37.8 (SD: 4.8) kg/m2 | 12 months | ESG had fewer adverse events and shorter hospital stay |
| Fayad, L. et al. (2019) [32] | Retrospective | 105 | 41.5 kg/m2, SD 8.2 | 9.9% (SD 2.4) | 12 months | 12 months |
| Fiorillo, C. et al. (2020) [33] Glaysher, M. A. et al. (2019) [34] | Retrospective Retrospective | 4632 | ESG: 35.7 (SD: 5.1) kg/m2 median 36.5 kg/m2 (range: 29.8–42.9) | ESG: %TWL: 15.1% (SD: 5.7%) at 6 m (range: 4.0–10.7%) | 6 months 6 months | ESG resulted in greater weight loss BMI reduction was also greater (p = 0.019) |
| Gudur et al. (2022) [35] | Retrospective study | 36,323 | BMI: 39.1 ± 5.0 kg/m2 | ESG: %TWL: 14.8% (SD: 4.9%) at 12 m; | 12 months | ESG had better safety profile |
| Hajifathalian et al. (2021) [36] | Prospective | 118 | Mean: 40.0 kg/m2 | %TWL: 16.3% at 12 m | 12 months | Significant improvement in insulin resistance and hepatic steatosis after ESG |
| Hill et al. (2017) [37] | Prospective | 21 | Mean BMI: 37.6 kg/m | %TWL: 16.2% at 12 m | 12 months | ESG proficiency improved significantly after 20 procedures |
| Jagtap, N. et al. (2021) [38] | Prospective | 26 | Mean: 39.1 kg/m2 | TWL: 17.4% at 12 m | 12 months | ESG found to be an effective treatment for obesity and non-alcoholic fatty liver disease |
| Brunaldi et al. (2022) [39] | Retrospective | 100 | BMI: 37.2 ± 5.3 kg/m2 | TWL: 13.5% at 12 m | 12 months | ESG is feasible in a non-academic community setting |
| Kumar, N. et al. (2018) [40] | Prospective | 122 | Mean BMI 37.4 ± 1.9 kg/m2 | 13.1 ± 1.3 kg | 12 months | ESG showed significant weight loss over 12 months |
| Li, R. et al. (2021) [41] | Prospective | 21 | 49.9 ± 14.4 kg/m2 | Weight loss: 17.5 ± 14.6 kg | 12 months | ESG was successfully performed in all patients without intraoperative complications |
| Lopez-Nava, G. et al. (2022) [42] | Retrospective | 435 | Mean 45.8 kg/m2 | 20.5% | 12 months | ESG was effective in all three obesity classes, with higher weight loss in class III patients |
| Lopez-Nava, G. et al. (2017) [43] | Prospective | 248 | 37.8 ± 5.6 kg/m2 | TBWL: 18.6% (95% CI: 15.7–21.5) | 24 months | ESG effectively induced weight loss up to 24 months in moderately obese patients |
| Neto, M. G. et al. (2019) [44] | Prospective | 233 | 34.7 ± 2.6 kg/m2 | 19.7% (±5.7) | 12 months | ESG resulted in significant short-term weight loss, with an average %TBWL of 19.7% at 12 months |
| Neto, M. G. et al. (2020) [45] | Prospective | 1828 | 30–40 kg/m2 | 18.2% | 12 months | ESG resulted in significant weight loss and had a low complication rate |
| Pizzicannella, M. et al. (2019) [46] | Prospective | 133 | 43.2 ± 8.6 kg/m2 | %EWL: 19.3 ± 13.4, %TWL: 8.9 ± 6.1 | 12 months | Weight loss correlated with ESG durability: Patients with intact ESG had the highest %EWL and %TWL at 6 and 12 months |
| Sarkar, A. et al. (2022) [47] | Retrospective | 91 | 38.7 kg/m2 (range: 31.2–57.6) | 17.4% | 12 months | ESG in new bariatric centers yielded comparable weight loss and metabolic outcomes to experienced centers |
| Sartoretto, A. et al. (2018) [48] | Retrospective | 112 | 37.9 ± 6.7 kg/m2 | %TBWL: 14.9 ± 6.1 | 6 months | Male patients and those with higher baseline BMI tended to lose more weight |
| Saumoy, M., y col (2017) [49] | Prospective | 128 | 38.92 ± 6.95 kg/m2 (range: 30.02–68.04) | %TBWL: 15.80 ± 9.50 | 12 months | ESG can be efficiently and safely mastered after approximately 55 cases |
| Sharaiha, R. Z. et al. (2020) [50] | Retrospective | 216 | 39 ± 6 kg/m2 | %TBWL: 15.9% (95% CI: 11.7–20.5, p < 0.001) | 5 years | 90% of patients maintained at least 5% TBWL at 5 years, meeting ASGE and ASMBS criteria for primary bariatric intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, V.P.S.; Thaher, O.; Elshafei, M.; Pouwels, S.; Pape-Köhler, C. Outcomes of Endoscopic Sleeve Gastroplasty: A Systematic Review. Medicina 2025, 61, 1821. https://doi.org/10.3390/medicina61101821
Vargas VPS, Thaher O, Elshafei M, Pouwels S, Pape-Köhler C. Outcomes of Endoscopic Sleeve Gastroplasty: A Systematic Review. Medicina. 2025; 61(10):1821. https://doi.org/10.3390/medicina61101821
Chicago/Turabian StyleVargas, Vanessa Pamela Salolin, Omar Thaher, Moustafa Elshafei, Sjaak Pouwels, and Carolina Pape-Köhler. 2025. "Outcomes of Endoscopic Sleeve Gastroplasty: A Systematic Review" Medicina 61, no. 10: 1821. https://doi.org/10.3390/medicina61101821
APA StyleVargas, V. P. S., Thaher, O., Elshafei, M., Pouwels, S., & Pape-Köhler, C. (2025). Outcomes of Endoscopic Sleeve Gastroplasty: A Systematic Review. Medicina, 61(10), 1821. https://doi.org/10.3390/medicina61101821







