Effect of Circadian Blood Pressure Variations on Retinal Microvascular Structures: Optical Coherence Tomography Angiography Analysis with the Nighttime Divided into Subintervals (Retinal Dawn Pattern)
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Parameters Assessed
- Vessel density values of the superficial and deep vascular layers, obtained automatically from 6 mm fovea-centered optical coherence tomography angiography (OCT-A) images using the device’s built-in software. Foveal avascular zone (FAZ) area, FAZ perimeter, and fractal dimension measurements, automatically calculated from the same OCT-A images.
- Twenty-four-hour mean systolic, diastolic, and mean arterial pressure (MAP) values.
- Daytime and nighttime systolic, diastolic, and mean arterial pressure values.
- Daytime and nighttime pulse pressure values.
- Variance, standard deviation, and coefficient of variation for systolic, diastolic, and mean arterial pressure values.
- Differences between daytime and nighttime measurements, such as the percentage decrease in nighttime systolic pressure.
- Classification of participants as dipper or non-dipper.
- Analysis of nighttime measurements by dividing them into two subintervals: 00:00–04:00 and 04:00–08:00.
- Available results of blood biochemistry and complete blood count tests.
2.4. Retinal Imaging Protocol
2.5. Blood Pressure Measurement Protocol
2.6. Statistical Analysis
2.7. Ethics Statement
3. Result
| Variable | S Systolic Variance | Systolic Standard Deviation | Systolic Coefficient of Variation | Diastolic Variance | Diastolic Standard Deviation | MAP Variance | MAP Standard Deviation | MAP Coefficient of Variation |
|---|---|---|---|---|---|---|---|---|
| D. Deep Whole Image | 0.586/−0.072 | 0.715/−0.049 | 0.657/0.059 | 0.812/0.032 | 0.814/0.031 | 0.846/−0.026 | 0.580/−0.074 | 0.915/−0.014 |
| D. Deep Superior- Hemi | 0.472/−0.096 | 0.539/−0.082 | 0.312/0.134 | 0.927/0.012 | 0.908/−0.015 | 0.826/−0.029 | 0.499/−0.09 | 0.863/0.023 |
| D. Deep Inferior- Hemi | 0.565/−0.076 | 0.356/−0.122 | 0.973/0.004 | 0.737/−0.045 | 0.832/−0.028 | 0.785/−0.036 | 0.304/−0.136 | 0.732/0.046 |
| D. Deep Fovea | 0.869/0.022 | 0.929/−0.012 | 0.809/−0.032 | 0.802/0.033 | 0.718/0.048 | 0.773/0.038 | 0.656/−0.059 | 0.641/0.062 |
| D. Deep Parafovea | 0.885/−0.019 | 0.311/−0.134 | 0.636/0.063 | 0.600/−0.07 | 0.776/−0.038 | 0.976/0.004 | 0.192/−0.172 | 0.701/0.051 |
| D. Deep Parafovea–Superior-Hemi | 0.720/−0.048 | 0.345/−0.125 | 0.565/0.076 | 0.729/−0.046 | 0.838/−0.027 | 0.992/−0.001 | 0.270/−0.146 | 0.978/0.004 |
| D. Deep Parafovea–Inferior-Hemi | 0.926/0.012 | 0.316/−0.133 | 0.709/0.05 | 0.528/−0.084 | 0.744/−0.043 | 0.922/0.013 | 0.158/−0.186 | 0.456/0.099 |
| D. Deep Parafovea–Tempo | 0.983/0.003 | 0.385/−0.115 | 0.850/0.025 | 0.645/−0.061 | 0.746/−0.043 | 0.943/0.009 | 0.200/−0.169 | 0.586/0.072 |
| D. Deep Parafovea–Superior | 0.614/−0.067 | 0.393/−0.113 | 0.447/0.101 | 0.771/−0.039 | 0.311/−0.134 | 0.859/−0.024 | 0.328/−0.13 | 0.937/0.011 |
| D. Deep Parafovea–Nazal | 0.903/0.016 | 0.319/−0.132 | 0.326/0.13 | 0.718/−0.048 | 0.897/0.017 | 0.682/0.055 | 0.249/−0.153 | 0.470/0.096 |
| D. Deep Parafovea–Inferior | 0.868/−0.022 | 0.282/−0.142 | 0.924/−0.013 | 0.430/−0.105 | 0.865/0.023 | 0.893/−0.018 | 0.146/−0.192 | 0.807/0.033 |
| D. Deep Perifovea | 0.365/−0.12 | 0.386/−0.115 | 0.745/0.043 | 0.815/−0.031 | 0.769/−0.039 | 0.614/−0.067 | 0.357/−0.122 | 0.815/0.031 |
| D. Deep Perifovea - Superior - Hemi | 0.306/−0.135 | 0.499/−0.09 | 0.389/0.114 | 0.981/0.003 | 0.662/−0.058 | 0.621/−0.066 | 0.521/−0.085 | 0.890/0.018 |
| D. Deep Perifovea–Inferior–Hemi | 0.472/−0.096 | 0.346/−0.125 | 0.925/−0.013 | 0.720/−0.048 | 0.923/−0.013 | 0.675/−0.056 | 0.298/−0.138 | 0.733/0.045 |
| D. Deep Perifovea–Tempo | 0.697/−0.052 | 0.670/−0.057 | 0.751/0.042 | 0.968/0.005 | 0.768/0.039 | 0.927/−0.012 | 0.665/−0.058 | 0.797/0.034 |
| D. Deep Perifovea–Superior | 0.277/−0.144 | 0.552/−0.079 | 0.252/0.152 | 0.981/0.003 | 0.552/−0.079 | 0.558/−0.078 | 0.588/−0.072 | 0.947/−0.009 |
| D. Deep Perifovea–Nazal | 0.523/−0.085 | 0.297/−0.138 | 0.992/−0.001 | 0.744/−0.043 | 0.872/−0.021 | 0.769/−0.039 | 0.228/−0.159 | 0.780/0.037 |
| D. Deep Perifovea–Inferior | 0.260/−0.149 | 0.276/−0.144 | 0.907/−0.016 | 0.579/−0.074 | 0.540/−0.081 | 0.429/−0.105 | 0.279/−0.143 | 0.951/0.008 |
| D. Superficial Whole Imaqe | 0.724/0.047 | 0.664/0.058 | 0.487/0.092 | 0.498/0.09 | 0.368/−0.119 | 0.659/0.059 | 0.475/0.095 | 0.368/0.119 |
| D. Superficial Superior–Hemi | 0.865/0.023 | 0.666/0.057 | 0.453/0.1 | 0.590/0.072 | 0.302/−0.137 | 0.867/0.022 | 0.476/0.095 | 0.572/0.075 |
| D. Superficial Inferior-Hemi | 0.623/0.065 | 0.670/0.057 | 0.528/0.084 | 0.456/0.099 | 0.420/−0.107 | 0.525/0.084 | 0.501/0.089 | 0.249/0.152 |
| D. Superficial Fovea | 0.733/−0.045 | 0.444/0.102 | 0.436/−0.103 | 0.544/0.081 | 0.559/−0.078 | 0.742/−0.044 | 0.412/0.109 | 0.845/−0.026 |
| D. Superficial Parafovea | 0.559/0.078 | 0.369/0.119 | 0.531/0.083 | 0.272/0.145 | 0.850/−0.025 | 0.523/0.085 | 0.324/0.131 | 0.158/0.186 |
| D. Superficial Parafovea–Superior–Hemi | 0.588/0.072 | 0.400/0.112 | 0.497/0.09 | 0.252/0.152 | 0.935/0.011 | 0.522/0.085 | 0.310/0.134 | 0.207/0.167 |
| D. Superficial Parafovea–Inferior–Hem | 0.508/0.088 | 0.379/0.117 | 0.620/0.066 | 0.286/0.141 | 0.853/−0.025 | 0.477/0.094 | 0.364/0.12 | 0.130/0.199 |
| D. Superficial Parafovea–Tempo | 0.700/0.051 | 0.421/0.107 | 0.851/−0.025 | 0.332/0.128 | 0.652/−0.06 | 0.712/0.049 | 0.450/0.1 | 0.150/0.19 |
| D. Superficial Parafovea–Superior | 0.458/0.098 | 0.247/0.153 | 0.333/0.128 | 0.208/0.166 | 0.393/−0.113 | 0.491/0.091 | 0.168/0.182 | 0.140/0.194 |
| D. Superficial Parafovea–Nazal | 0.597/0.07 | 0.633/0.063 | 0.210/0.166 | 0.414/0.108 | 0.888/0.019 | 0.516/0.086 | 0.602/0.069 | 0.133/0.198 |
| D. Superficial Parafovea–Inferior | 0.607/0.068 | 0.328/0.13 | 0.874/0.021 | 0.267/0.147 | 0.728/0.046 | 0.514/0.087 | 0.272/0.145 | 0.367/0.12 |
| D. Superficial Perifovea | 0.891/0.018 | 0.791/0.035 | 0.535/0.082 | 0.629/0.064 | 0.295/−0.139 | 0.792/0.035 | 0.538/0.082 | 0.416/0.108 |
| D. Superficial Perifovea–Superior–Hemi | 0.946/−0.009 | 0.859/0.024 | 0.473/0.095 | 0.808/0.032 | 0.223/−0.161 | 0.947/−0.009 | 0.593/0.071 | 0.609/0.068 |
| D. Superficial Perifovea–Inferior–Hemi | 0.725/0.047 | 0.689/0.053 | 0.585/0.073 | 0.417/0.108 | 0.435/−0.104 | 0.519/0.086 | 0.443/0.102 | 0.243/0.154 |
| D. Superficial Perifovea–Tempo | 0.894/0.018 | 0.327/0.13 | 0.899/−0.017 | 0.289/0.14 | 0.649/−0.061 | 0.774/0.038 | 0.226/0.16 | 0.351/0.124 |
| D. Superficial Perifovea–Superior | 0.804/−0.033 | 0.848/−0.025 | 0.270/0.146 | 0.792/−0.035 | 0.149/−0.19 | 0.635/−0.063 | 0.912/0.015 | 0.864/0.023 |
| D. Superficial Perifovea–Nazal | 0.675/0.056 | 0.835/0.028 | 0.543/0.081 | 0.559/0.078 | 0.477/−0.094 | 0.516/0.086 | 0.562/0.077 | 0.422/0.107 |
| D. Superficial Perifovea–Inferior | 0.643/0.062 | 0.806/0.033 | 0.372/0.118 | 0.470/0.096 | 0.388/−0.114 | 0.474/0.095 | 0.565/0.076 | 0.113/0.208 |
| FAZ (Mm2) | 0.526/0.084 | 0.979/−0.004 | 0.503/0.089 | 0.584/−0.073 | 0.663/−0.058 | 0.869/0.022 | 0.993/0.001 | 0.892/0.018 |
| PERIM (Mm) | 0.260/0.149 | 0.605/0.069 | 0.566/0.076 | 0.898/−0.017 | 0.957/0.007 | 0.638/0.062 | 0.622/0.065 | 0.830/0.029 |
| FD | 0.276/0.144 | 0.987/−0.002 | 0.707/0.05 | 0.567/0.076 | 0.896/0.017 | 0.226/0.16 | 0.950/−0.008 | 0.047/0.260 * |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPM | Ambulatory Blood Pressure Monitoring |
| OCT | Optical Coherence Tomography |
| FAZ | Foveal Avascular Zone |
| PERIM | FAZ Perimeter |
| FD | Flow density |
| MAP | Mean Arterial Pressure |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| PP | Pulse Pressure |
| SCP | Superficial Capillary Plexus |
| DCP | Deep Capillary Plexus |
| RNFL | Retinal Nerve Fiber Layer |
| VLDL | Very Low-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| TG | Triglyceride |
| SD | Standard Deviation |
| bpm | Beats Per Minute |
| Hb | Hemoglobin |
| Cr | Creatinine |
| BUN | Blood Urea Nitrogen |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| GFR | Glomerular Filtration Rate |
| BMI | Body Mass Index |
References
- Silva, B.V.; Sousa, C.; Caldeira, D.; Abreu, A.; Pinto, F.J. Management of arterial hypertension: Challenges and opportunities. Clin. Cardiol. 2022, 45, 1094–1099. [Google Scholar] [CrossRef]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16 (Suppl. 1), S61–S63. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, J.H.; Kim, W.; Kim, W.S.; Park, S.; Lee, S.J.; Shin, J. Clinical and life style factors related to the nighttime blood pressure, nighttime dipping and their phenotypes in Korean hypertensive patients. Clin. Hypertens. 2023, 29, 21. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Bruno, R.M.; McEvoy, J.W.; Touyz, R.M. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: What is new in pharmacotherapy? Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 7–9. [Google Scholar] [CrossRef]
- Huart, J.; Persu, A.; Lengelé, J.P.; Krzesinski, J.M.; Jouret, F.; Stergiou, G.S. Pathophysiology of the Nondipping Blood Pressure Pattern. Hypertension 2023, 80, 719–729. [Google Scholar] [CrossRef]
- Beyaz Coskun, A.; Elibol, E.; Duyar Ozer, S. Evaluation of Cardiovascular Disease Risk According to the Morningness-Eveningness Status in Adult Individuals. Cureus 2025, 17, e86751. [Google Scholar] [CrossRef]
- Arora, D.; Sharvi, S.; Singh, A.; Srivastava, S.; Tyagi, A. Assessment of Circadian Variations in Blood Pressure and Their Correlation with Sleep Patterns in Medical Students. J. Heart Valve Dis. 2025, 30, 31–34. [Google Scholar]
- Shen, Y.; Xiang, W.; Chen, S.; Hou, Z.; Hong, D. Association Between the Circadian Rhythm of Arterial Blood Pressure and White Matter Lesions in Hospitalized Hypertensive Patients: A Cross-Sectional Study. Int. J. Gen. Med. 2025, 18, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Oshima, T.; Ozono, R.; Higashi, Y.; Sasaki, S.; Matsumoto, T. Non-dipper phenomenon in essential hypertension is related to blunted nocturnal rise and fall of sympatho-vagal nervous activity and progress in retinopathy. Auton. Neurosci. 2001, 88, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kuzeytemiz, M.; Karaagac, K.; Vatansever, F.; Ozluk, O.A.; Yilmaz, M.; Arslan, B.; Peker, T. The effect of non-dipper and dipper blood pressure patterns on aortic elasticity in patients with metabolic syndrome. Clin. Exp. Hypertens. 2013, 35, 632–636. [Google Scholar] [CrossRef]
- Carollo, C.; Vadalà, M.; Sorce, A.; Sinatra, N.; Orlando, E.; Cirafici, E. Relationship Between Renal Resistive Index and Retinal Vascular Density in Individuals with Hypertension. Biomedicines 2025, 13, 312. [Google Scholar] [CrossRef]
- Cirillo, A.; Huart, J.; Taminiau, B.; Descy, J.; Saint-Remy, A.; Daube, G. Human Stool Metabolome Differs upon 24 h Blood Pressure Levels and Blood Pressure Dipping Status: A Prospective Longitudinal Study. Metabolites 2021, 11, 282. [Google Scholar] [CrossRef]
- Zeman, M.; Dulková, K.; Bada, V.; Herichová, I. Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci. 2005, 76, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Mitchell, P. The eye in hypertension. Lancet 2007, 369, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Fraser, J.A. Retinal vasculature: A window on the brain. Hypertension 2013, 62, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Anjos, R.; Ferreira, A.; Barkoudah, E.; Claggett, B.; Abegão Pinto, L.; Miguel, A. Application of Optical Coherence Tomography Angiography Macular Analysis for Systemic Hypertension. A Systematic Review and Meta-analysis. Am. J. Hypertens. 2022, 35, 356–364. [Google Scholar] [CrossRef]
- Fraser-Bell, S.; Symes, R.; Vaze, A. Hypertensive eye disease: A review. Clin. Exp. Ophthalmol. 2017, 45, 45–53. [Google Scholar] [CrossRef]
- Mulè, G.; Vadalà, M.; Geraci, G.; Cottone, S. Retinal vascular imaging in cardiovascular medicine: New tools for an old examination. Atherosclerosis 2018, 268, 188–190. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ikram, M.K.; Sabanayagam, C.; Wong, T.Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 2012, 60, 1094–1103. [Google Scholar] [CrossRef]
- Runsewe, O.I.; Srivastava, S.K.; Sharma, S.; Chaudhury, P.; Tang, W.H.W. Optical coherence tomography angiography in cardiovascular disease. Prog. Cardiovasc. Dis. 2024, 87, 60–72. [Google Scholar] [CrossRef]
- Donati, S.; Maresca, A.M.; Cattaneo, J.; Grossi, A.; Mazzola, M.; Caprani, S.M. Optical coherence tomography angiography and arterial hypertension: A role in identifying subclinical microvascular damage? Eur. J. Ophthalmol. 2021, 31, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Niro, A.; Sborgia, G.; Lampignano, L.; Giuliani, G.; Castellana, F.; Zupo, R. Association of Neuroretinal Thinning and Microvascular Changes with Hypertension in an Older Population in Southern Italy. J. Clin. Med. 2022, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.H.; Won, Y.; Lee, M.W.; Shin, Y.I.; Jo, Y.J.; Kim, J.Y. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Garg, I.; Bannai, D.; Kasetty, M.; Katz, R.; Park, J.Y. Retinal microvasculature and vasoreactivity changes in hypertension using optical coherence tomography-angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3505–3515. [Google Scholar] [CrossRef]
- Shin, Y.I.; Nam, K.Y.; Lee, W.H.; Ryu, C.K.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Peripapillary microvascular changes in patients with systemic hypertension: An optical coherence tomography angiography study. Sci. Rep. 2020, 10, 6541. [Google Scholar] [CrossRef]
- Seçkin, Ö.; Ünlü, S.; Yalçın, M.R. The hidden role of left atrial strain: Insights into end-organ damage in dipper and nondipper hypertension. J. Hum. Hypertens. 2025, 39, 425–431. [Google Scholar] [CrossRef]
- Guzel, D.; Kalkan, E.A.; Eren, F.; Zengin, O.; Erel, O.; Sahiner, E.S. Can Serum Endocan Levels be Used as an Early Prognostic Marker for Endothelial Dysfunction in COVID-19? Angiology 2022, 73, 438–444. [Google Scholar] [CrossRef]
- Vlachakis, P.K.; Theofilis, P.; Manios, E.; Tentolouris, A.; Drakopoulou, M.; Karakasis, P.; Vordoni, A.; Korompoki, E.; Oikonomou, E.; Tsioufis, C.; et al. Endothelial Function Biomarkers in Hypertension. Curr. Med. Chem. 2025, 32, 8835–8854. [Google Scholar] [CrossRef]
- Nolde, J.M.; Frost, S.; Kannenkeril, D.; Lugo-Gavidia, L.M.; Chan, J.; Joyson, A. Capillary vascular density in the retina of hypertensive patients is associated with a non-dipping pattern independent of mean ambulatory blood pressure. J. Hypertens. 2021, 39, 1826–1834. [Google Scholar] [CrossRef]
- Park, J.; Song, W.K.; Baek, M.S.; Yoon, J.; Lee, A.; Kim, K.E.; Kook, M.S. Relationship between nocturnal blood pressure dip and β-parapapillary atrophy zone choroidal vessel density in normal-tension glaucoma patients. PLoS ONE 2025, 20, e0317468. [Google Scholar] [CrossRef]
- Bursali, Ö.; Altintaş, Ö.; Ağir, A.; Yüksel, N.; Özkan, B. Optical coherence tomography measurements in patients with systemic hypertension. Scott. Med. J. 2021, 66, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kario, K. Morning surge in blood pressure and cardiovascular risk: Evidence and perspectives. Hypertension 2010, 56, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Lv, C.; Zhang, H. Effect of blood pressure variability on hypertensive retinopathy. Clin. Exp. Hypertens. 2023, 45, 2205050. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Yao, X.; Le, T.T.; Tan, B.; Schmetterer, L.; Chua, J. The New Era of Retinal Imaging in Hypertensive Patients. Asia Pac. J. Ophthalmol. 2022, 11, 149–159. [Google Scholar] [CrossRef]
- He, Y.; Qin, M.Z.; Cao, K.; Zhang, Y.P.; Jiao, X.; Zhang, Z.; Guo, C.X. Assessment of the impact of low-density lipoprotein cholesterol on retinal vessels using optical coherence tomography angiography. Lipids Health Dis. 2024, 23, 301. [Google Scholar] [CrossRef]
- Wang-Evers, M.; Casper, M.J.; Glahn, J.; Luo, T.; Doyle, A.E.; Karasik, D. Assessing the impact of aging and blood pressure on dermal microvasculature by reactive hyperemia optical coherence tomography angiography. Sci. Rep. 2021, 11, 13411. [Google Scholar] [CrossRef]
- Hua, D.; Xu, Y.; Zeng, X.; Yang, N.; Jiang, M.; Zhang, X.; Xing, Y. Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy. Microvasc. Res. 2020, 129, 103969. [Google Scholar] [CrossRef]
- Choi, J.; Kook, M.S. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. Biomed. Res. Int. 2015, 2015, 141905. [Google Scholar] [CrossRef]
- Milani, P.; Bochicchio, S.; Urbini, L.E.; Bulone, E.; Callegarin, S.; Pisano, L. Diurnal Measurements of Macular Thickness and Vessel Density on OCT Angiography in Healthy Eyes and Those with Ocular Hypertension and Glaucoma. J. Glaucoma 2020, 29, 918–925. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P.; Alward, W.L. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol. 1994, 117, 603–624. [Google Scholar] [CrossRef]
- Liu, K.; Xu, H.; Jiang, H.; Wang, H.; Wang, P.; Xu, Y. Macular vessel density and foveal avascular zone parameters in patients after acute primary angle closure determined by OCT angiography. Sci. Rep. 2020, 10, 18717. [Google Scholar] [CrossRef]
- van Koeverden, A.K.; He, Z.; Nguyen, C.T.O.; Vingrys, A.J.; Bui, B.V. Systemic hypertension is not protective against chronic intraocular pressure elevation in a rodent model. Sci. Rep. 2018, 8, 7107. [Google Scholar] [CrossRef]
- Hanssen, H.; Streese, L.; Vilser, W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog. Retin. Eye Res. 2022, 91, 101095. [Google Scholar] [CrossRef]
- Harris, A.; Rechtman, E.; Siesky, B.; Jonescu-Cuypers, C.; McCranor, L.; Garzozi, H.J. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol. Clin. N. Am. 2005, 18, 345–353. [Google Scholar] [CrossRef]
- Harris, A.; Kagemann, L.; Ehrlich, R.; Rospigliosi, C.; Moore, D.; Siesky, B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can. J. Ophthalmol. 2008, 43, 328–336. [Google Scholar] [CrossRef]


| Variable | Category | Blood Pressure | p | |||
|---|---|---|---|---|---|---|
| Non-Dipper (n = 34) | Dipper (n = 26) | |||||
| n | % | n | % | |||
| Sex | Female | 20 | 58.82 | 13 | 50.00 | 0.603 |
| Male | 14 | 41.18 | 13 | 50.00 | ||
| Hypertension | No | 18 | 52.94 | 13 | 50.00 | 0.514 |
| Yes | 16 | 47.06 | 13 | 50.00 | ||
| Variable | Mean ± SD | Median—IQR | Mean ± SD | Median—IQR | p | |
| Age (Years) | 50.68 ± 12.73 | 48.50—22.00 | 44.85 ± 13.76 | 46.00—19.50 | 0.095 | |
| Variable | Blood Pressure | p | |||
|---|---|---|---|---|---|
| Non-Dipper (n = 34) | Dipper (n = 26) | ||||
| Mean ± SS | Median —IQR | Mean ± SS | Median —IQR | ||
| D. Deep Whole Image | 44.66 ± 9.53 | 45.60—10.25 | 45.97 ± 4.20 | 46.30—6.48 | 0.720 u |
| D. Deep Superior–Hemi | 46.18 ± 5.72 | 46.50—9.12 | 46.13 ± 4.16 | 47.00—6.52 | 0.969 t |
| D. Deep Inferior–Hemi | 45.52 ± 6.72 | 45.30—11.33 | 45.77 ± 4.62 | 46.30—7.63 | 0.690 t |
| D. Deep Fovea | 34.15 ± 8.05 | 34.95—10.28 | 35.69 ± 8.05 | 34.00—11.2 | 0.466 t |
| D. Deep Parafovea | 52.24 ± 5.89 | 52.05—8.00 | 52.21 ± 3.69 | 51.65—6.82 | 0.822 t |
| D. Deep Parafovea–Superior-Hemi | 52.55 ± 5.75 | 52.25—6.95 | 52.25 ± 3.91 | 52.40—7.08 | 0.963 t |
| D. Deep Parafovea–Inferior-Hemi | 51.94 ± 6.29 | 51.85—8.42 | 52.15 ± 3.71 | 51.00—6.92 | 0.829 u |
| D. Deep Parafovea–Tempo | 53.31 ± 6.13 | 53.65—7.05 | 53.25 ± 3.66 | 53.95—5.68 | 0.972 t |
| D. Deep Parafovea–Superior | 51.74 ± 6.33 | 51.90—7.72 | 51.78 ± 3.63 | 51.00—6.17 | 0.884 t |
| D. Deep Parafovea–Nazal | 52.86 ± 6.05 | 52.65—9.1 | 52.65 ± 4.23 | 52.30—7.38 | 0.672 t |
| D. Deep Parafovea–Inferior | 51.09 ± 6.29 | 50.65—6.98 | 51.18 ± 4.72 | 49.45—8.05 | 0.835 u |
| D. Deep Perifovea | 46.83 ± 6.72 | 47.10—10.58 | 47.48 ± 4.57 | 48.60—7.57 | 0.768 t |
| D. Deep Perifovea–Superior–Hemi | 47.22 ± 6.11 | 48.00—9.68 | 47.64 ± 4.56 | 48.55—7.15 | 0.755 t |
| D. Deep Perifovea–Inferior–Hemi | 46.44 ± 7.75 | 45.75—12.52 | 46.98 ± 5.08 | 47.40—9.05 | 0.695 t |
| D. Deep Perifovea–Tempo | 50.85 ± 6.47 | 50.30—8.63 | 50.27 ± 4.39 | 50.50—7.43 | 0.576 t |
| D. Deep Perifovea–Superior | 45.80 ± 7.01 | 45.85—12.1 | 46.73 ± 5.36 | 47.65—9.92 | 0.978 t |
| D. Deep Perifovea–Nazal | 45.61 ± 6.53 | 44.60—11.10 | 45.57 ± 5.45 | 43.95—7.5 | 0.712 t |
| D. Deep Perifovea–Inferior | 45.22 ± 8.63 | 45.10—13.73 | 45.95 ± 5.69 | 47.15—10.22 | 0.928 t |
| D. Superficial Whole Image | 47.04 ± 4.51 | 48.20—7.40 | 47.13 ± 4.26 | 48.60—6.70 | 0.946 u |
| D. Superficial Superior–Hemi | 46.98 ± 4.42 | 47.70—7.73 | 47.24 ± 4.43 | 48.60—5.45 | 0.704 u |
| D. Superficial Inferior-Hemi | 47.11 ± 4.69 | 47.90—7.45 | 47.21 ± 4.14 | 48.50—4.95 | 0.712 t |
| D. Superficial Fovea | 19.80 ± 7.81 | 19.35—11.88 | 19.07 ± 8.62 | 17.15—7.00 | 0.928 t |
| D. Superficial Parafovea | 47.85 ± 5.94 | 49.10—9.57 | 48.19 ± 5.31 | 49.20—7.07 | 0.735 t |
| D. Superficial Parafovea–Superior–Hemi | 47.85 ± 5.97 | 49.10—10.52 | 47.36 ± 7.65 | 49.10—7.95 | 0.818 t |
| D. Superficial Parafovea–Inferior–Hem | 47.84 ± 6.13 | 49.45—9.15 | 48.12 ± 5.66 | 49.50—8.2 | 0.771 u |
| D. Superficial Parafovea–Tempo | 48.34 ± 5.33 | 49.00—7.9 | 48.80 ± 5.19 | 49.35—6.27 | 0.720 u |
| D. Superficial Parafovea–Superior | 48.21 ± 6.54 | 49.05—11.35 | 49.36 ± 5.28 | 50.75—5.27 | 0.779 t |
| D. Superficial Parafovea–Nazal | 45.61 ± 7.03 | 46.95—11.92 | 46.38 ± 6.19 | 47.05—9.63 | 0.469 t |
| D. Superficial Parafovea–Inferior | 49.02 ± 6.22 | 51.10—8.00 | 48.23 ± 6.11 | 49.60—7.20 | 0.658 t |
| D. Superficial Perifovea | 47.76 ± 4.57 | 48.85—6.65 | 48.27 ± 3.82 | 48.95—5.63 | 0.627 t |
| D. Superficial Perifovea–Superior–Hemi | 47.45 ± 4.46 | 47.70—7.58 | 48.12 ± 3.98 | 49.10—5.33 | 0.651 t |
| D. Superficial Perifovea–Inferior–Hemi | 47.95 ± 4.64 | 48.60—7.72 | 48.30 ± 3.87 | 49.20—6.28 | 0.547 t |
| D. Superficial Perifovea–Tempo | 43.15 ± 5.24 | 44.20—6.68 | 44.39 ± 4.44 | 44.50—7.17 | 0.300 u |
| D. Superficial Perifovea–Superior | 47.55 ± 4.52 | 46.80—7.95 | 48.66 ± 4.18 | 49.70—8.18 | 0.755 t |
| D. Superficial Perifovea–Nazal | 51.92 ± 4.30 | 52.40—8.48 | 51.30 ± 5.29 | 53.05—4.05 | 0.335 t |
| D. Superficial Perifovea–Inferior | 47.97 ± 5.01 | 48.05—6.68 | 48.47 ± 3.73 | 48.95—5.68 | 0.618 t |
| FAZ (Mm2) | 0.29 ± 0.08 | 0.29—0.11 | 0.30 ± 0.08 | 0.31—0.10 | 0.671 t |
| PERIM (Mm) | 2.08 ± 0.30 | 2.14—0.41 | 2.09 ± 0.30 | 2.14—0.38 | 0.578 t |
| FD | 51.24 ± 5.34 | 51.41—8.48 | 52.39 ± 5.43 | 52.98—6.79 | 0.927 t |
| Variable | Total Cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL Cholesterol (mg/dL) | LDL Cholesterol (mg/dL) | VDL Cholesterol (mg/dL) | NON-HDL Cholesterol (mg/dL) | White Blood Cell Count (×103/µL) | Neutrophil Count (×103/µL) | Lymphocyte Count (×103/µL) | Monocyte Count (×103/µL) | Red Blood Cell Count (×106/µL) | Red Cell Distribution Width (%) | Platelet Count (×103/µL) | Hemoglobin (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. Deep Whole Image | 0.080/0.228 | 0.386/0.114 | 0.798/0.034 | 0.051/0.253 | 0.203/0.167 | 0.028/0.283 * | 0.182/−0.175 | 0.138/−0.194 | 0.451/−0.099 | 0.318/−0.131 | 0.939/0.01 | 0.836/0.027 | 0.078/−0.229 | 0.409/0.109 |
| D. Deep Superior–Hemi | 0.059/0.245 | 0.285/0.14 | 0.737/0.044 | 0.047/0.258 * | 0.211/0.164 | 0.021/0.297 * | 0.219/−0.161 | 0.124/−0.201 | 0.145/−0.19 | 0.213/−0.163 | 0.803/0.033 | 0.749/0.042 | 0.488/−0.091 | 0.328/0.129 |
| D. Deep Inferior–Hemi | 0.093/0.219 | 0.490/0.091 | 0.783/0.036 | 0.064/ 0.24 | 0.163/0.182 | 0.039/0.267 * | 0.252/−0.15 | 0.183/−0.174 | 0.324/−0.129 | 0.069/−0.237 | 0.722/0.047 | 0.700/0.051 | 0.935/0.011 | 0.106/0.211 |
| D. Deep Fovea | 0.406/−0.109 | 0.566/−0.076 | 0.750/−0.042 | 0.443/−0.101 | 0.654/0.059 | 0.601/−0.069 | 0.692/−0.052 | 0.581/−0.073 | 0.367/−0.118 | 0.841/0.026 | 0.465/0.096 | 0.337/−0.126 | 0.990/−0.002 | 0.627/0.064 |
| D. Deep Parafovea | 0.103/0.213 | 0.288/0.139 | 0.354/0.122 | 0.060/0.244 | 0.079/0.228 | 0.029/0.281 * | 0.289/−0.139 | 0.159/−0.184 | 0.712/−0.049 | 0.273/−0.144 | 0.946/−0.009 | 0.467/0.096 | 0.802/0.033 | 0.499/0.089 |
| D. Deep Parafovea–Superior–Hemi | 0.111/0.208 | 0.207/0.165 | 0.198/0.168 | 0.050/0.254 | 0.078/0.229 | 0.026/0.288 * | 0.187/−0.173 | 0.097/−0.216 | 0.576/−0.074 | 0.256/−0.149 | 0.851/−0.025 | 0.561/0.077 | 0.843/−0.026 | 0.431/0.104 |
| D. Deep Parafovea–Inferior–Hemi | 0.109/0.209 | 0.401/0.11 | 0.584/0.072 | 0.084/0.225 | 0.095/0.217 | 0.042/0.263 * | 0.437/−0.102 | 0.262/−0.147 | 0.870/−0.022 | 0.313/−0.133 | 0.960/0.007 | 0.406/0.109 | 0.507/0.087 | 0.598/0.07 |
| D. Deep Parafovea–Tempo | 0.039/0.268 * | 0.339/0.125 | 0.753/ 0.042 | 0.026/0.288 * | 0.100/0.215 | 0.010/0.329 * | 0.305/−0.135 | 0.173/−0.178 | 0.960/−0.007 | 0.180/−0.176 | 0.930/0.012 | 0.481/0.093 | 0.679/0.055 | 0.432/0.103 |
| D. Deep Parafovea–Superior | 0.166/0.181 | 0.187/0.173 | 0.317/0.131 | 0.135/0.195 | 0.085/0.224 | 0.058/0.246 | 0.250/−0.151 | 0.148/−0.189 | 0.271/−0.144 | 0.165/−0.181 | 0.997/−0.001 | 0.708/0.049 | 0.494/−0.09 | 0.571/0.075 |
| D. Deep Parafovea–Nazal | 0.123/0.202 | 0.374/0.117 | 0.320/0.131 | 0.059/0.246 | 0.103/0.213 | 0.040/0.266 * | 0.229/−0.158 | 0.096/−0.217 | 0.843/−0.026 | 0.298/−0.136 | 0.918/−0.014 | 0.420/0.106 | 0.917/−0.014 | 0.446/0.1 |
| D. Deep Parafovea–Inferior | 0.333/0.127 | 0.435/0.103 | 0.318/0.131 | 0.178/0.176 | 0.129/0.198 | 0.125/0.2 | 0.499/−0.089 | 0.363/−0.12 | 0.942/−0.01 | 0.681/−0.054 | 0.743/−0.043 | 0.384/0.114 | 0.206/0.165 | 0.844/0.026 |
| D. Deep Perifovea | 0.077/0.23 | 0.276/0.143 | 0.484/0.092 | 0.052/0.252 | 0.106/0.211 | 0.025/0.290 * | 0.132/−0.197 | 0.086/−0.223 | 0.164/−0.182 | 0.082/−0.226 | 0.783/0.036 | 0.644/0.061 | 0.620/−0.065 | 0.076/0.231 |
| D. Deep Perifovea–Superior–Hemi | 0.031/0.278 * | 0.224/0.159 | 0.585/ 0.072 | 0.050/0.255 * | 0.145/0.19 | 0.016/0.309 * | 0.152/−0.187 | 0.090/−0.221 | 0.059/−0.246 | 0.092/−0.219 | 0.560/0.077 | 0.660/0.058 | 0.418/−0.106 | 0.176/0.177 |
| D. Deep Perifovea–Inferior–Hemi | 0.164/0.182 | 0.362/0.12 | 0.432/0.103 | 0.060/0.144 | 0.106/0.211 | 0.041/0.265 * | 0.174/−0.178 | 0.118/−0.204 | 0.408/−0.109 | 0.098/−0.215 | 0.983/−0.003 | 0.629/0.064 | 0.974/0.004 | 0.066/0.239 |
| D. Deep Perifovea–Tempo | 0.099/0.215 | 0.102/0.213 | 0.497/0.089 | 0.060/0.245 | 0.043/0.263 * | 0.018/0.304 * | 0.735/−0.045 | 0.509/−0.087 | 0.533/−0.082 | 0.200/−0.168 | 0.946/−0.009 | 0.572/0.074 | 0.691/0.052 | 0.275/0.143 |
| D. Deep Perifovea–Superior | 0.043/0.263 * | 0.299/0.136 | 0.979/ 0.004 | 0.068/0.2637 | 0.305/0.135 | 0.025/0.289 * | 0.147/−0.19 | 0.096/−0.217 | 0.022/−0.296 * | 0.096/−0.217 | 0.432/0.103 | 0.782/0.036 | 0.270/−0.145 | 0.293/0.138 |
| D. Deep Perifovea–Nazal | 0.121/0.202 | 0.641/0.061 | 0.404/ 0.11 | 0.073/0.233 | 0.297/0.137 | 0.067/0.238 | 0.148/−0.189 | 0.101/−0.214 | 0.266/−0.146 | 0.025/−0.288 * | 0.836/0.027 | 0.532/0.082 | 0.924/−0.013 | 0.064/0.241 |
| D. Deep Perifovea–Inferior | 0.263/0.147 | 0.468/0.096 | 0.304/0.135 | 0.105/0.111 | 0.149/0.189 | 0.092/0.219 | 0.104/−0.212 | 0.070/−0.236 | 0.390/−0.113 | 0.294/−0.138 | 0.923/−0.013 | 0.666/0.057 | 0.929/−0.012 | 0.097/0.216 |
| D. Superficial Whole Image | 0.271/0.144 | 0.939/0.01 | 0.079/0.228 | 0.641/0.161 | 0.601/−0.069 | 0.474/0.094 | 0.734/0.045 | 0.931/0.011 | 0.238/−0.155 | 0.060/−0.244 | 0.897/0.017 | 0.277/0.143 | 0.566/0.076 | 0.487/−0.092 |
| D. Superficial Superior–Hemi | 0.237/0.155 | 0.936/0.011 | 0.069/0.236 | 0.622/0.165 | 0.707/−0.05 | 0.408/0.109 | 0.731/0.045 | 0.921/0.013 | 0.328/−0.128 | 0.129/−0.198 | 0.911/0.015 | 0.248/0.151 | 0.546/0.08 | 0.452/−0.099 |
| D. Superficial Inferior–Hemi | 0.357/0.121 | 0.839/0.027 | 0.085/0.225 | 0.749/0.142 | 0.546/−0.079 | 0.605/0.068 | 0.783/0.036 | 0.945/0.009 | 0.149/−0.189 | 0.022/−0.295 * | 0.858/0.024 | 0.330/0.128 | 0.760/0.04 | 0.627/−0.064 |
| D. Superficial Fovea | 0.054/−0.25 | 0.732/−0.045 | 0.066/−0.239 | 0.044/−0.261 * | 0.947/0.009 | 0.111/−0.208 | 0.739/0.044 | 0.431/0.104 | 0.366/−0.119 | 0.080/−0.228 | 0.963/0.006 | 0.683/−0.054 | 0.780/−0.037 | 0.653/−0.059 |
| D. Superficial Parafovea | 0.121/0.203 | 0.792/0.035 | 0.034/0.274 * | 0.722/0.047 | 0.982/−0.003 | 0.356/0.121 | 0.340/−0.125 | 0.282/−0.141 | 0.159/−0.184 | 0.015/−0.313 * | 0.262/0.147 | 0.322/0.13 | 0.718/0.048 | 0.940/−0.01 |
| D. Superficial Parafovea–Superior–Hemi | 0.151/0.188 | 0.983/0.003 | 0.142/0.192 | 0.593/0.17 | 0.701/−0.051 | 0.397/0.111 | 0.565/−0.076 | 0.394/−0.112 | 0.374/−0.117 | 0.137/−0.194 | 0.480/0.093 | 0.346/0.124 | 0.261/0.147 | 0.604/−0.068 |
| D. Superficial Parafovea–Inferior–Hem | 0.117/0.205 | 0.636/0.062 | 0.024/0.290 * | 0.649/0.06 | 0.792/0.035 | 0.276/0.143 | 0.393/−0.112 | 0.328/−0.129 | 0.172/−0.179 | 0.005/−0.361 ** | 0.243/0.153 | 0.361/0.12 | 0.775/0.038 | 0.926/−0.012 |
| D. Superficial Parafovea–Tempo | 0.020/0.300 * | 0.934/0.011 | 0.022/0.294 * | 0.451/0.0099 | 0.699/−0.051 | 0.198/0.168 | 0.551/−0.079 | 0.343/−0.125 | 0.355/−0.121 | 0.122/−0.202 | 0.041/0.264 * | 0.396/0.111 | 0.367/0.118 | 0.862/−0.023 |
| D. Superficial Parafovea–Superior | 0.467/0.096 | 0.892/0.018 | 0.217/0.162 | 0.685/−0.005 | 0.642/−0.061 | 0.967/−0.005 | 0.409/−0.108 | 0.388/−0.114 | 0.132/−0.197 | 0.120/−0.203 | 0.711/0.049 | 0.226/0.159 | 0.660/0.058 | 0.712/−0.049 |
| D. Superficial Parafovea–Nazal | 0.081/0.227 | 0.664/0.057 | 0.032/0.277 * | 0.465/ 0. 96 | 0.840/0.027 | 0.192/0.171 | 0.372/−0.117 | 0.283/−0.141 | 0.092/−0.22 | 0.005/−0.356 ** | 0.235/0.156 | 0.437/0.102 | 0.922/0.013 | 0.631/0.063 |
| D. Superficial Parafovea–Inferior | 0.310/0.133 | 0.454/0.099 | 0.023/0.293 * | 0.905/0.016 | 0.595/0.07 | 0.421/0.106 | 0.220/−0.161 | 0.268/−0.145 | 0.259/−0.148 | 0.002/−0.395 ** | 0.465/0.096 | 0.355/0.122 | 0.985/−0.002 | 0.824/−0.029 |
| D. Superficial Perifovea | 0.262/0.147 | 0.941/0.01 | 0.130/0.197 | 0.497/0.089 | 0.720/−0.047 | 0.360/0.12 | 0.561/0.077 | 0.753/0.041 | 0.208/−0.165 | 0.062/−0.242 | 0.963/−0.006 | 0.303/0.135 | 0.747/0.042 | 0.765/−0.039 |
| D. Superficial Perifovea–Superior–Hemi | 0.164/0.182 | 0.716/0.048 | 0.087/0.223 | 0.465/0.096 | 0.871/−0.021 | 0.253/0.15 | 0.626/0.064 | 0.815/0.031 | 0.325/−0.129 | 0.105/−0.211 | 0.889/0.018 | 0.281/0.142 | 0.700/0.051 | 0.676/−0.055 |
| D. Superficial Perifovea–Inferior–Hemi | 0.472/0.095 | 0.857/0.024 | 0.203/0.167 | 0.637/0.062 | 0.597/−0.07 | 0.585/0.072 | 0.494/0.09 | 0.668/0.057 | 0.135/−0.195 | 0.043/−0.262 * | 0.837/−0.027 | 0.312/0.133 | 0.721/0.047 | 0.851/−0.025 |
| D. Superficial Perifovea–Tempo | 0.572/0.074 | 0.731/0.045 | 0.127/0.199 | 0.755/0.0041 | 0.982/0.003 | 0.878/0.02 | 0.804/0.033 | 0.906/0.016 | 0.160/−0.184 | 0.470/−0.095 | 0.825/0.029 | 0.324/0.13 | 0.568/0.075 | 0.620/−0.065 |
| D. Superficial Perifovea–Superior | 0.092/0.219 | 0.721/0.047 | 0.073/0.233 | 0.260/0.148 | 0.990/−0.002 | 0.125/ 0.2 | 0.528/0.083 | 0.759/0.04 | 0.543/−0.08 | 0.117/−0.205 | 0.881/0.02 | 0.243/0.153 | 0.989/0.002 | 0.782/−0.036 |
| D. Superficial Perifovea–Nazal | 0.358/0.121 | 0.932/0.011 | 0.193/0.17 | 0.370/0.118 | 0.547/−0.079 | 0.353/0.122 | 0.561/0.077 | 0.735/0.045 | 0.155/−0.186 | 0.001/−0.417 ** | 0.894/−0.018 | 0.491/0.091 | 0.785/0.036 | 0.955/−0.007 |
| D. Superficial Perifovea–Inferior | 0.306/0.134 | 0.760/0.04 | 0.460/0.097 | 0.364/0.119 | 0.448/ −0.1 | 0.407/0.109 | 0.587/0.071 | 0.900/0.017 | 0.358/−0.121 | 0.269/−0.145 | 0.639/−0.062 | 0.310/0.133 | 0.449/0.1 | 0.895/−0.017 |
| FAZ (Mm2) | 0.025/0.289 * | 0.898/0.017 | 0.771/0.038 | 0.099/0.215 | 0.724/0.046 | 0.083/0.226 | 0.696/0.052 | 0.875/0.021 | 0.497/0.089 | 0.651/0.06 | 0.392/0.113 | 0.538/0.081 | 0.992/−0.001 | 0.360/0.12 |
| PERIM (Mm) | 0.046/0.259 * | 0.733/0.045 | 0.537/0.081 | 0.130/0.198 | 0.784/−0.036 | 0.183/0.174 | 0.609/0.067 | 0.801/0.033 | 0.240/0.154 | 0.055/0.249 | 0.626/0.064 | 0.551/0.079 | 0.499/0.089 | 0.953/−0.008 |
| FD | 0.013/0.319 * | 0.308/0.134 | 0.557/0.077 | 0.111/0.208 | 0.633/0.063 | 0.036/0.271 * | 0.716/0.048 | 0.753/−0.042 | 0.441/−0.101 | 0.085/−0.224 | 0.224/0.159 | 0.764/0.039 | 0.219/0.161 | 0.233/0.156 |
| Variable | 00:00–04:00 Systolic | 00:00–04:00 Diastolic | 00:00–04:00 MAP | 04:00–08:00 Systolic | 04:00–08:00 Diastolic | 04:00–08:00 MAP | Systolic Change Ratio |
|---|---|---|---|---|---|---|---|
| D. Deep Whole Image | 0.187/0.173 | 0.297/0.137 | 0.226/0.159 | 0.581/0.073 | 0.274/0.145 | 0.162/0.184 | 0.571/−0.075 |
| D. Deep Superior–Hemi | 0.136/0.195 | 0.121/0.202 | 0.087/0.223 | 0.487/0.092 | 0.055/0.252 | 0.018/0.307 * | 0.921/−0.013 |
| D. Deep Inferior–Hemi | 0.060/0.244 | 0.276/0.143 | 0.306/0.135 | 0.456/0.099 | 0.237/0.156 | 0.164/0.183 | 0.678/−0.055 |
| D. Deep Fovea | 0.602/0.069 | 0.361/0.12 | 0.174/0.178 | 0.128/0.2 | 0.052/0.255 | 0.047/0.260 * | 0.334/0.127 |
| D. Deep Parafovea | 0.364/0.119 | 0.538/0.081 | 0.539/0.081 | 0.723/0.047 | 0.272/0.145 | 0.128/0.2 | 0.872/−0.021 |
| D. Deep Parafovea–Superior–Hemi | 0.309/0.134 | 0.267/0.146 | 0.251/0.151 | 0.474/0.095 | 0.081/0.229 | 0.023/0.296 * | 0.941/−0.01 |
| D. Deep Parafovea–Inferior–Hemi | 0.435/0.103 | 0.907/0.015 | 0.937/0.01 | 0.994/−0.001 | 0.661/0.058 | 0.452/0.1 | 0.809/−0.032 |
| D. Deep Parafovea–Tempo | 0.472/0.095 | 0.976/0.004 | 0.873/0.021 | 0.924/0.013 | 0.587/0.072 | 0.291/0.14 | 0.613/−0.067 |
| D. Deep Parafovea–Superior | 0.343/0.124 | 0.428/0.104 | 0.475/0.094 | 0.766/0.04 | 0.132/0.198 | 0.088/0.224 | 0.867/−0.022 |
| D. Deep Parafovea–Nazal | 0.322/0.13 | 0.394/0.112 | 0.399/0.111 | 0.612/0.067 | 0.277/0.144 | 0.084/0.227 | 0.896/−0.017 |
| D. Deep Parafovea–Inferior | 0.450/0.099 | 0.600/0.069 | 0.620/0.065 | 0.711/0.049 | 0.390/0.114 | 0.252/0.152 | 0.857/0.024 |
| D. Deep Perifovea | 0.112/0.207 | 0.309/0.134 | 0.329/0.128 | 0.782/0.037 | 0.234/0.157 | 0.166/0.183 | 0.441/−0.101 |
| D. Deep Perifovea–Superior–Hemi | 0.198/0.169 | 0.221/0.16 | 0.189/0.172 | 0.828/0.029 | 0.126/0.202 | 0.070/0.238 | 0.577/−0.074 |
| D. Deep Perifovea–Inferior–Hemi | 0.074/0.232 | 0.347/0.124 | 0.398/0.111 | 0.605/0.069 | 0.332/0.129 | 0.241/0.155 | 0.586/−0.072 |
| D. Deep Perifovea–Tempo | 0.099/0.215 | 0.200/0.168 | 0.103/0.212 | 0.130/0.199 | 0.110/0.21 | 0.041/0.266 * | 0.345/0.124 |
| D. Deep Perifovea–Superior | 0.180/0.176 | 0.205/0.166 | 0.185/0.173 | 0.837/0.027 | 0.057/0.249 | 0.039/0.269 * | 0.532/−0.082 |
| D. Deep Perifovea–Nazal | 0.267/0.146 | 0.402/0.11 | 0.445/0.1 | 0.923/−0.013 | 0.822/0.03 | 0.510/0.087 | 0.154/−0.186 |
| D. Deep Perifovea–Inferior | 0.059/0.245 | 0.231/0.157 | 0.305/0.135 | 0.722/0.047 | 0.176/0.179 | 0.186/0.175 | 0.751/−0.042 |
| D. Superficial Whole Image | 0.213/0.163 | 0.724/0.046 | 0.252/0.15 | 0.059/0.247 | 0.327/0.13 | 0.298/0.138 | 0.970/−0.005 |
| D. Superficial Superior–Hemi | 0.210/0.164 | 0.837/0.027 | 0.304/0.135 | 0.052/0.255 | 0.319/0.132 | 0.254/0.151 | 0.877/−0.02 |
| D. Superficial Inferior-Hemi | 0.242/0.153 | 0.727/0.046 | 0.291/0.139 | 0.104/0.214 | 0.386/0.115 | 0.441/0.102 | 0.798/−0.034 |
| D. Superficial Fovea | 0.555/0.078 | 0.461/0.097 | 0.368/0.118 | 0.517/0.086 | 0.396/0.113 | 0.520/0.085 | 0.985/−0.002 |
| D. Superficial Parafovea | 0.369/0.118 | 0.930/0.012 | 0.361/0.12 | 0.110/0.21 | 0.664/0.058 | 0.615/0.067 | 0.642/−0.061 |
| D. Superficial Parafovea–Superior–Hemi | 0.377/0.116 | 0.651/0.06 | 0.166/0.181 | 0.030/0.282 * | 0.436/0.103 | 0.233/0.158 | 0.490/0.091 |
| D. Superficial Parafovea–Inferior–Hem | 0.389/0.113 | 0.877/0.02 | 0.366/0.119 | 0.216/0.164 | 0.797/0.034 | 0.812/0.032 | 0.632/−0.063 |
| D. Superficial Parafovea–Tempo | 0.506/0.088 | 0.866/0.022 | 0.358/0.121 | 0.418/0.107 | 0.657/0.059 | 0.605/0.069 | 0.674/−0.055 |
| D. Superficial Parafovea–Superior | 0.724/0.047 | 0.931/−0.011 | 0.361/0.12 | 0.022/0.297 * | 0.549/0.08 | 0.438/0.103 | 0.491/−0.091 |
| D. Superficial Parafovea–Nazal | 0.492/0.09 | 0.806/−0.032 | 0.637/0.062 | 0.339/0.127 | 0.820/0.03 | 0.847/0.026 | 0.459/−0.097 |
| D. Superficial Parafovea–Inferior | 0.457/0.098 | 0.627/0.064 | 0.241/0.154 | 0.056/0.25 | 0.732/0.046 | 0.688/0.053 | 0.885/−0.019 |
| D. Superficial Perifovea | 0.066/0.239 | 0.990/0.002 | 0.551/0.079 | 0.125/0.202 | 0.413/0.109 | 0.481/0.093 | 0.609/−0.067 |
| D. Superficial Perifovea–Superior–Hemi | 0.213/0.163 | 0.717/−0.048 | 0.754/0.041 | 0.158/0.186 | 0.573/0.075 | 0.580/0.074 | 0.477/−0.094 |
| D. Superficial Perifovea–Inferior–Hemi | 0.232/0.157 | 0.704/0.05 | 0.354/0.122 | 0.080/0.229 | 0.264/0.148 | 0.350/0.124 | 0.694/−0.052 |
| D. Superficial Perifovea–Tempo | 0.319/0.131 | 0.661/0.058 | 0.260/0.148 | 0.054/0.253 | 0.307/0.135 | 0.403/0.111 | 0.957/−0.007 |
| D. Superficial Perifovea–Superior | 0.224/0.159 | 0.539/−0.081 | 0.959/−0.007 | 0.346/0.125 | 0.666/0.057 | 0.702/0.051 | 0.236/−0.155 |
| D. Superficial Perifovea–Nazal | 0.320/0.131 | 0.903/−0.016 | 0.590/0.071 | 0.183/0.176 | 0.748/0.043 | 0.655/0.059 | 0.606/−0.068 |
| D. Superficial Perifovea–Inferior | 0.170/0.179 | 0.878/0.02 | 0.463/0.097 | 0.122/0.204 | 0.288/0.141 | 0.371/0.119 | 0.811/−0.032 |
| FAZ (Mm2) | 0.325/−0.129 | 0.554/−0.078 | 0.304/−0.135 | 0.170/−0.181 | 0.364/−0.12 | 0.230/−0.159 | 0.093/−0.219 |
| PERIM (Mm) | 0.551/−0.079 | 0.933/0.011 | 0.826/−0.029 | 0.460/−0.098 | 0.730/−0.046 | 0.755/−0.041 | 0.651/−0.06 |
| FD | 0.778/−0.037 | 0.316/−0.132 | 0.594/−0.07 | 0.899/−0.017 | 0.498/−0.09 | 0.408/−0.11 | 0.209/−0.165 |
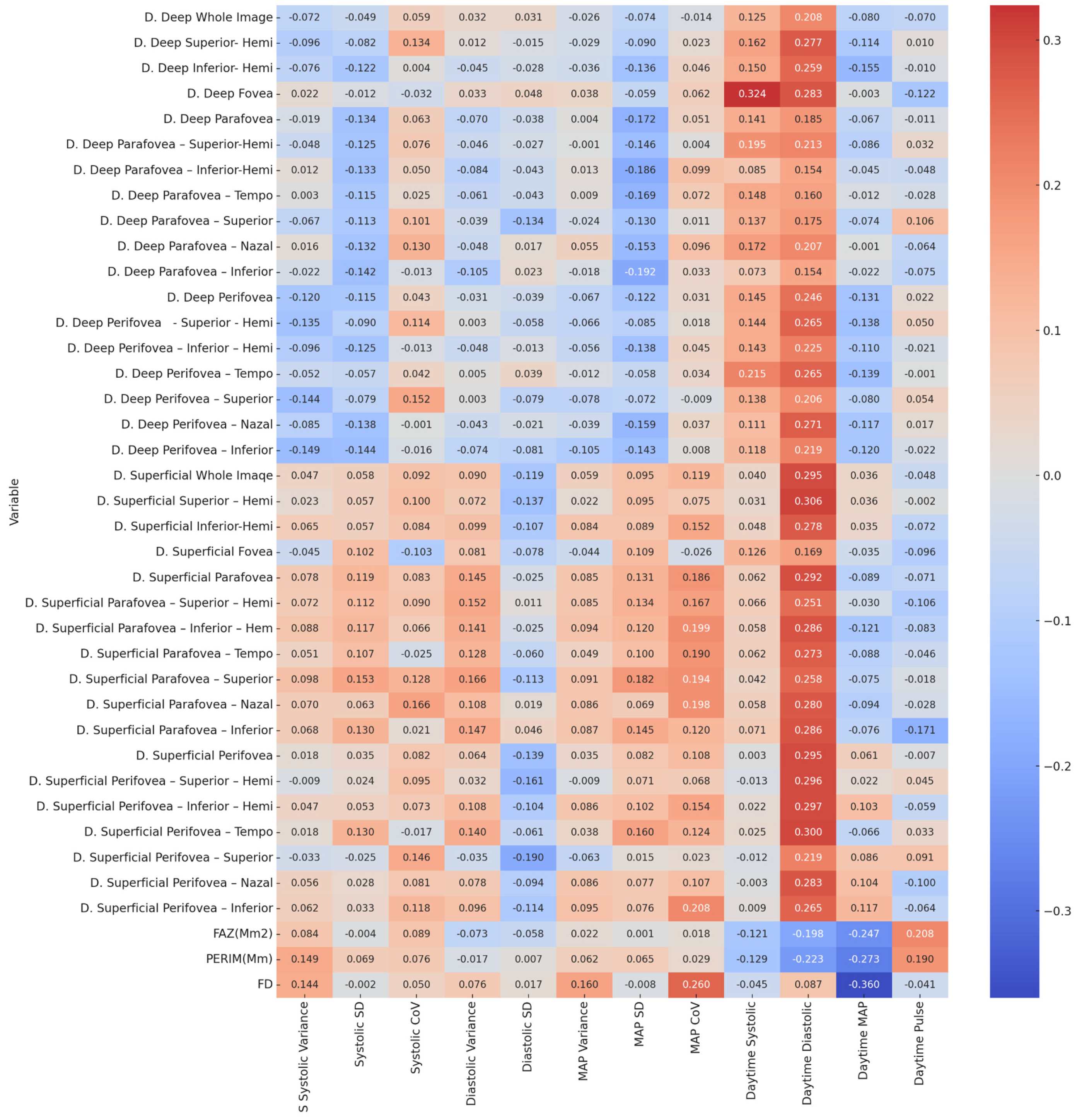
| Variable | Nighttime Systolic | Nighttime Diastolic | Nighttime MAP | Nighttime Pulse | Daytime Systolic | Daytime Diastolic | Daytime MAP | Daytime Pulse |
|---|---|---|---|---|---|---|---|---|
| D. Deep Whole Image | 0.350/0.123 | 0.209/0.164 | 0.026/0.289 * | 0.216/−0.162 | 0.344/0.125 | 0.111/0.208 | 0.546/−0.08 | 0.597/−0.07 |
| D. Deep Superior–Hemi | 0.204/0.166 | 0.045/0.260 * | 0.001/0.426 ** | 0.215/−0.162 | 0.220/0.162 | 0.032/0.277 * | 0.389/−0.114 | 0.937/0.01 |
| D. Deep Inferior–Hemi | 0.306/0.134 | 0.161/0.183 | 0.008/0.343 ** | 0.411/−0.108 | 0.257/0.15 | 0.045/0.259 * | 0.240/−0.155 | 0.940/−0.01 |
| D. Deep Fovea | 0.108/0.21 | 0.141/0.192 | 0.112/0.209 | 0.717/0.048 | 0.012/0.324 * | 0.029/0.283 * | 0.979/−0.003 | 0.355/−0.122 |
| D. Deep Parafovea | 0.379/0.116 | 0.363/0.119 | 0.028/0.287 * | 0.249/−0.151 | 0.286/0.141 | 0.156/0.185 | 0.614/−0.067 | 0.936/−0.011 |
| D. Deep Parafovea–Superior–Hemi | 0.137/0.194 | 0.137/0.194 | 0.008/0.340 ** | 0.272/−0.144 | 0.138/0.195 | 0.103/0.213 | 0.517/−0.086 | 0.807/0.032 |
| D. Deep Parafovea–Inferior–Hemi | 0.790/0.035 | 0.746/0.043 | 0.091/0.222 | 0.263/−0.147 | 0.522/0.085 | 0.241/0.154 | 0.735/−0.045 | 0.714/−0.048 |
| D. Deep Parafovea–Tempo | 0.421/0.106 | 0.779/0.037 | 0.071/0.237 | 0.315/−0.132 | 0.263/0.148 | 0.222/0.16 | 0.931/−0.012 | 0.834/−0.028 |
| D. Deep Parafovea–Superior | 0.354/0.122 | 0.238/0.155 | 0.020/0.303 * | 0.067/−0.238 | 0.302/0.137 | 0.181/0.175 | 0.578/−0.074 | 0.420/0.106 |
| D. Deep Parafovea–Nazal | 0.265/0.146 | 0.338/0.126 | 0.056/0.25 | 0.465/−0.096 | 0.194/0.172 | 0.112/0.207 | 0.995/−0.001 | 0.629/−0.064 |
| D. Deep Parafovea–Inferior | 0.686/0.053 | 0.380/0.115 | 0.033/0.279 * | 0.314/−0.132 | 0.582/0.073 | 0.240/0.154 | 0.872/−0.022 | 0.566/−0.075 |
| D. Deep Perifovea | 0.441/0.101 | 0.179/0.176 | 0.010/0.333 ** | 0.291/−0.139 | 0.273/0.145 | 0.058/0.246 | 0.324/−0.131 | 0.867/0.022 |
| D. Deep Perifovea–Superior–Hemi | 0.365/0.119 | 0.105/0.211 | 0.005/0.365 ** | 0.196/−0.169 | 0.275/0.144 | 0.041/0.265 * | 0.296/−0.138 | 0.705/0.05 |
| D. Deep Perifovea–Inferior–Hemi | 0.382/0.115 | 0.231/0.157 | 0.014/0.317 * | 0.363/−0.119 | 0.280/0.143 | 0.084/0.225 | 0.405/−0.11 | 0.875/−0.021 |
| D. Deep Perifovea–Tempo | 0.080/0.228 | 0.081/0.227 | 0.002/0.391 ** | 0.643/−0.061 | 0.102/0.215 | 0.041/0.265 * | 0.294/−0.139 | 0.993/−0.001 |
| D. Deep Perifovea–Superior | 0.370/0.118 | 0.070/0.236 | 0.002/0.396 ** | 0.150/−0.188 | 0.296/0.138 | 0.114/0.206 | 0.546/−0.08 | 0.681/0.054 |
| D. Deep Perifovea–Nazal | 0.638/0.062 | 0.411/0.108 | 0.063/0.243 | 0.512/−0.086 | 0.401/0.111 | 0.036/0.271 * | 0.378/−0.117 | 0.899/0.017 |
| D. Deep Perifovea–Inferior | 0.345/0.124 | 0.141/0.192 | 0.008/0.342 ** | 0.201/−0.167 | 0.374/0.118 | 0.093/0.219 | 0.367/−0.12 | 0.867/−0.022 |
| D. Superficial Whole Imaqe | 0.170/0.179 | 0.428/0.104 | 0.069/0.239 | 0.693/−0.052 | 0.764/0.04 | 0.022/0.295 * | 0.788/0.036 | 0.717/−0.048 |
| D. Superficial Superior–Hemi | 0.179/0.176 | 0.447/0.1 | 0.052/0.254 | 0.738/−0.044 | 0.816/0.031 | 0.018/0.306 * | 0.787/0.036 | 0.988/−0.002 |
| D. Superficial Inferior–Hemi | 0.236/0.155 | 0.502/0.088 | 0.143/0.193 | 0.780/−0.037 | 0.721/0.048 | 0.032/0.278 * | 0.792/0.035 | 0.584/−0.072 |
| D. Superficial Fovea | 0.364/0.119 | 0.385/0.114 | 0.604/0.069 | 0.257/0.149 | 0.341/0.126 | 0.196/0.169 | 0.790/−0.035 | 0.466/−0.096 |
| D. Superficial Parafovea | 0.237/0.155 | 0.655/0.059 | 0.131/0.199 | 0.609/−0.067 | 0.643/0.062 | 0.024/0.292 * | 0.504/−0.089 | 0.588/−0.071 |
| D. Superficial Parafovea–Superior–Hemi | 0.076/0.231 | 0.396/0.111 | 0.043/0.265 * | 0.317/−0.131 | 0.619/0.066 | 0.053/0.251 | 0.819/−0.03 | 0.419/−0.106 |
| D. Superficial Parafovea–Inferior–Hem | 0.285/0.14 | 0.698/0.051 | 0.177/0.178 | 0.738/−0.044 | 0.662/0.058 | 0.027/0.286 * | 0.359/−0.121 | 0.528/−0.083 |
| D. Superficial Parafovea–Tempo | 0.368/0.118 | 0.562/0.076 | 0.110/0.21 | 0.408/−0.109 | 0.638/0.062 | 0.035/0.273 * | 0.508/−0.088 | 0.726/−0.046 |
| D. Superficial Parafovea–Superior | 0.242/0.153 | 0.723/0.047 | 0.090/0.222 | 0.307/−0.134 | 0.750/0.042 | 0.047/0.258 * | 0.575/−0.075 | 0.894/−0.018 |
| D. Superficial Parafovea–Nazal | 0.531/0.083 | 0.854/0.024 | 0.204/0.168 | 0.868/−0.022 | 0.662/0.058 | 0.030/0.280 * | 0.479/−0.094 | 0.834/−0.028 |
| D. Superficial Parafovea–Inferior | 0.089/0.221 | 0.581/0.073 | 0.273/0.145 | 0.994/0.001 | 0.594/0.071 | 0.027/0.286 * | 0.568/−0.076 | 0.193/−0.171 |
| D. Superficial Perifovea | 0.417/0.107 | 0.597/0.07 | 0.150/0.19 | 0.754/−0.041 | 0.984/0.003 | 0.022/0.295 * | 0.649/0.061 | 0.959/−0.007 |
| D. Superficial Perifovea–Superior–Hemi | 0.534/0.082 | 0.786/0.036 | 0.153/0.188 | 0.835/−0.027 | 0.924/−0.013 | 0.022/0.296 * | 0.871/0.022 | 0.732/0.045 |
| D. Superficial Perifovea–Inferior–Hemi | 0.301/0.136 | 0.418/0.106 | 0.145/0.192 | 0.654/−0.059 | 0.866/0.022 | 0.021/0.297 * | 0.436/0.103 | 0.655/−0.059 |
| D. Superficial Perifovea–Tempo | 0.443/0.101 | 0.300/0.136 | 0.073/0.235 | 0.912/−0.015 | 0.852/0.025 | 0.020/0.300 * | 0.617/−0.066 | 0.803/0.033 |
| D. Superficial Perifovea–Superior | 0.686/0.053 | 0.996/−0.001 | 0.242/0.155 | 0.788/−0.036 | 0.925/−0.012 | 0.093/0.219 | 0.517/0.086 | 0.491/0.091 |
| D. Superficial Perifovea–Nazal | 0.298/0.136 | 0.894/0.018 | 0.358/0.122 | 0.648/−0.06 | 0.983/−0.003 | 0.028/0.283 * | 0.433/0.104 | 0.447/−0.1 |
| D. Superficial Perifovea–Inferior | 0.380/0.115 | 0.546/0.08 | 0.173/0.18 | 0.538/−0.081 | 0.949/0.009 | 0.040/0.265 * | 0.378/0.117 | 0.629/−0.064 |
| FAZ (Mm2) | 0.059/−0.245 | 0.544/−0.08 | 0.947/−0.009 | 0.931/−0.011 | 0.363/−0.121 | 0.128/−0.198 | 0.059/−0.247 | 0.110/0.208 |
| PERIM (Mm) | 0.180/−0.175 | 0.921/0.013 | 0.577/0.074 | 0.873/−0.021 | 0.329/−0.129 | 0.087/−0.223 | 0.037/−0.273 * | 0.146/0.19 |
| FD | 0.408/−0.109 | 0.541/−0.08 | 0.326/0.13 | 0.706/−0.05 | 0.734/−0.045 | 0.510/0.087 | 0.005/−0.360 ** | 0.758/−0.041 |
| Variable | B | Sh | Beta | t | P | R2 | F | p |
|---|---|---|---|---|---|---|---|---|
| Constant | 21.181 | 5.675 | − | 3.732 | 0.000 | 0.437 | 5.537 | 0.001 |
| Age (years) | −0.068 | 0.044 | −0.178 | −1.539 | 0.130 | |||
| LDL cholesterol (mg/dL) | 0.050 | 0.039 | 0.310 | 1.282 | 0.206 | |||
| Non-HDL cholesterol (mg/dL) | 0.010 | 0.035 | 0.071 | 0.293 | 0.770 | |||
| Daytime Diastolic BP (mmHg) | 0.077 | 0.046 | 0.197 | 1.674 | 0.100 | |||
| Nighttime Diastolic BP (mmHg) | −0.165 | 0.128 | −0.332 | −1.286 | 0.204 | |||
| 04:00–08:00 MAP (mmHg) | 0.253 | 0.106 | 0.604 | 2.384 | 0.021 | |||
| Nighttime MAP (mmHg) | 0.058 | 0.080 | 0.147 | 0.729 | 0.469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, O.; Polat, Ş.N.; Satılmış, C.; Göre, B.; Yakut, M.; Aydoğmuş, İ.; Çelik, M.; Önen, M.; Ateş, İ. Effect of Circadian Blood Pressure Variations on Retinal Microvascular Structures: Optical Coherence Tomography Angiography Analysis with the Nighttime Divided into Subintervals (Retinal Dawn Pattern). Medicina 2025, 61, 1801. https://doi.org/10.3390/medicina61101801
Zengin O, Polat ŞN, Satılmış C, Göre B, Yakut M, Aydoğmuş İ, Çelik M, Önen M, Ateş İ. Effect of Circadian Blood Pressure Variations on Retinal Microvascular Structures: Optical Coherence Tomography Angiography Analysis with the Nighttime Divided into Subintervals (Retinal Dawn Pattern). Medicina. 2025; 61(10):1801. https://doi.org/10.3390/medicina61101801
Chicago/Turabian StyleZengin, Oğuzhan, Şule Nur Polat, Canan Satılmış, Burak Göre, Melike Yakut, İrem Aydoğmuş, Merve Çelik, Mehmet Önen, and İhsan Ateş. 2025. "Effect of Circadian Blood Pressure Variations on Retinal Microvascular Structures: Optical Coherence Tomography Angiography Analysis with the Nighttime Divided into Subintervals (Retinal Dawn Pattern)" Medicina 61, no. 10: 1801. https://doi.org/10.3390/medicina61101801
APA StyleZengin, O., Polat, Ş. N., Satılmış, C., Göre, B., Yakut, M., Aydoğmuş, İ., Çelik, M., Önen, M., & Ateş, İ. (2025). Effect of Circadian Blood Pressure Variations on Retinal Microvascular Structures: Optical Coherence Tomography Angiography Analysis with the Nighttime Divided into Subintervals (Retinal Dawn Pattern). Medicina, 61(10), 1801. https://doi.org/10.3390/medicina61101801







