Predictive Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Dal Maso, L.; Pizzato, M.; Vaccarella, S. Evolving epidemiological patterns of thyroid cancer and estimates of overdiagnosis in 2013-17 in 63 countries worldwide: A population-based study. Lancet Diabetes Endocrinol. 2024, 12, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Houten, P.V.; Netea-Maier, R.T.; Smit, J.W. Differentiated thyroid carcinoma: An update. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101687. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Che, W.; Yu, Z.; Zheng, S.; Xie, S.; Chen, C.; Qiao, M.; Lyu, J. The Incidence Trend of Papillary Thyroid Carcinoma in the United States During 2003–2017. Cancer Control 2022, 29, 10732748221135447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Ryu, H.J.; Park, H.; Kim, T.H.; Kim, S.W.; Oh, Y.L.; Chung, J.H. Mortality rate and causes of death in papillary thyroid microcarcinoma. Endocrine 2024, 83, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, E.; Campopiano, M.C.; Elisei, R. MANAGEMENT OF ENDOCRINE DISEASE: Papillary thyroid microcarcinoma: Toward an active surveillance strategy. Eur. J. Endocrinol. 2021, 185, R23–R34. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Guglielmi, R.; Novizio, R.; Pontecorvi, A.; Durante, C. Management of low-risk papillary thyroid cancer. Minimally-invasive treatments dictate a further paradigm shift? Endocrine 2024, 85, 584–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, X.; Jin, Q.; Cen, X.; Qiu, M.; Wu, Z. Clinicopathologic predictors of central lymph node metastases in clinical node-negative papillary thyroid microcarcinoma: A systematic review and meta-analysis. World J. Surg. Oncol. 2022, 20, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dismukes, J.; Fazendin, J.; Obiarinze, R.; Márquez, G.C.H.; Ramonell, K.M.; Buczek, E.; Lindeman, B.; Chen, H. Prophylactic Central Neck Dissection in Papillary Thyroid Carcinoma: All Risks, No Reward. J. Surg. Res. 2021, 264, 230–235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baud, G.; Jannin, A.; Marciniak, C.; Chevalier, B.; Do Cao, C.; Leteurtre, E.; Beron, A.; Lion, G.; Boury, S.; Aubert, S.; et al. Impact of Lymph Node Dissection on Postoperative Complications of Total Thyroidectomy in Patients with Thyroid Carcinoma. Cancers 2022, 14, 5462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.; Li, B.L.; Zheng, C.J.; He, X.D. Predictive factors for central lymph node metastases in papillary thyroid microcarcinoma. World J. Clin. Cases 2020, 8, 1350–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, S.; Han, Z.; Lu, Q.; Wang, P.; Chen, G. Clinical and Ultrasonic Risk Factors for Lateral Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boutzios, G.; Tsourouflis, G.; Garoufalia, Z.; Alexandraki, K.; Kouraklis, G. Long-term sequelae of the less than total thyroidectomy procedures for benign thyroid nodular disease. Endocrine 2019, 63, 247–251. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, K.; Xu, J.; Li, S.; Liu, S.; Zhang, L. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer among patients with papillary thyroid microcarcinoma. Cancer Med. 2019, 8, 6977–6985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvalho, A.Y.; Kohler, H.F.; Gomes, C.C.; Vartanian, J.G.; Kowalski, L.P. Predictive Factors of Recurrence of Papillary Thyroid Microcarcinomas: Analysis of 2,538 Patients. Int. Arch. Otorhinolaryngol. 2021, 25, e585–e593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Wang, Z.; Meng, X.; Duh, Q.Y.; Chen, G. Predictors for central lymph node metastases in CN0 papillary thyroid microcarcinoma (mPTC): A retrospective analysis of 1304 cases. Asian J. Surg. 2019, 42, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Jiwang, L.; Yahong, L.; Kai, L.; Bo, H.; Yuejiao, Z.; Haotian, W.; Tao, Y. Clinicopathologic factors and preoperative ultrasonographic characteristics for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A single center retrospective study. Braz. J. Otorhinolaryngol. 2022, 88, 36–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, J.; Lee, H.S.; Heo, J.H.; Song, Y.S. Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Endocrinol. Metab. 2024, 39, 324–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, J.W.; Ye, J.; Wu, W.X.; Qu, Z.; Qin, A.-C.; Jiang, Y. Management of cN0 papillary thyroid microcarcinoma patients according to risk-scoring model for central lymph node metastasis and predictors of recurrence. J. Endocrinol. Investig. 2020, 43, 1807–1817. [Google Scholar] [CrossRef]
- Ringel, M.D.; Sosa, J.A.; Baloch, Z.; Bischoff, L.; Bloom, G.; Brent, G.A.; Brock, P.L.; Chou, R.; Flavell, R.R.; Goldner, W.; et al. 2025 American Thyroid Association Management Guidelines for Adult Patients with Differentiated Thyroid Cancer. Thyroid 2025, 35, 841–985. [Google Scholar] [CrossRef]
- Wu, Z.; Han, L.; Li, W.; Wang, W.; Chen, L.; Yao, Y.; Wang, Y. Which is preferred for initial treatment of papillary thyroid cancer, total thyroidectomy or lobotomy? Cancer Med. 2021, 10, 1614–1622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shou, J.-D.; Li, F.-B.; Shi, L.-H.; Zhou, L.; Xie, L.; Wang, J.-B. Predicting non-small-volume central lymph node metastases (>5 or ≥2 mm) preoperatively in cN0 papillary thyroid microcarcinoma without extrathyroidal extension. Medicine 2020, 99, e22338. [Google Scholar] [CrossRef]
- Rosario, P.W.; Mourão, G.F.; Calsolari, M.R. Active Surveillance in Adults with Low-Risk Papillary Thyroid Microcarcinomas: A Prospective Study. Horm. Metab. Res. 2019, 51, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zheng, B.; Li, C.; Zhou, J.; Tong, J.; Ye, L.; He, Y. Active Surveillance for Low-Risk Papillary Thyroid Microcarcinoma in China: A Prospective Study on Progression, Influencing Factors, and Cost-Effectiveness. World. J. Surg. 2025, 49, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Moon, J.H.; Hwangbo, Y.; Ryu, C.H.; Cho, S.W.; Choi, J.Y.; Chung, E.J.; Jeong, W.J.; Jung, Y.S.; Ryu, J.; et al. Progression of Low-Risk Papillary Thyroid Microcarcinoma During Active Surveillance: Interim Analysis of a Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma in Korea. Thyroid 2022, 32, 1328–1336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Zhu, J.; Ai, X.; Dang, M.; Huang, P. An ultrasound-based nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma. Med. Ultrason. 2024, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ding, Y.; Meng, C.; Li, P.; Bai, N.; Li, X. Patient’s age with papillary thyroid cancer: Is it a key factor for cervical lymph node metastasis? Eur. J. Surg. Oncol. 2023, 49, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- HS, O.; Park, S.; Kim, M.; Kwon, H.; Song, E.; Sung, T.Y.; Lee, Y.M.; Kim, W.G.; Kim, T.Y.; Shong, Y.K.; et al. Young Age and Male Sex Are Predictors of Large-Volume Central Neck Lymph Node Metastasis in Clinical N0 Papillary Thyroid Microcarcinomas. Thyroid 2017, 27, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhao, Y.; Chen, K.; Shen, J.; Shi, J.; Lu, S.; Lei, J.; Li, Z.; Luo, D. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J. Endocrinol. Investig. 2019, 42, 227–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tacchi, G.; Pedicini, F.; Crucitti, P.; Carlini, M. Evaluation of predictive factors for lymph node metastasis in thyroid microcarcinoma: A two-year experience from two high-volume centers. Updates Surg. 2025, 77, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Deng, C.; Yang, Z.; Hu, D.; Shu, X.; Yu, P.; Su, X. Preoperative and pathological predictive factors of central lymph node metastasis in papillary thyroid microcarcinoma. Auris Nasus Larynx 2022, 49, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, X.F.; Yang, M.; Huang, L.P. Analysis of the risk factors for central lymph-node metastasis of cN0 papillary thyroid microcarcinoma: A retrospective study. Asian J. Surg. 2022, 45, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Medas, F.; Canu, G.L.; Cappellacci, F.; Boi, F.; Lai, M.L.; Erdas, E.; Calò, P.G. Predictive Factors of Lymph Node Metastasis in Patients with Papillary Microcarcinoma of the Thyroid: Retrospective Analysis on 293 Cases. Front. Endocrinol. 2020, 11, 551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Li, X.; Liu, C.; Liu, R.; Shi, X.; Ma, L.; Zhao, H.; Xia, Y.; Jiang, Y. What Are the Characteristics of Papillary Thyroid Microcarcinoma Prone to High-Volume Lateral Lymph Node Metastasis?—An Analysis of 2981 Consecutive Cases. J. Investig. Surg. 2022, 35, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Li, M.Y.; Zhou, C.P.; Ao, W.; Huang, W.Y.; Wang, S.S.; Yu, J.F.; Tang, Z.H.; Abdelhamid Ahmed, A.H.; Wang, T.Y.; et al. Accurate preoperative prediction of nodal metastasis in papillary thyroid microcarcinoma: Towards optimal management of patients. Head Neck 2024, 46, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Guo, Q.; Pan, K.; Lin, J. Development of a nomogram for prediction of central lymph node metastasis of papillary thyroid microcarcinoma. BMC Cancer 2024, 24, 235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Liao, L.; Yan, D.; Liu, J.; Liu, W.; Liu, S.; Huang, H. The impact of age at diagnosis on central lymph node metastasis in clinically low-risk papillary thyroid microcarcinoma patients. Thyroid Res. 2025, 18, 6. [Google Scholar] [CrossRef]
- Shukla, N.; Osazuwa-Peters, N.; Megwalu, U.C. Association Between Age and Nodal Metastasis in Papillary Thyroid Carcinoma. Otolaryngol. Head Neck Surg. 2021, 165, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zhao, K.; Li, D.; Chen, Z.; Jiang, R.; Wang, X.; He, X. Lymph node metastasis in young and middle-aged papillary thyroid carcinoma patients: A SEER-based cohort study. BMC Cancer 2020, 20, 181. [Google Scholar] [CrossRef]
- Chen, B.D.; Zhang, Z.; Wang, K.K.; Shang, M.Y.; Zhao, S.S.; Ding, W.B.; Du, R.; Yu, Z.; Xu, X.M. A multivariable model of BRAFV600E and ultrasonographic features for predicting the risk of central lymph node metastasis in cN0 papillary thyroid microcarcinoma. Cancer Manag. Res. 2019, 11, 7211–7217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kayhan, Y.; Azizova, L.; Yılmaz, M.; Bakış, M.; Kefeli, M.; Kan, E.K.; Atmaca, A.; Çolak, R. Prognostic factors for aggressiveness in subcentimeter papillary thyroid carcinoma: Impact of tumor size and lymph node metastases. Arch. Endocrinol. Metab. 2024, 68, e230422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Liu, H.; Bian, C.; Yao, X.Y.; Chen, S.J.; Wu, S.P. Preoperative risk factors and recommendations for surgical intervention in cN0 papillary thyroid microcarcinoma. Neoplasma 2021, 68, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.-L.; Davies, L. Thyroid cancer incidence differences between men and women. Curr. Opin. Endocr. Metab. Res. 2023, 31, 100472. [Google Scholar] [CrossRef]
- Du, J.; Yang, Q.; Sun, Y.; Shi, P.; Xu, H.; Chen, X.; Dong, T.; Shi, W.; Wang, Y.; Song, Z.; et al. Risk factors for central lymph node metastasis in patients with papillary thyroid carcinoma: A retrospective study. Front. Endocrinol. 2023, 14, 1288527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, T.; Wang, L.; Zhang, X.; Shi, Y. A clinical and molecular pathology prediction model for central lymph node metastasis in cN0 papillary thyroid microcarcinoma. Front. Endocrinol. 2023, 14, 1075598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y.; Pu, W.; Zhou, H.; Cui, M.; Zhao, W.; Ma, J.; Wang, C.; Sun, Z. Risk factors for central lymph node metastasis in papillary thyroid microcarcinoma—A retrospective study of 1433 cases from a single center. Pol. J. Pathol. 2022, 73, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Chen, Z.; Chen, S.; Xu, Z.; Wen, L.; Mao, Z.; Shen, J.; Liu, J.; Wang, W. Lateral lymph node metastasis in papillary thyroid microcarcinoma: A study of 5241 follow-up patients. Endocrine 2024, 83, 414–421. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Xia, X. Risk Factor Analysis for Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Int. J. Gen. Med. 2021, 14, 9923–9929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz Pardo, J.; Ríos, A.; Rodríguez, J.M.; Paredes, M.; Soriano, V.; Oviedo, M.I.; Hernández, A.M.; Parrilla, P. Risk Factors of Metastatic Lymph Nodes in Papillary Thyroid Microcarcinoma. Cir. Esp. 2020, 98, 219–225, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Ruiz Pardo, J.; Ríos Zambudio, A.; Rodríguez González, J.M.; Paredes Quiles, M.; Soriano Giménez, V.; Oviedo Ramírez, M.I.; Hernández Martínez, A.M.; Parrilla Paricio, P. Papillary thyroid microcarcinoma with minimal extrathyroidal extension. Is its course so indolent that it requires a less aggressive treatment? Rev. Clin. Esp. 2021, 221, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, Y.; Xu, L.; Wen, J.; Chen, G. Exploring risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: Construction of a novel population-based predictive model. BMC Endocr. Disord. 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, S.; Ni, S.; Wang, X.; Liu, S. A nomogram for predicting lateral lymph node metastasis in cN0 unifocal papillary thyroid microcarcinoma. BMC Cancer 2023, 23, 718. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Ji, X.; Zhang, H.; Sun, W. Clinical and molecular features of progressive papillary thyroid microcarcinoma. Int. J. Surg. 2024, 110, 2313–2322. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Ni, S.; Liu, S. A prediction model for identifying high-risk lymph node metastasis in clinical low-risk papillary thyroid microcarcinoma. BMC Endocr. Disord. 2023, 23, 260. [Google Scholar] [CrossRef]
- Amendola, S.; Piticchio, T.; Scappaticcio, L.; Sellasie, S.W.; Volpe, S.; Le Moli, R.; Coppola, L.; Guidobaldi, L.; Pedicini, F.; Carbone, C.; et al. Papillary thyroid carcinoma: ≤ 10 mm does not always mean pN0. A multicentric real-world study. Updates Surg. 2024, 76, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, T.; Qiu, W.; Song, J.; Ying, T.; Fan, Y.; Yang, Z. Bilateral multifocality, a marker for aggressive disease, is not an independent prognostic factor for papillary thyroid microcarcinoma: A propensity score matching analysis. Clin. Endocrinol. 2021, 95, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, R.; Ebina, A.; Toda, K.; Jikuzono, T.; Saitou, M.; Sen, M.; Kazusaka, H.; Matsui, M.; Yamada, K.; Mitani, H.; et al. Multifocality and Progression of Papillary Thyroid Microcarcinoma During Active Surveillance. World J. Surg. 2021, 45, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.-c.; Lin, B.; Zhang, Y.; Zhao, L.-q.; Luo, D.-c. Total tumor diameter is a better indicator of multifocal papillary thyroid microcarcinoma: A propensity score matching analysis. Front. Endocrinol. 2022, 13, 974755. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, S.; Ni, S.; Liu, W.; Liu, S. Hashimoto’s thyroiditis is negatively associated with lymph node metastasis in PTMC. J. Cancer Res. Clin. Oncol. 2023, 149, 15525–15533. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Um, I.C.; Kwon, S.R.; Lee, J.H.; Lim, H.W.; Jeong, Y.U.; Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C.; et al. Predictive factors of lymph node metastasis in papillary thyroid cancer. PLoS ONE 2023, 18, e0294594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, J.H.; Park, J.Y.; Hong, A.R.; Kim, H.K.; Kang, H.C. Predictors of lateral lymph node metastasis and skip metastasis in patients with papillary thyroid microcarcinoma. Front. Endocrinol. 2024, 15, 1392247, Erratum in: Front. Endocrinol. 2024, 15, 1480460. https://doi.org/10.3389/fendo.2024.1480460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer-Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spyroglou, A.; Kostopoulos, G.; Tseleni, S.; Toulis, K.; Bramis, K.; Mastorakos, G.; Konstadoulakis, M.; Vamvakidis, K.; Alexandraki, K.I. Hobnail Papillary Thyroid Carcinoma, A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2785. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.S.; Yun, H.J.; Kim, S.M.; Chang, H.; Lee, Y.S.; Chang, H.-S.; Park, C.S. Aggressive Subtypes of Papillary Thyroid Carcinoma Smaller Than 1 cm. J. Clin. Endocrinol. Metab. 2023, 108, 1370–1375. [Google Scholar] [CrossRef]
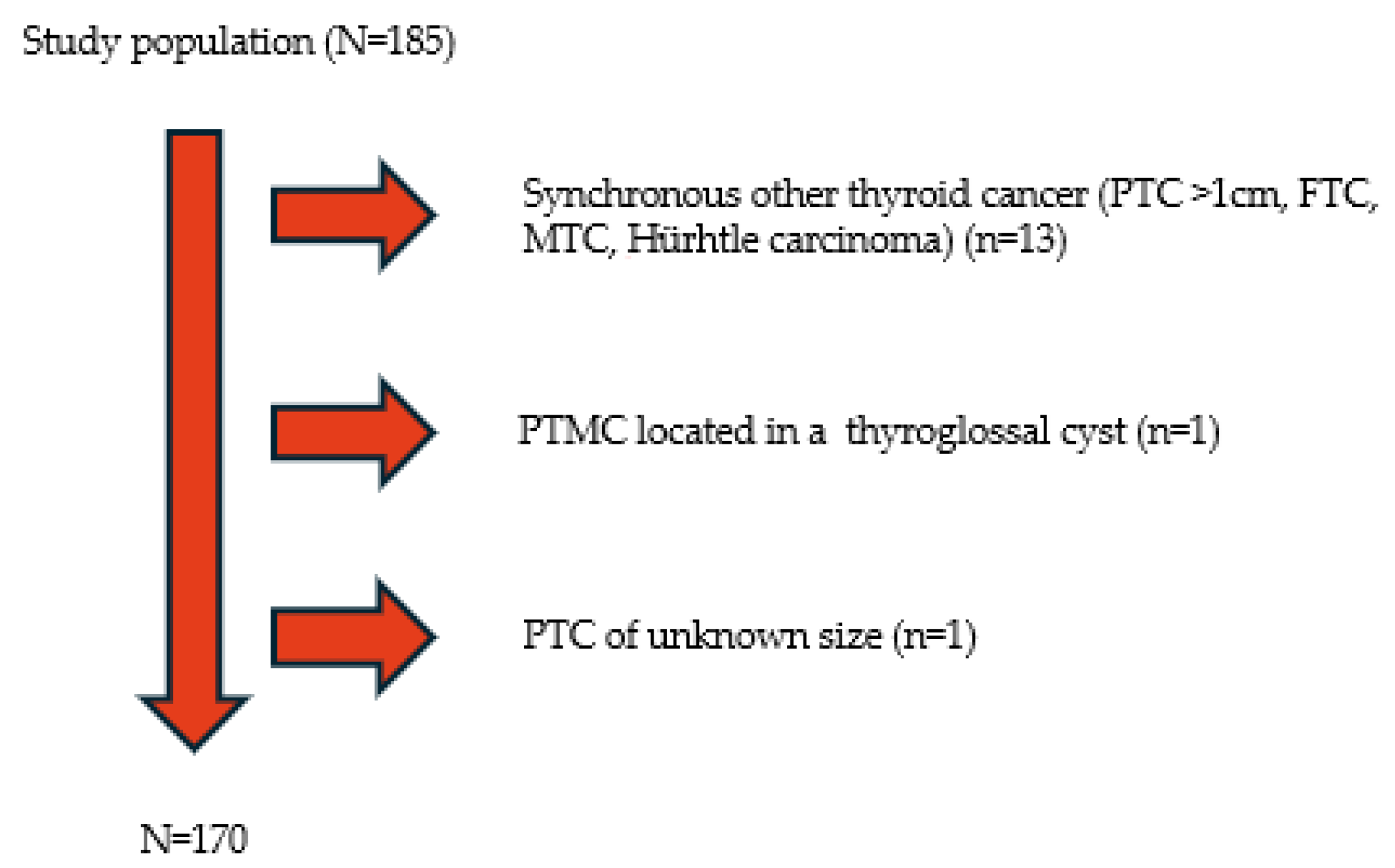
| Variables | Total of Patients (Proportion) |
|---|---|
| Gender | |
| Female | 133 (78.2%) |
| Male | 37 (21.8%) |
| Age, years | |
| Mean ± SD | 47.14 ± 12.81 |
| Max-min | 17–78 |
| Size | |
| Median (IQR/range) | 5 (4.25/0.15–10) |
| BMI (Mean ± SD) | 26.97 ± 4.61 |
| Position | |
| Right lobe | 54 (32.7%) |
| Left lobe | 60 (35.3%) |
| Isthmus | 6 (3.5%) |
| Bilateral | 45 (27.3%) |
| Multifocality | |
| Present | 63(37.1%) |
| Absent | 107 (62.9%) |
| Extrathyroidal extension | |
| Present | 22 (12.9%) |
| Absent | 148(87.1%) |
| Thyroid Capsule Invasion | |
| Present | 39 (22.9%) |
| Absent | 131 (77%) |
| Aggressive histology | 15 (8.8%) |
| Tall cell | 10 (5.9%) |
| Sclerosing | 2 (1.2%) |
| Oncocytic | 3 (1.8%) |
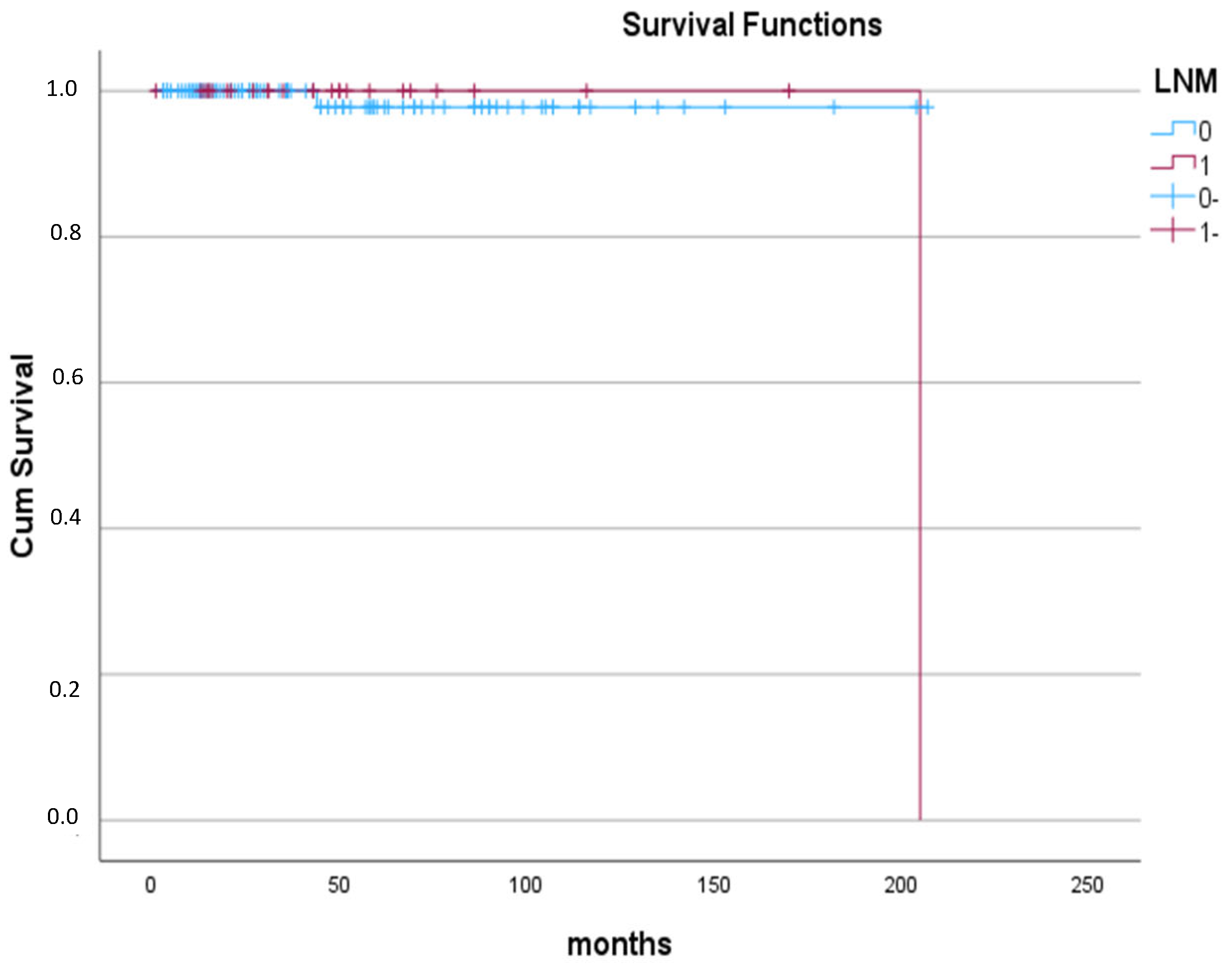
| Lymph node metastasis | |
| Present | 27 (15.9%) |
| Absent | 143 (84.1%) |
| Autoimmune thyroid disease | |
| Present | 44 (41.5%) |
| Absent | 62 (58.5%) |
| Coexisting diseases | |
| Follicular adenoma | 5 (3%) |
| NIFPT | 8 (4.8%) |
| Grave’s disease | 3 (1.8%) |
| Surgical Procedure | |
| Total Thyroidectomy | 168 (98.8%) |
| Other | 2 (1.2%) |
| Lymphadenectomy | |
| Yes | 40 (23.5%) |
| No | 130 (76.5%) |
| RAI | |
| Yes | 72 (58.5%) |
| Variables | Without LNM | With LNM | Significance |
|---|---|---|---|
| Gender | |||
| Female vs. Male | 114 vs. 29 | 19 vs. 8 | 0.280 |
| Age at diagnosis (years) | |||
| <55 vs. ≥55 | 93 vs. 47 | 25 vs. 2 | 0.006 |
| Tumor size (mm) | |||
| ≤5 vs. >5 | 73 vs. 68 | 9 vs. 18 | 0.079 |
| Position | |||
| Unilateral vs. Bilateral | 104 vs. 36 | 18 vs. 9 | 0.414 |
| Multifocality | 52 | 11 | 0.666 |
| TTD of multifocal PTMC | 0.507 | ||
| ≤1 cm | 31 | 5 | |
| >1 cm | 21 | 6 | |
| Autoimmune thyroid disease | 33 | 11 | 0.625 |
| Extrathyroidal extension | 15 | 7 | 0.054 |
| Thyroid capsule invasion | 28 | 11 | 0.016 |
| Aggressive histology | 14 | 1 | 0.470 |
| Tall cell carcinoma | 9 | 1 | 1.000 |
| BMI | 26.77 ± 4.65 | 27.63 ± 4.54 | 0.482 |
| Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio | Significance | 95% CI | Odds Ratio | Significance | 95% CI |
| Age at diagnosis < 55 | 6.317 | 0.015 | 1.435–27.817 | 6.309 | 0.016 | 1.403–28.372 |
| Extrathyroidal extension | 2.987 | 0.034 | 1.084–8.228 | 2.350 | 0.128 | 0.782–7.066 |
| Thyroid capsule invasion | 2.824 | 0.020 | 1.181–6.751 | 2.460 | 0.068 | 0.937–6.459 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violetis, O.; Sfakiotaki, M.; Spyroglou, A.; Pissadaki, E.; Iliakopoulos, K.; Syntzanaki, E.-K.; Konstantakou, P.; Chouliara, E.; Nastos, C.; Dafnios, N.; et al. Predictive Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC). Medicina 2025, 61, 1800. https://doi.org/10.3390/medicina61101800
Violetis O, Sfakiotaki M, Spyroglou A, Pissadaki E, Iliakopoulos K, Syntzanaki E-K, Konstantakou P, Chouliara E, Nastos C, Dafnios N, et al. Predictive Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC). Medicina. 2025; 61(10):1800. https://doi.org/10.3390/medicina61101800
Chicago/Turabian StyleVioletis, Odysseas, Maria Sfakiotaki, Ariadni Spyroglou, Evangelia Pissadaki, Konstantinos Iliakopoulos, Eleni-Konstantina Syntzanaki, Panagiota Konstantakou, Eleni Chouliara, Constantinos Nastos, Nikolaos Dafnios, and et al. 2025. "Predictive Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC)" Medicina 61, no. 10: 1800. https://doi.org/10.3390/medicina61101800
APA StyleVioletis, O., Sfakiotaki, M., Spyroglou, A., Pissadaki, E., Iliakopoulos, K., Syntzanaki, E.-K., Konstantakou, P., Chouliara, E., Nastos, C., Dafnios, N., Simeakis, G., Bramis, K., Myoteri, D., Mastorakos, G., Xekouki, P., & Alexandraki, K. I. (2025). Predictive Factors of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC). Medicina, 61(10), 1800. https://doi.org/10.3390/medicina61101800








