Combined ACL–MCL Injuries: Anatomy, Biomechanics, and Clinical Management
Abstract
1. Introduction
2. Anatomy
2.1. The Anterior Cruciate Ligament
2.2. Biomechanics of the ACL
3. Medial Side of the Knee Complex
3.1. Anatomy of Medial Complex
- Superficial or Layer I, which comprises the crural fascia extending from the fascia of the quadriceps to the tibial periosteum;
- Intermediate or Layer II, which consists of the superficial medial collateral ligament (sMCL) and the medial patellofemoral ligament (MPFL);
- Deep or Layer III, which includes the deep MCL and the posterior oblique ligament (POL) [27].
3.2. Biomechanics of Medial Complex
4. Injury Mechanism and Clinical Assessment
4.1. Injury Mechanism
4.2. Clinical Evaluation of Combined MCL and ACL Injuries
4.3. Instrumental Evaluation
4.4. Magnetic Resonance Imaging (MRI) in the Diagnosis of ACL and MCL Injuries
4.5. Laximeter Imaging Analysis Software
5. Treatment
5.1. MCL
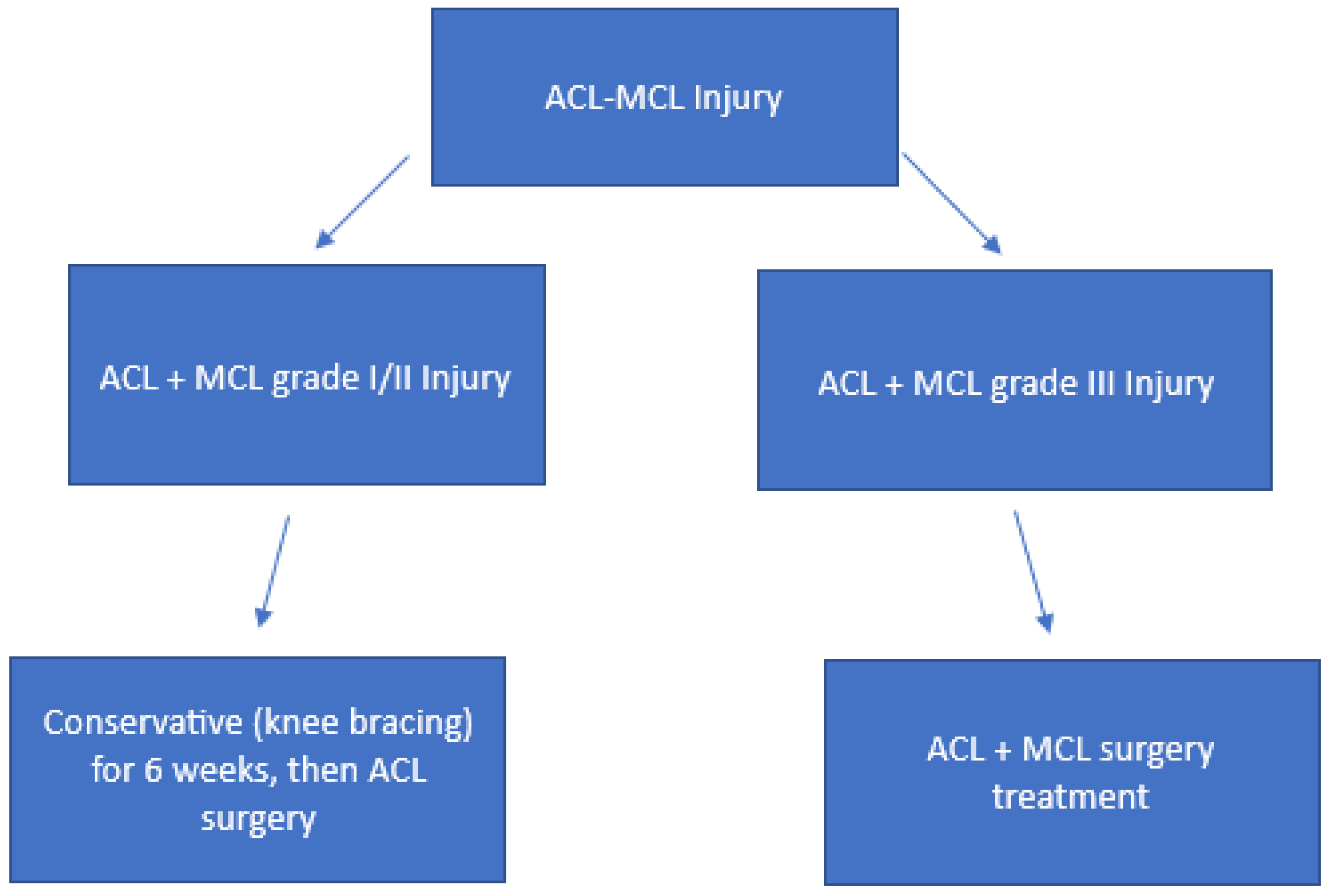
- Grade I injuries are characterized by a medial joint line opening of 3–5 mm, indicating a mild sprain with minimal fiber disruption and no loss of ligamentous integrity (i.e., a stretch injury).
- Grade II injuries exhibit a medial opening of 6–10 mm, representing a moderate injury with a partial tear of the MCL and increased joint laxity.
- Grade III injuries involve a medial opening of ≥10 mm and reflect a severe injury, with a complete tear of the MCL and marked ligamentous instability [52].
- Direct repair: reattaching the MCL using screws and washers or a suture anchor [4].
- Lind (Danish) reconstruction: a semi-anatomic “double-bundle” reconstruction of the sMCL and POL. The patient’s semitendinosus autograft is harvested, preserving the tibial insertion, and is fixed proximally at the sMCL insertion to recreate the sMCL and distally on the tibia to mimic the POL. This method does not aim for exact replication of ligament insertions but reconstructs two structures with a single autograft and uses only one femoral tunnel [8].
- Hughston reconstruction: focuses on capsular and POL “plication” to restore natural soft-tissue tension. It leverages anatomical features of the posteromedial corner, notably the proximal portion of the sMCL, which has significant soft-tissue adhesions to the medial femoral condyle that can help disperse tension. It is valued for simplicity and cost-effectiveness [54].
- New reconstruction option: the latest “triple-stranded” MCL reconstruction uses three different grafts aimed at recreating the sMCL, POL, and dMCL [55].
5.2. ACL
- Bone–Patellar Tendon–Bone (BPTB) Autograft: This is a technique where surgeon uses a strip of the patient’s own patellar tendon (usually 25–30 mm length and 7–10 mm width), along with small bone blocks from the kneecap and shinbone. It provides strong fixation and is often favored for athletes due to its durability [61].
- Hamstring Tendon Autograft: Surgeon uses tendons from the patient’s hamstring muscles (the gracilis and semitendinosus tendons). Less invasive to the kneecap area, which can reduce anterior knee pain [62].
- Quadriceps Tendon Autograft: It consists of harvesting a portion of the quadriceps tendon, sometimes with a small piece of the kneecap. Offers a large graft size and good strength. Usually used in revision surgeries [63].
- Allograft Reconstruction: Where surgeon uses donor tissue from a cadaver. Avoids harvesting from the patient, leading to less initial pain and quicker recovery. However, there is a slightly higher risk of graft failure [64].
5.3. Combined ACL-MCL Injury Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willinger, L.; Balendra, G.; Pai, V.; Lee, J.; Mitchell, A.; Jones, M.; Williams, A. High Incidence of Superficial and Deep Medial Collateral Ligament Injuries in ‘Isolated’ Anterior Cruciate Ligament Ruptures: A Long Overlooked Injury. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 167–175. [Google Scholar] [CrossRef]
- Andrews, K.; Lu, A.; Mckean, L.; Ebraheim, N. Review: Medial Collateral Ligament Injuries. J. Orthop. 2017, 14, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Svantesson, E.; Hamrin Senorski, E.; Alentorn-Geli, E.; Westin, O.; Sundemo, D.; Grassi, A.; Čustović, S.; Samuelsson, K. Increased Risk of ACL Revision with Non-Surgical Treatment of a Concomitant Medial Collateral Ligament Injury: A Study on 19,457 Patients from the Swedish National Knee Ligament Registry. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Jakobsen, B.; Lund, B.; Hansen, M.; Abdallah, O.; Christiansen, S. Anatomical Reconstruction of the Medial Collateral Ligament and Posteromedial Corner of the Knee in Patients with Chronic Medial Instability. Am. J. Sports Med. 2009, 37, 1116–1122. [Google Scholar] [CrossRef]
- Halinen, J.; Lindahl, J.; Hirvensalo, E. Operative and Nonoperative Treatments of Medial Collateral Ligament Rupture with Early Anterior Cruciate Ligament Reconstruction: A Prospective Randomized Study. Am. J. Sports Med. 2006, 34, 1134–1140. [Google Scholar] [CrossRef]
- Willinger, L.; Shinohara, S.; Athwal, K. Medial Collateral Ligament Injuries Increase Forces on the Anterior Cruciate Ligament Graft. Am. J. Sports Med. 2021, 49, 2102–2110. [Google Scholar]
- Jiang, D.; Ao, Y.; Gong, X. Effects of Medial Collateral Ligament Injury on Anterior Cruciate Ligament Graft Force and Knee Kinematics: A Finite Element Study. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 405–411. [Google Scholar]
- LaPrade, R.; Wijdicks, C. Surgical Technique: Development of an Anatomic Medial Knee Reconstruction. Clin. Orthop. 2012, 470, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kothari, M.; Shah, H. Comparative Outcomes of MCL Repair versus Reconstruction in Multiligamentous Knee Injuries: A Systematic Review. Knee Surg. Relat. Res. 2020, 32, 37. [Google Scholar]
- DeLong, J.; Waterman, B. Surgical Techniques for Medial Collateral Ligament Injury: Current Concepts and Review of the Literature. J. Knee Surg. 2015, 28, 387–394. [Google Scholar]
- Amis, A.A.; Dawkins, G.P. Functional Anatomy of the Anterior Cruciate Ligament. Fibre Bundle Actions Related to Ligament Replacements and Injuries. J. Bone Joint Surg. Br. 1991, 73, 260–267. [Google Scholar] [CrossRef]
- Markatos, K.; Kaseta, M.K.; Lallos, S.N.; Korres, D.S.; Efstathopoulos, N. The Anatomy of the ACL and Its Importance in ACL Reconstruction. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Insall, J.N.; Scott, W.N. Insall & Scott Surgery of the Knee; Churchill Livingstone/Elsevier: Amsterdam, The Netherlands, 2006; Volume 2. [Google Scholar]
- Kopf, S.; Pombo, M.W.; Szczodry, M.; Irrgang, J.J.; Fu, F.H. Size Variability of the Human Anterior Cruciate Ligament Insertion Sites. Am. J. Sports Med. 2011, 39, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Cone, S.G.; Howe, D.; Fisher, M.B. Size and Shape of the Human Anterior Cruciate Ligament and the Impact of Sex and Skeletal Growth: A Systematic Review. JBJS Rev. 2019, 7, e8. [Google Scholar] [CrossRef] [PubMed]
- Gali, J.C.; Camargo, D.B.; de Oliveira, F.A.M.; Pereira, R.H.N.; da Silva, P.A.C. Anatomia Descritiva da Inserção Femoral do Ligamento Cruzado Anterior. Rev. Bras. Ortop. 2018, 53, 421–426. [Google Scholar] [CrossRef]
- Boisgard, S.; Levai, J.P.; Geiger, B.; Saidane, K.; Landjerit, B. Study of the Variations in Length of the Anterior Cruciate Ligament during Flexion of the Knee: Use of a 3D Model Reconstructed from MRI Sections. Surg. Radiol. Anat. 1999, 21, 313–317. [Google Scholar] [CrossRef]
- Sakane, M.; Fox, R.J.; Woo, S.L.; Livesay, G.A.; Li, G.; Fu, F.H. In Situ Forces in the Anterior Cruciate Ligament and Its Bundles in Response to Anterior Tibial Loads. J. Orthop. Res. 1997, 15, 285–293. [Google Scholar] [CrossRef]
- Colombet, P.; Robinson, J.; Christel, P.; Franceschi, J.-P.; Djian, P.; Bellier, G.; Sbihi, A. Morphology of Anterior Cruciate Ligament Attachments for Anatomic Reconstruction: A Cadaveric Dissection and Radiographic Study. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 984–992. [Google Scholar] [CrossRef]
- Kondo, E.; Merican, A.M.; Yasuda, K.; Amis, A.A. Biomechanical Analysis of Knee Laxity with Isolated Anteromedial or Posterolateral Bundle-Deficient Anterior Cruciate Ligament. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 335–343. [Google Scholar] [CrossRef]
- Zantop, T.; Herbort, M.; Raschke, M.J.; Fu, F.H.; Petersen, W. The Role of the Anteromedial and Posterolateral Bundles of the Anterior Cruciate Ligament in Anterior Tibial Translation and Internal Rotation. Am. J. Sports Med. 2007, 35, 223–227. [Google Scholar] [CrossRef]
- Fukubayashi, T.; Torzilli, P.A.; Sherman, M.F.; Warren, R.F. An In Vitro Biomechanical Evaluation of Anterior-Posterior Motion of the Knee. Tibial Displacement, Rotation, and Torque. J. Bone Jt. Surg. Am. 1982, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Stephen, J.M.; El-Daou, H.; Williams, A.; Amis, A.A. The Medial Ligaments and the ACL Restrain Anteromedial Laxity of the Knee. Knee Surg. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3700–3708. [Google Scholar] [CrossRef]
- Amis, A.A.; Bull, A.M.J.; Lie, D.T.T. Biomechanics of Rotational Instability and Anatomic Anterior Cruciate Ligament Reconstruction. Oper. Tech. Orthop. 2005, 15, 29–35. [Google Scholar] [CrossRef]
- Kim, Y.H.; Purevsuren, T.; Kim, K.; Oh, K.-J. Contribution of Posterolateral Corner Structures to Knee Joint Translational and Rotational Stabilities: A Computational Study. Proc. Inst. Mech. Eng. Part H 2013, 227, 968–975. [Google Scholar] [CrossRef]
- Wymenga, A.B.; Kats, J.J.; Kooloos, J.; Hillen, B. Surgical Anatomy of the Medial Collateral Ligament and the Posteromedial Capsule of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 229–234. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Engebretsen, A.H.; Ly, T.V.; Johansen, S.; Wentorf, F.A.; Engebretsen, L. The Anatomy of the Medial Part of the Knee. J. Bone Jt. Surg. Am. 2007, 89, 2000–2010. [Google Scholar] [CrossRef]
- Wijdicks, C.A.; Griffith, C.J.; Johansen, S.; Engebretsen, L.; LaPrade, R.F. Injuries to the Medial Collateral Ligament and Associated Medial Structures of the Knee. J. Bone Jt. Surg. Am. 2010, 92, 1266–1280. [Google Scholar] [CrossRef]
- Coobs, B.R.; Wijdicks, C.A.; Armitage, B.M.; Spiridonov, S.I.; Westerhaus, B.D.; Johansen, S.; Engebretsen, L.; Laprade, R.F. An In Vitro Analysis of an Anatomical Medial Knee Reconstruction. Am. J. Sports Med. 2010, 38, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R.; Bull, A.M.J.; Amis, A.A. Structural Properties of the Medial Collateral Ligament Complex of the Human Knee. J. Biomech. 2005, 38, 1067–1074. [Google Scholar] [CrossRef]
- De Maeseneer, M.; Van Roy, F.; Lenchik, L.; Barbaix, E.; De Ridder, F.; Osteaux, M. Three Layers of the Medial Capsular and Supporting Structures of the Knee: MR Imaging-Anatomic Correlation. Radiographics 2000, 20 (Suppl. 1), S83–S89. [Google Scholar] [CrossRef] [PubMed]
- Casp, A.J.; Bryniarski, A.; Brady, A.W.; Fossum, B.W.; Godin, J.A. Reanalysis of the Posterior Oblique Ligament: Quantitative Anatomy, Radiographic Markers, and Biomechanical Properties. Orthop. J. Sports Med. 2023, 11, 23259671231174857. [Google Scholar] [CrossRef]
- Wierer, G.; Milinkovic, D.; Robinson, J.R.; Raschke, M.J.; Weiler, A.; Fink, C.; Herbort, M.; Kittl, C. The Superficial Medial Collateral Ligament Is the Major Restraint to Anteromedial Instability of the Knee. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.; Muhmann, R.J.; Raschke, M.J.; Katthagen, J.C.; Oeckenpöhler, S.; Wermers, J.; Glasbrenner, J.; Robinson, J.R.; Kittl, C. The Anterior Fibers of the Superficial MCL and the ACL Restrain Anteromedial Rotatory Instability. Am. J. Sports Med. 2023, 51, 2928–2935. [Google Scholar] [CrossRef]
- Beel, W.; Doughty, C.; Vivacqua, T.; Getgood, A.; Willing, R. Load Sharing of the Deep and Superficial Medial Collateral Ligaments, the Effect of a Partial Superficial Medial Collateral Injury, and Implications on ACL Load. Am. J. Sports Med. 2024, 52, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Moslemian, A.; Arakgi, M.E.; Roessler, P.P.; Sidhu, R.S.; Degen, R.M.; Willing, R.; Getgood, A.M.J. The Medial Structures of the Knee Have a Significant Contribution to Posteromedial Rotational Laxity Control in the PCL-Deficient Knee. Knee Surg. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 4172–4181. [Google Scholar] [CrossRef]
- Ateschrang, A.; Döbele, S.; Freude, T.; Stöckle, U.; Schröter, S.; Kraus, T.M. Acute MCL and ACL Injuries: First Results of Minimal-Invasive MCL Ligament Bracing with Combined ACL Single-Bundle Reconstruction. Arch. Orthop. Trauma Surg. 2016, 136, 1265–1272. [Google Scholar] [CrossRef]
- Phisitkul, P.; James, S.L.; Wolf, B.R.; Amendola, A. MCL Injuries of the Knee: Current Concepts Review. Iowa Orthop. J. 2006, 26, 77–90. [Google Scholar]
- Themes, U.F.O. Medial Ligamentous Injuries of the Knee: Acute and Chronic. In Musculoskeletal Key; E. James Swenson Jr.: Rochester, NY, USA, 2016; Available online: https://musculoskeletalkey.com/medial-ligamentous-injuries-of-the-knee-acute-and-chronic-2/ (accessed on 30 September 2025).
- Encinas-Ullán, C.A.; Rodríguez-Merchán, E.C. Isolated Medial Collateral Ligament Tears: An Update on Management. EFORT Open Rev. 2018, 3, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Braaten, J.A.; Banovetz, M.T.; Rodriguez, A.N.; Thomas, P.; LaPrade, R.F. From Anatomy to Complex Reconstruction: A Modern Review on the Medial Collateral Ligament of the Knee. Arch. Bone Jt. Surg. 2022, 10, 818–826. [Google Scholar] [CrossRef]
- Slocum, D.B.; Larson, R.L. Rotatory Instability of the Knee. Its Pathogenesis and a Clinical Test to Demonstrate Its Presence. J. Bone Jt. Surg. Am. 1968, 50, 211–225. [Google Scholar] [CrossRef]
- Robinson, J.R.; Bull, A.M.J.; Thomas, R.R.D.; Amis, A.A. The Role of the Medial Collateral Ligament and Posteromedial Capsule in Controlling Knee Laxity. Am. J. Sports Med. 2006, 34, 1815–1823. [Google Scholar] [CrossRef]
- Benjaminse, A.; Gokeler, A.; van der Schans, C.P. Clinical Diagnosis of an Anterior Cruciate Ligament Rupture: A Meta-Analysis. J. Orthop. Sports Phys. Ther. 2006, 36, 267–288. [Google Scholar] [CrossRef]
- Griffith, C.J.; LaPrade, R.F.; Johansen, S.; Armitage, B.; Wijdicks, C.; Engebretsen, L. Medial Knee Injury: Part 1, Static Function of the Individual Components of the Main Medial Knee Structures. Am. J. Sports Med. 2009, 37, 1762–1770. [Google Scholar] [CrossRef]
- Hess, T.; Rupp, S.; Hopf, T.; Gleitz, M.; Liebler, J. Lateral Tibial Avulsion Fractures and Disruptions to the Anterior Cruciate Ligament: A Clinical Study of Their Incidence and Correlation. Clin. Orthop. Relat. Res. (1976–2007) 1994, 303, 193–197. [Google Scholar] [CrossRef]
- Musahl, V.; Karlsson, J. Anterior Cruciate Ligament Tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.; Weaver, J.S.; Kinne, E.; Omar, I. Magnetic Resonance Imaging of the Knee. Pol. J. Radiol. 2020, 85, e509–e531. [Google Scholar] [CrossRef]
- Willinger, L.; Runer, A.; Vieider, R.; Muench, L.N.; Siebenlist, S.; Winkler, P.W. Noninvasive and Reliable Quantification of Anteromedial Rotatory Knee Laxity: A Pilot Study on Healthy Individuals. Am. J. Sports Med. 2024, 52, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Signorelli, C.; Grassi, A.; Yue, H.; Raggi, F.; Urrizola, F.; Bonanzinga, T.; Marcacci, M. Assessment of the Pivot Shift Using Inertial Sensors. Curr. Rev. Musculoskelet. Med. 2016, 9, 160–163. [Google Scholar] [CrossRef]
- Indelicato, P.A. Isolated Medial Collateral Ligament Injuries in the Knee. J. Am. Acad. Orthop. Surg. 1995, 3, 9–14. [Google Scholar] [CrossRef]
- Reider, B.; Sathy, M.R.; Talkington, J.; Blyznak, N.; Kollias, S. Treatment of Isolated Medial Collateral Ligament Injuries in Athletes with Early Functional Rehabilitation: A Five-Year Follow-up Study. Am. J. Sports Med. 1994, 22, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Guenther, D.; Pfeiffer, T.; Petersen, W.; Imhoff, A.; Herbort, M.; Achtnich, A.; Stein, T.; Kittl, C.; Schoepp, C.; Akoto, R.; et al. Treatment of Combined Injuries to the ACL and the MCL Complex: A Consensus Statement of the Ligament Injury Committee of the German Knee Society (DKG). Orthop. J. Sports Med. 2021, 9, 23259671211050929. [Google Scholar] [CrossRef]
- Miyaji, N.; Holthof, S.R.; Bastos, R.P.S.; Ball, S.V.; Espregueira-Mendes, J.; Williams, A.; Amis, A.A. A Triple-Strand Anatomic Medial Collateral Ligament Reconstruction Restores Knee Stability More Completely Than a Double-Strand Reconstruction: A Biomechanical Study In Vitro. Am. J. Sports Med. 2022, 50, 1832–1842. [Google Scholar] [CrossRef]
- Papalia, R.; Osti, L.; Del Buono, A.; Denaro, V.; Maffulli, N. Management of Combined ACL-MCL Tears: A Systematic Review. Br. Med. Bull. 2010, 93, 201–215. [Google Scholar] [CrossRef]
- Trofa, D.P.; Sonnenfeld, J.J.; Song, D.J.; Lynch, T.S. Distal Knee Medial Collateral Ligament Repair With Suture Augmentation. Arthrosc. Tech. 2018, 7, e921–e926. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Bhattacharyya, R.; Andrews, B.; Varma, R.; Chen, A. The Management of Combined ACL and MCL Injuries: A Systematic Review. J. Orthop. 2022, 34, 21–30. [Google Scholar] [CrossRef]
- Frobell, R.B.; Roos, E.M.; Roos, H.P.; Ranstam, J.; Lohmander, L.S. A Randomized Trial of Treatment for Acute Anterior Cruciate Ligament Tears. N. Engl. J. Med. 2010, 363, 331–342. [Google Scholar] [CrossRef]
- Papaleontiou, A.; Poupard, A.M.; Mahajan, U.D.; Tsantanis, P. Conservative vs Surgical Treatment of Anterior Cruciate Ligament Rupture: A Systematic Review. Cureus 2024, 16, e56532. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.M.; Higgins, J.; Bernardoni, E.; Cvetanovich, G.; Bush-Joseph, C.A.; Verma, N.N.; Bach, B.R. Anterior Cruciate Ligament Reconstruction Basics: Bone-Patellar Tendon-Bone Autograft Harvest. Arthrosc. Tech. 2017, 6, e1189–e1194. [Google Scholar] [CrossRef]
- Vinagre, G.; Kennedy, N.I.; Chahla, J.; Cinque, M.E.; Hussain, Z.B.; Olesen, M.L.; LaPrade, R.F. Hamstring Graft Preparation Techniques for Anterior Cruciate Ligament Reconstruction. Arthrosc. Tech. 2017, 6, e2079–e2084. [Google Scholar] [CrossRef] [PubMed]
- Slone, H.S.; Romine, S.E.; Premkumar, A.; Xerogeanes, J.W. Quadriceps Tendon Autograft for Anterior Cruciate Ligament Reconstruction: A Comprehensive Review of Current Literature and Systematic Review of Clinical Results. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 541–554. [Google Scholar] [CrossRef]
- Iosifidis, M.I.; Tsarouhas, A. Allografts in Anterior Cruciate Ligament Reconstruction. In Sports Injuries: Prevention, Diagnosis, Treatment and Rehabilitation; Springer: Berlin Heidelberg, Germany, 2010; pp. 421–430. [Google Scholar] [CrossRef]
- Cox, C.L.; Spindler, K.P. Multiligamentous Knee Injuries—Surgical Treatment Algorithm. N. Am. J. Sports Phys. Ther. NAJSPT 2008, 3, 198–203. [Google Scholar] [PubMed]
- Wright, M.L.; Coladonato, C.; Ciccotti, M.G.; Tjoumakaris, F.P.; Freedman, K.B. Combined Anterior Cruciate Ligament and Medial Collateral Ligament Reconstruction Shows High Rates of Return to Activity and Low Rates of Recurrent Valgus Instability: An Updated Systematic Review. Arthrosc. Sports Med. Rehabil. 2023, 5, e867–e879. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.J.; Newman, A.P.; Burks, R.T. Postoperative Return of Motion in Anterior Cruciate Ligament and Medial Collateral Ligament Injuries. The Effect of Medial Collateral Ligament Rupture Location. Am. J. Sports Med. 1993, 21, 20–25. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Yagishita, K.; Ju, Y.-J.; Sekiya, I. Surgical Management of Grade 3 Medial Knee Injuries Combined with Cruciate Ligament Injuries. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 88–94. [Google Scholar] [CrossRef]
- Ibrahim, S.A. Primary Repair of the Cruciate and Collateral Ligaments after Traumatic Dislocation of the Knee. J. Bone Jt. Surg. Br. 1999, 81, 987–990. [Google Scholar] [CrossRef]






| Name | Technique | Advantages/Features |
|---|---|---|
| Direct repair | Suture of the native tissue in acute injuries ± anchor placement | In proximal/distal lesions with good quality of tissue |
| Hughston technique | Plication | In acute massive lesions, simple and cheap technique |
| Lind technique | Semi-anatomic reconstruction | Only one autograft |
| Laprade | Anatomic reconstruction | high stability and low rate of knee stiffness |
| New technique | “Triple-stranded” MCL reconstruction | showed superior results in terms of valgus stability and control of external rotation [55] |
| Graft | Advantages | Disadvantages |
|---|---|---|
| BPTB | High strength and stability; good biological integration; long-term proven results | Pain and chronic discomfort at the harvest site; risk of patellar fracture; joint stiffness |
| Hamstring Tendon | Less pain at the harvest site; lower risk of fractures; good residual muscle strength | Potential reduction in hamstring strength; less rigid than patellar tendon |
| Quadriceps Tendon | High strength; good integration capacity; adequate size | Pain at the harvest site; risk of injury to the quadriceps muscle; possible loss of muscle strength |
| Allograft | Eliminates pain at the harvest site; shorter surgical time; no muscle strength loss in the patient | Risk of rejection or infection; potentially lower integration capacity; higher costs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiretti, R.; Panzavolta, F.; Lucidi, G.A.; Zaffagnini, S. Combined ACL–MCL Injuries: Anatomy, Biomechanics, and Clinical Management. Medicina 2025, 61, 1788. https://doi.org/10.3390/medicina61101788
Ghiretti R, Panzavolta F, Lucidi GA, Zaffagnini S. Combined ACL–MCL Injuries: Anatomy, Biomechanics, and Clinical Management. Medicina. 2025; 61(10):1788. https://doi.org/10.3390/medicina61101788
Chicago/Turabian StyleGhiretti, Riccardo, Francesco Panzavolta, Gian Andrea Lucidi, and Stefano Zaffagnini. 2025. "Combined ACL–MCL Injuries: Anatomy, Biomechanics, and Clinical Management" Medicina 61, no. 10: 1788. https://doi.org/10.3390/medicina61101788
APA StyleGhiretti, R., Panzavolta, F., Lucidi, G. A., & Zaffagnini, S. (2025). Combined ACL–MCL Injuries: Anatomy, Biomechanics, and Clinical Management. Medicina, 61(10), 1788. https://doi.org/10.3390/medicina61101788





