Morphological Spectrum of the Lateral Pterygoid Muscle: Radioanatomical Analysis, Systematic Review, and Meta-Analytic Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Original Study
2.2. Systematic Review and Meta-Analysis
3. Results
3.1. Original Study
3.2. Systematic Review
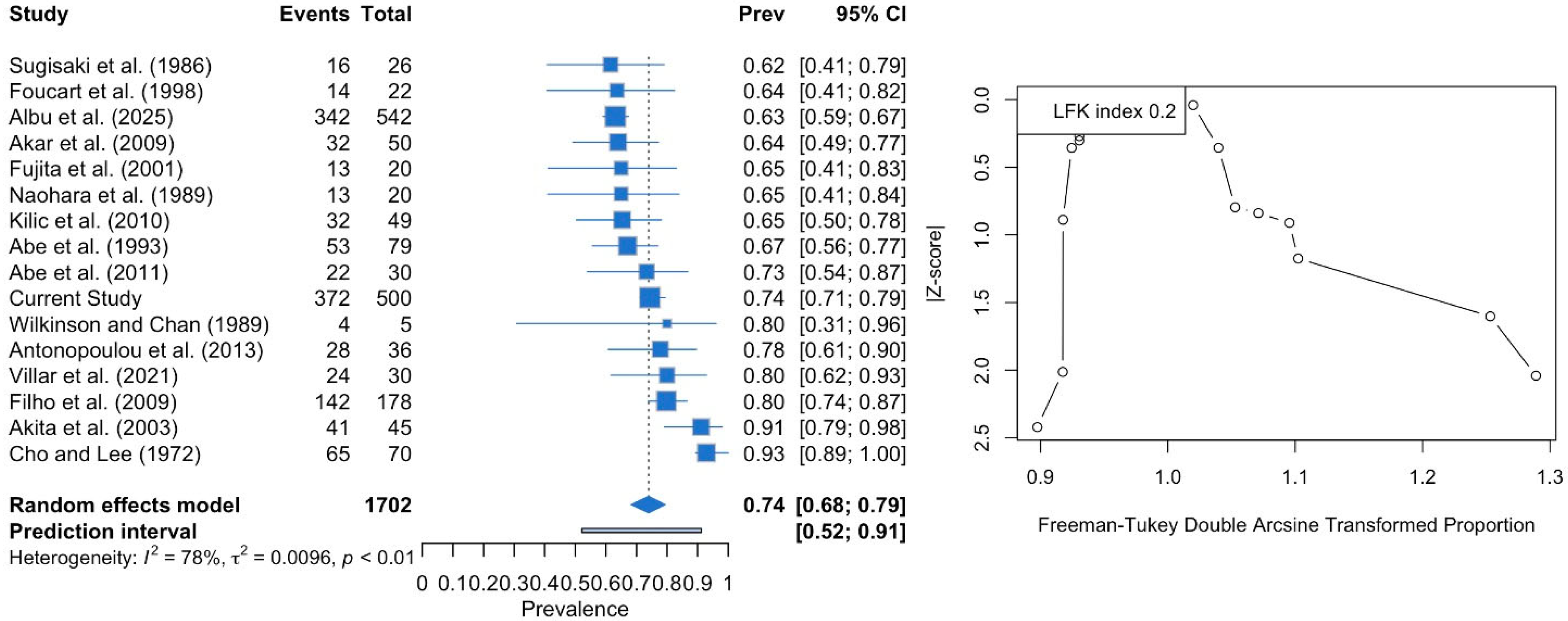
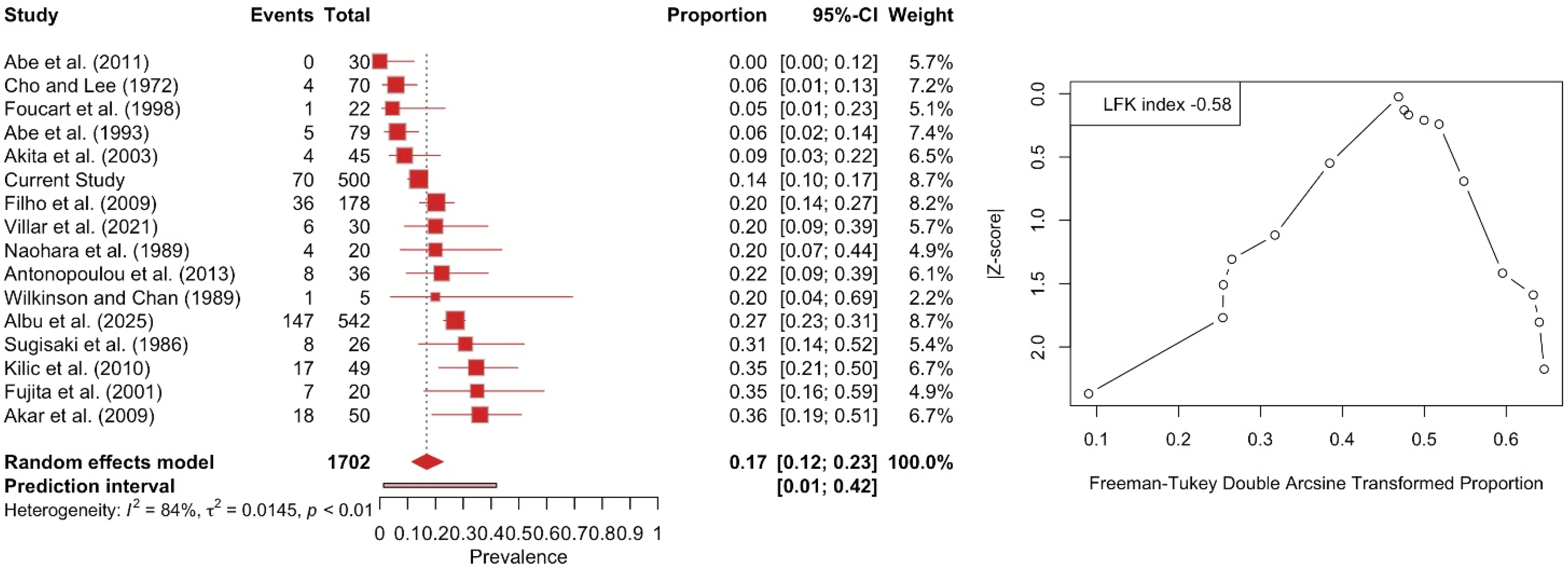
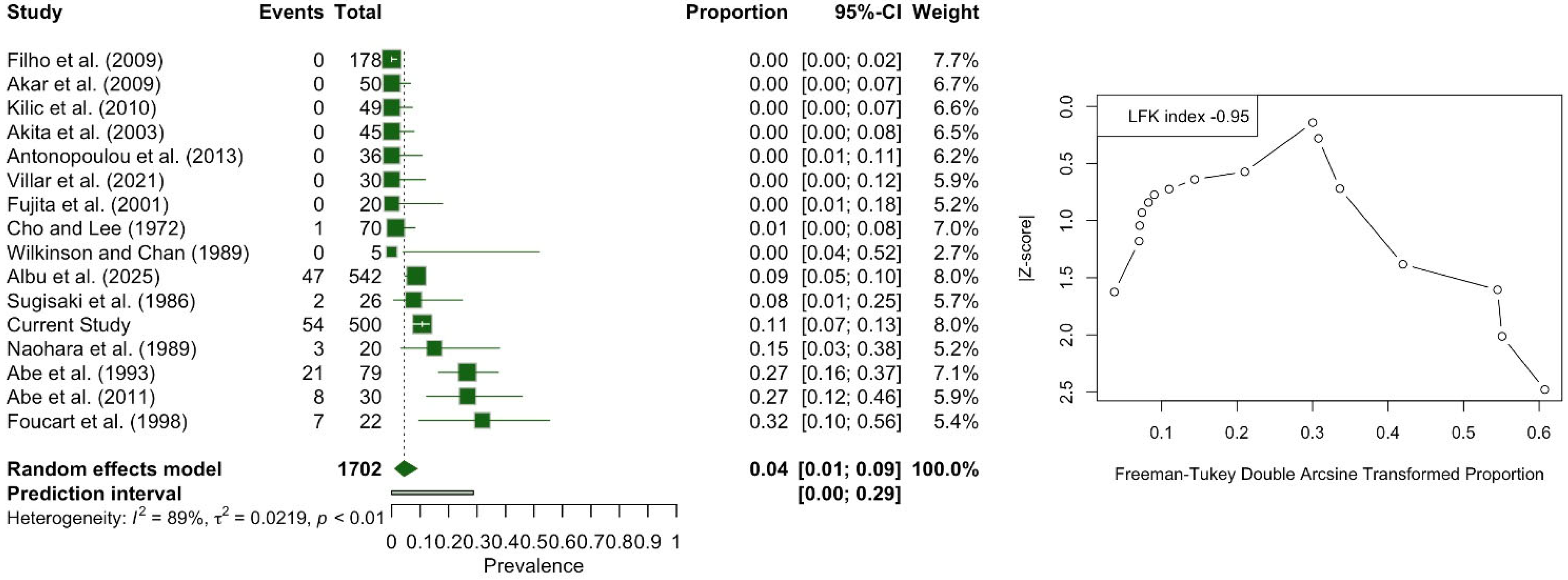
3.3. Meta-Analysis
4. Discussion
4.1. Anatomical Considerations of the Lateral Pterygoid Muscle (LPM) Variability
4.2. Clinical and Surgical Considerations
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piagkou, M.; Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Demetriou, F.; Tsakotos, G.; Olewnik, Ł.; Duparc, F. Mapping the Maxillary Artery and Lateral Pterygoid Muscle Relationship: Insights from Radiological and Meta-Analytic Evidence. Medicina 2025, 61, 1201. [Google Scholar] [CrossRef]
- Akita, K.; Sakaguchi-Kuma, T.; Fukino, K.; Ono, T. Masticatory Muscles and Branches of Mandibular Nerve: Positional Relationships Between Various Muscle Bundles and Their Innervating Branches. Anat. Rec. 2019, 302, 609–619. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Luzzi, S.; Olewnik, Ł.; Tsakotos, G.; Zielinska, N.; Galzio, R.; Tudose, R.C.; Rusu, M.C.; Piagkou, M. Foramen ovale morphology and relationship with the lateral pterygoid process plate: Proposal for a new classification system. Anat. Sci. Int. 2025, 100, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Stöckle, M.; Fanghänel, J.; Knüttel, H.; Alamanos, C.; Behr, M. The morphological variations of the lateral pterygoid muscle: A systematic review. Ann. Anat.-Anat. Anz. 2019, 222, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: London, UK, 2016. [Google Scholar]
- Albu, A.C.; Tudose, R.C.; Vrapciu, A.D.; Rusu, M.C. Beyond two heads: An imaging-based analysis of the lateral pterygoid muscle’s heads. Ann. Anat.-Anat. Anz. 2025, 259, 152387. [Google Scholar] [CrossRef]
- Usui, A.; Akita, K.; Yamaguchi, K. An anatomic study of the divisions of the lateral pterygoid muscle based on the findings of the origins and insertions. Surg. Radiol. Anat. 2008, 30, 327–333. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Iatrou, I.; Paraschos, A.; Anagnostopoulou, S. Variations of the attachment of the superior head of human lateral pterygoid muscle. J. Cranio-Maxillofac. Surg. 2013, 41, e91–e97. [Google Scholar] [CrossRef]
- Melke, G.S.d.F.; Costa, A.L.F.; Lopes, S.L.P.d.C.; Fuziy, A.; Ferreira-Santos, R.I. Three-dimensional lateral pterygoid muscle volume: MRI analyses with insertion patterns correlation. Ann. Anat.-Anat. Anz. 2016, 208, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Teng, H.; Shao, B.; Liu, Z. Biomechanical study of temporomandibular joints of patients with temporomandibular disorders under incisal clenching: A finite element analysis. J. Biomech. 2024, 166, 112065. [Google Scholar] [CrossRef]
- Murray, G.M.; Orfanos, T.; Chan, J.Y.; Wanigaratne, K.; Klineberg, I.J. Electromyographic activity of the human lateral pterygoid muscle during contralateral and protrusive jaw movements. Arch. Oral Biol. 1999, 44, 269–285. [Google Scholar] [CrossRef]
- Henry, B.M.; Tomaszewski, K.A.; Walocha, J.A. Methods of Evidence-Based Anatomy: A guide to conducting systematic reviews and meta-analysis of anatomical studies. Ann. Anat. 2016, 205, 16–21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Tomaszewski, K.A.; Ramakrishnan, P.K.; Roy, J.; Vikse, J.; Loukas, M.; Tubbs, R.S.; Walocha, J.A. Development of the anatomical quality assessment (AQUA) tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin. Anat. 2017, 30, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A.R. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, Y.S. A variety of number of heads of external pterygoid muscle. Taehan Chikkwa Uisa Hyophoe Chi 1972, 10, 305–306. [Google Scholar]
- Sugisaki, M.; Komori, E.; Nakazawa, M.; Tanabe, H.; Kato, S. Anatomical studies of the lateral pterygoid muscle by the superior approach and a review of the literature. Jpn. J. Oral Maxillofac. Surg. 1986, 32, 718–730. [Google Scholar] [CrossRef]
- Naohara, H. The macroscopic and microscopic study of the human lateral pterygoid muscle. Tsurumi Shigaku 1989, 15, 1–26. [Google Scholar]
- Wilkinson, T.; Chan, E.K.K. The anatomic relationship of the insertion of the superior lateral pterygoid muscle to the articular disc in the temporomandibular joint of human cadavers. Aust. Dent. J. 1989, 34, 315–322. [Google Scholar] [CrossRef]
- Abe, S.; Takasaki, I.; Ichikawa, K.; Ide, Y. Investigations of the run and the attachment of the lateral pterygoid muscle in Japanese. Bull. Tokyo Dent. Coll. 1993, 34, 135–139. [Google Scholar]
- Foucart, J.M.; Girin, J.P.; Carpentier, P. Innervation of the human lateral pterygoid muscle. Surg. Radiol. Anat. 1998, 20, 185–189. [Google Scholar] [CrossRef]
- Fujita, S.; Iizuka, T.; Dauber, W. Variation of heads of lateral pterygoid muscle and morphology of articular disc of human temporomandibular joint—Anatomical and histological analysis. J. Oral Rehabil. 2001, 28, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Shimokawa, T.; Sato, T. An anatomic study of the positional relationships between the lateral pterygoid muscle and its surrounding nerves. Eur. J. Anat. 2003, 7, 5–14. [Google Scholar]
- Coskun Akar, G.; Govsa, F.; Ozgur, Z. Examination of the Heads of the Lateral Pterygoid Muscle on the Temporomandibular Joint. J. Craniofacial Surg. 2009, 20, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Filho, H.P.; Guimarães, A.S.; Galdames, I.C.S. Prevalence of the Third Head of the Lateral Pterygoid Muscle: A Magnetic Resonance Image Study. Int. J. Morphol. 2009, 27, 1043–1046. [Google Scholar] [CrossRef]
- Kiliç, C.; Dergïn, G.; Yazar, F.; Kurt, B.; Kutoğlu, T.; Ozan, H.; Balcioğlu, H.A. Insertions of the lateral pterygoid muscle to the disc-capsule complex of the temporomandibular joint and condyle. Turk. J. Med. Sci. 2010, 40, 435–441. [Google Scholar] [CrossRef]
- Abe, S.; Naito, H.; Nakao, T.; Yamamoto, M.; Won, S.-Y.; Kim, H.-J.; Ide, Y. Analysis of the Intramuscular Innervation of the Lateral Pterygoid Muscle. J. Hard Tissue Biol. 2011, 20, 259–264. [Google Scholar] [CrossRef]
- Villar, C.T.; Deana, N.F.; Sousa-Rodrigues, C.F.; Alves, N. Evaluation of the Presence of the Third Head on the Lateral Pterygoid Muscle in Adult Individuals. Int. J. Morphol. 2021, 39, 1270–1273. [Google Scholar] [CrossRef]
- Katori, Y.; Yamamoto, M.; Asakawa, S.; Maki, H.; Rodríguez-Vázquez, J.F.; Murakami, G.; Abe, S. Fetal developmental change in topographical relationship between the human lateral pterygoid muscle and buccal nerve. J. Anat. 2012, 220, 384–395. [Google Scholar] [CrossRef]
- Rusu, M.C.; Toader, C.; Tudose, R.C.; Grigoriţă, L.O. Debate on the Morphological Variability of the Lateral Pterygoid Muscle—Discrepancies, Speculations and New Original Anatomical Samples. Medicina 2024, 60, 1913. [Google Scholar] [CrossRef]
- Mandal, G.; Montalbano, M.; Natsis, K.; Piagkou, M.; Tubbs, R.S.; Loukas, M. Musculus pterygoideus proprius: A meta-analysis. Clin. Anat. 2023, 37, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Stoenescu, M.D.; Săndulescu, M. The Maxillomandibularis Muscle. J. Craniofacial Surg. 2024, 35, e85–e86. [Google Scholar] [CrossRef]
- Monemi, M.; Thornell, L.-E.; Eriksson, P.-O. Diverse changes in fibre type composition of the human lateral pterygoid and digastric muscles during aging. J. Neurol. Sci. 1999, 171, 38–48. [Google Scholar] [CrossRef]
- Davies, J.; Charles, M.; Cantelmi, D.; Liebgott, B.; Ravichandiran, M.; Ravichandiran, K.; Agur, A. Lateral pterygoid muscle: A three-dimensional analysis of neuromuscular partitioning. Clin. Anat. 2012, 25, 576–583. [Google Scholar] [CrossRef]
- McNamara, J.A. The independent functions of the two heads of the lateral pterygoid muscle. Am. J. Anat. 1973, 138, 197–205. [Google Scholar] [CrossRef]
- Piagkou, M.N.; Demesticha, T.; Piagkos, G.; Androutsos, G.; Skandalakis, P. Mandibular nerve entrapment in the infratemporal fossa. Surg. Radiol. Anat. 2011, 33, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gimillo, P.; Valverde-Navarro, A.; Margaix-Muñoz, M.; Poveda-Roda, R.; Delgado-Navarro, C.; Puig-Bernabeu, J. Lateral pterygoid muscle ultrasound-guided injection: A technical note. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101547. [Google Scholar] [CrossRef] [PubMed]
- Martenot, A.; Devoti, J.-F.; Pons, M.; Meyer, C.; Brumpt, E.; Louvrier, A.; Bertin, E. Persistent myogenic temporomandibular disorders: Are navigation-guided botulinum toxin-A injections into the lateral pterygoid muscles effective? J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101715. [Google Scholar] [CrossRef] [PubMed]
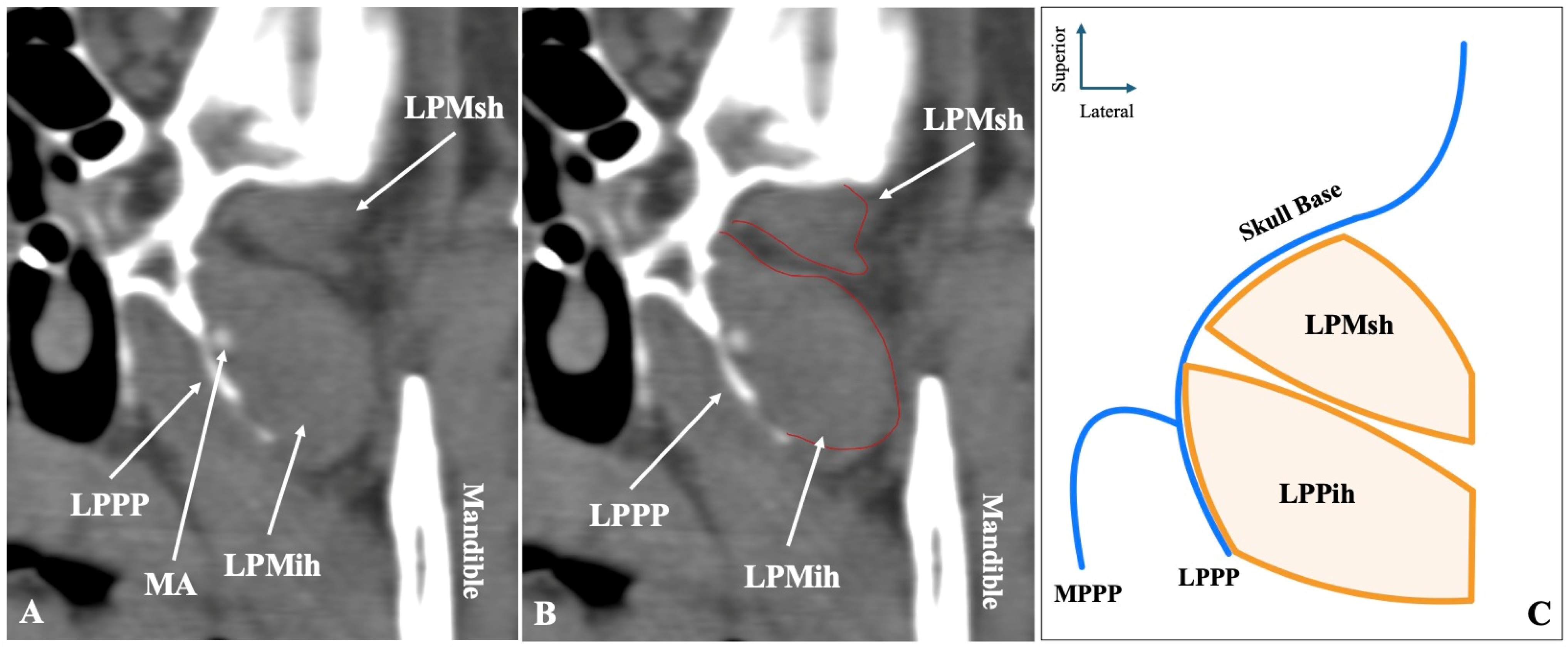
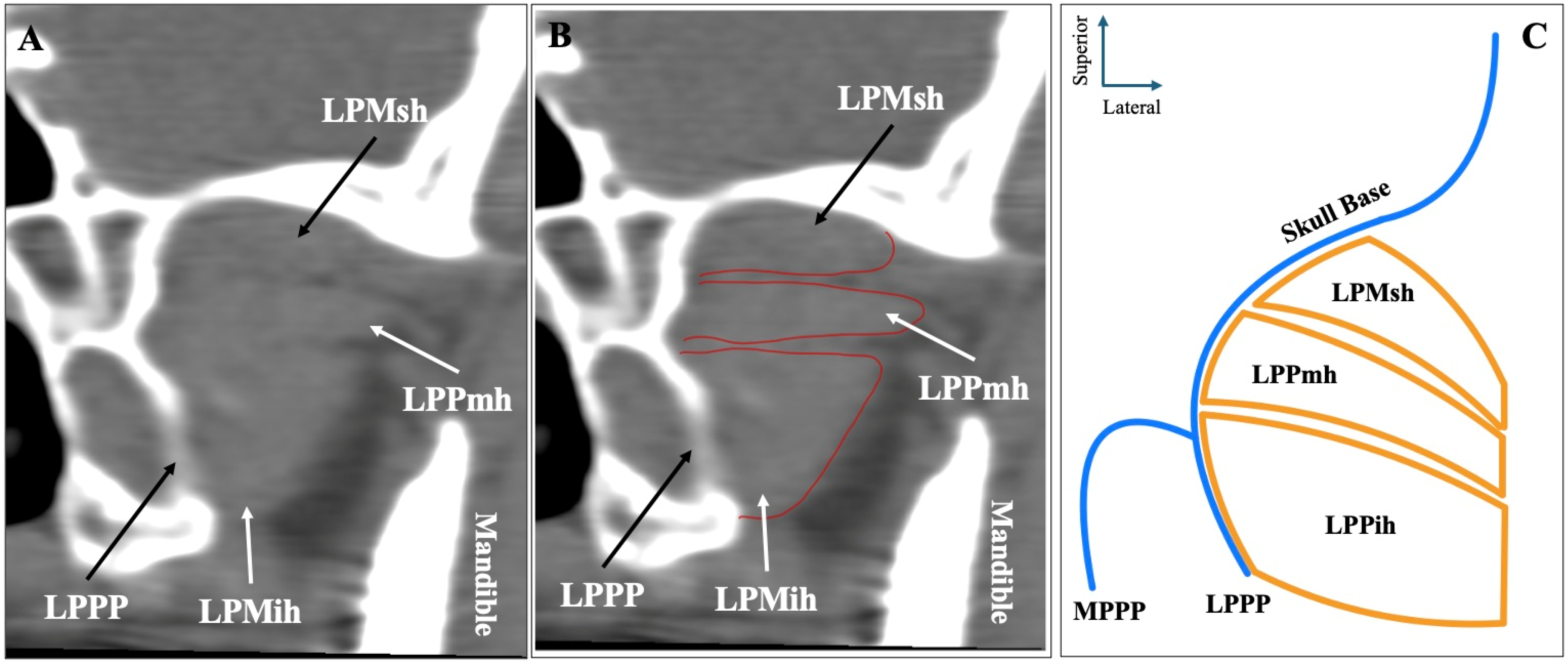





| LPM Morphology | Total (n = 500) | Left (n = 250) | Right (n = 250) | p-Value | Males (n = 272) | Females (n = 228) | p-Value |
| Two-Headed | 372 (74.4%) | 188 (75.2%) | 184 (73.6%) | 0.555 | 192 (70.6%) | 180 (78.9%) | 0.095 |
| Three-Headed | 70 (14%) | 36 (14.4%) | 34 (13.6%) | 48 (17.6%) | 22 (9.7%) | ||
| One-Headed | 54 (10.8%) | 26 (10.4%) | 24 (11.2%) | 30 (11%) | 24 (10.5%) | ||
| Four-Headed | 4 (0.8) | 0 (0%) | 4 (1.6%) | 2 (0.7%) | 2 (1.8%) |
| Study | Year | Population | Type of Study | Sides | Risk of Bias |
| Cho and Lee [16] | 1972 | Asia | Cadaveric | 70 | Low |
| Sugisaki et al. [17] | 1986 | Asia | Cadaveric | 26 | High |
| Naohara et al. [18] | 1989 | Asia | Cadaveric | 20 | High |
| Wilkinson and Chan [19] | 1989 | America | Cadaveric | 5 | High |
| Abe et al. [20] | 1993 | Asia | Cadaveric | 79 | Low |
| Foucart et al. [21] | 1998 | Europe | Cadaveric | 22 | High |
| Fujita et al. [22] | 2001 | Asia | Cadaveric | 20 | Low |
| Akita et al. [23] | 2003 | Asia | Cadaveric | 45 | Low |
| Akar et al. [24] | 2009 | Asia | Cadaveric | 50 | Low |
| Filho et al. [25] | 2009 | America | Imaging (MRI) | 178 | Low |
| Kilic et al. [26] | 2010 | Asia | Cadaveric | 49 | Low |
| Abe et al. [27] | 2011 | Asia | Cadaveric | 30 | High |
| Antonopoulou et al. [8] | 2013 | Europe | Cadaveric | 36 | Low |
| Villar et al. [28] | 2021 | America | Cadaveric | 30 | Low |
| Albu et al. [6] | 2025 | Europe | Imaging (CT, CTA, CBCT) | 542 | Low |
| Current Study | 2025 | Europe | Imaging (CTA) | 500 | - |
| Parameters | Two-Headed | Three-Headed | One-Headed |
| Asia (n = 9) | 73.51 | 16.54 | 5.06 |
| Europe (n = 4) | 69.96 | 17.35 | 8.83 |
| America (n = 3) | 81.64 | 18.36 | 0.00 |
| p-value | 0.1227 | 0.8197 | <0.0001 |
| Cadaveric (n = 13) | 74.25 | 16.00 | 4.41 |
| Imaging (n = 3) | 72.41 | 20.19 | 4.97 |
| p-value | 0.8783 | 0.6297 | 0.8140 |
| Sample over 50 muscles (n = 6) | 74.29 | 16.94 | 5.32 |
| Sample under 50 muscles (n = 10) | 73.54 | 17.07 | 4.01 |
| p-value | 0.7640 | 0.8173 | 0.9412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Arkoudis, N.-A.; Velonakis, G.; Samolis, A.; Vassiou, K.; Fiska, A.; Piagkou, M. Morphological Spectrum of the Lateral Pterygoid Muscle: Radioanatomical Analysis, Systematic Review, and Meta-Analytic Synthesis. Medicina 2025, 61, 1780. https://doi.org/10.3390/medicina61101780
Triantafyllou G, Papadopoulos-Manolarakis P, Arkoudis N-A, Velonakis G, Samolis A, Vassiou K, Fiska A, Piagkou M. Morphological Spectrum of the Lateral Pterygoid Muscle: Radioanatomical Analysis, Systematic Review, and Meta-Analytic Synthesis. Medicina. 2025; 61(10):1780. https://doi.org/10.3390/medicina61101780
Chicago/Turabian StyleTriantafyllou, George, Panagiotis Papadopoulos-Manolarakis, Nikolaos-Achilleas Arkoudis, Georgios Velonakis, Alexandros Samolis, Katerina Vassiou, Aliki Fiska, and Maria Piagkou. 2025. "Morphological Spectrum of the Lateral Pterygoid Muscle: Radioanatomical Analysis, Systematic Review, and Meta-Analytic Synthesis" Medicina 61, no. 10: 1780. https://doi.org/10.3390/medicina61101780
APA StyleTriantafyllou, G., Papadopoulos-Manolarakis, P., Arkoudis, N.-A., Velonakis, G., Samolis, A., Vassiou, K., Fiska, A., & Piagkou, M. (2025). Morphological Spectrum of the Lateral Pterygoid Muscle: Radioanatomical Analysis, Systematic Review, and Meta-Analytic Synthesis. Medicina, 61(10), 1780. https://doi.org/10.3390/medicina61101780










