Abstract
Background and Objectives: The objectives of this review were as follows: to measure changes in renal biomarker levels before, immediately after, and 24 h post-marathon; to identify promising biomarkers for the diagnosis of acute kidney injury; and to describe the temporal patterns of biomarker dynamics in relation to the marathon. Materials and Methods: Studies of marathon runners reporting AKI-related biomarkers were included. Four databases (PubMed, EMBASE, Web of Science, and LILACS) were searched. Data on study design, participant characteristics, and biomarker values (pre-, post-, and 24 h post-race) were extracted, and a random effects meta-analysis was performed. Risk of bias was assessed with the National Heart, Lung, and Blood Institute pre–post tool. Results: The study showed significant increases in most biomarkers immediately after the marathon compared to baseline values. The largest increases were observed in Tissue Inhibitor of Metalloproteinases-2* Insulin-like Growth Factor Binding Protein-7 (TIMP-2*IGFBP), copeptin, urinary Liver-type Fatty Acid Binding Protein (L-FABP), urinary Monocyte Chemoattractant Protein-1 (MCP-1), IGFBP-7, urinary Chitinase 3-like Protein 1 (YKL-40), and TIMP-2, suggesting that these biomarkers are promising candidates for future research. Several patterns of biomarker evolution were observed: some increased without decreasing even at 24 h after the marathon; others increased post-marathon and decreased at 24 h while remaining above baseline; some increased after the marathon and then fell below baseline at 24 h. Conclusions: Marathon running causes significant increases in kidney injury biomarkers, with different patterns of evolution.
1. Introduction
Recently, running has gained popularity [1,2,3]. Long-distance running is one of the forms of running, and can include half marathons, marathons, and ultramarathons. Physical activity is acknowledged as a health-protective factor [2,4]. Nevertheless, long-distance running can induce high stress on different organs and systems [5,6]. Studies have found out that the kidneys can be affected during a marathon. Some runners may experience stage 1 acute kidney injury (AKI) [7,8]. AKI is defined as an unexpected and often temporary decline in kidney function, as indicated by a rise in creatinine or a fall in urine volume [9]. Stage 1 AKI is defined by KDIGO as a rise in serum creatinine by ≥0.3 mg/dL within 48 h, or a rise up to 1.5–1.9 times baseline within the prior 7 days, or a decrease in urine excretion <0.5 mL/kg/h for 6–12 h [10]. These episodes are mainly reversible, but when repeated, or maybe in cases with comorbidities or in the presence of other risk factors, a long-term renal injury can happen [11]. It is yet uncertain if recurrent renal insults that fit the criteria of AKI cause an accelerated development of long-term renal problems in long-distance running [12]. In addition to serum creatinine and urine output, there are many biomarkers for AKI. A classification by Oh et al. [13] identifies three main categories: functional biomarkers: serum creatinine, serum cystatin C (sCys C); tubular enzymes; damage biomarkers: urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL), and serum NGAL (sNGAL), urinary Kidney Injury Molecule-1 (uKIM-1), Interleukin 18, urinary liver-type fatty acid binding protein (uL-FABP); and pre-injury phase biomarkers: urinary Tissue Inhibitor of Metalloproteinases-2 (uTIMP-2) and urinary Insulin-like Growth Factor Binding Protein-7 (uIGFBP-7).
Although many observational studies have shown biomarker modifications after a marathon, no systematic synthesis has assessed the biomarkers’ temporal patterns and diagnosis over time.
The study aimed to investigate changes in urinary, serum, and plasma biomarkers indicative of acute kidney injury in individuals participating in marathons. The objectives were as follows: to measure changes in renal biomarker levels before, immediately after, and 24 h post-marathon; to identify promising biomarkers for the diagnosis of acute kidney injury; and to describe the temporal patterns of biomarker dynamics in relation to the marathon.
2. Materials and Methods
The present manuscript followed the “Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocols (PRISMA)” [14].
2.1. Eligibility Criteria
We included prospective and retrospective observational studies involving (P) individuals who participated in (I) running a marathon, with the primary outcome (O) being the changes in urinary, serum, or plasma biomarkers that may be indicative of acute kidney injury (serum creatinine, urinary creatinine, BUN-to-creatinine ratio, serum urea, TIMP-2, IGFBP-7, TIMP-2*IGFBP, urinary L-FABP, urinary NGAL, plasma NGAL, serum cystatin C, plasma KIM-1, urinary KIM-1, plasma TNF-alpha, urinary TNF-alpha, plasma MCP-1, urinary MCP-1, plasma YKL-40, and urinary YKL-40). Secondary outcomes included serum C-reactive protein, copeptin, and serum creatine kinase. Studies involving other types of marathons (e.g., half-marathon, ultramarathon, or other distances), as well as reviews, meta-analyses, editorials, letters to the editor, and conference abstracts, were excluded.
2.2. Information Sources
To identify studies that met our selection criteria, we searched four databases: PubMed, EMBASE, Web of Science, and LILACS. Additionally, the reference lists of the selected articles and reviews were examined to identify further relevant studies.
2.3. Search Strategy
The search strategy included the following terms: acute kidney injury and marathon, as well as the relevant biomarkers, using MeSH terms, synonyms, singular and plural forms, and abbreviations. The search was conducted from inception until 29 May 2024. No language restrictions were applied in the search strategies or in the selection of articles. The complete search strategy for each database is provided in Supplementary Table S1.
2.4. Selection Process
An initial semi-automated removal of duplicate studies was performed using Zotero version 7.0.11 (Corporation for Digital Scholarship, Vienna, VA, USA) [15]. Subsequently, titles and abstracts were manually screened to exclude articles that did not meet the eligibility criteria, as well as any remaining duplicates. Full-text versions of the remaining articles were then manually reviewed by two authors (D.-C.L., L.-I.T.), and irrelevant studies, non-eligible article types, and duplicates were excluded.
2.5. Data Collection Process
From each selected study, data was manually extracted by several authors (D.-C.L., L.-I.T., O.A., Ș.L.P., and A.I.). The information included study characteristics, country, region, marathon location, study design, age, percentage of female marathon participants (sex), body mass index, the marathon event, previous marathons completed, weekly running distance (km/week), running history (years), AKI criteria, AKI stage 1 reporting, and values of urinary, serum, and plasma biomarkers, and indicators of acute kidney injury.
2.6. Effect Size
For each outcome, the mean and standard deviation were extracted. When this data was unavailable, values were estimated from the median and interquartile range using formulas provided in the Cochrane Handbook [16]. The effect size of interest was represented by the mean values of the biomarkers. Measurements were collected at three time points: before the marathon, immediately after the marathon, and 24 h post-marathon.
2.7. Risk of Bias Assessment
Several authors assessed the methodological quality (D.-C.L., O.A., Ș.L.P., and A.I.) of the selected articles with the quality assessment tool for before and after (pre–post) studies with no control group from the National Heart, Lung, and Blood Institute (NHLBI) [17]. In addition to the questions in the NHLBI assessment tool, we added 4 supplementary original questions to broaden and clarify the risk of bias assessment: Clear exclusion criteria for the following: alcohol or food abstinence 12 h before baseline measurement; exclusion of comorbidities that could alter measurements; exclusion of medication that could alter measurements; and presenting the use of water, electrolytes, or food during the marathon.
2.8. Synthesis Methods
Means and standard deviations were entered into meta-analyses using the meta package in R [18]. Due to clinical heterogeneity among studies, the mean and 95% confidence intervals (CIs) for each variable were calculated using a random-effects model. Results were presented as forest plots. The chi-squared-based Q test and the I2 statistic were used to assess statistical heterogeneity between studies. Statistical significance was defined as a p-value less than 0.05. All analyses were performed using R statistical software, version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria) [19].
2.9. Assessment of Publication Bias
Publication bias was assessed using Egger’s test.
During the preparation of this manuscript, the authors used ChatGPT version 4.0 (OpenAI, San Francisco, CA, USA, accessed in June 2025) as a brainstorming tool to generate suggestions for perspectives to include in the Discussion section. Also, it was used to improve the scientific writing of the manuscript. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
A protocol for the publication can be found at protocols.io with the ID 225510 (registered on 26 August 2025).
3. Results
A total of 119 records were retrieved from the four databases searched, and one additional study was identified through reference screening of the selected articles. The identification and selection process is illustrated in Figure 1. After removing 37 duplicates, 83 records were screened based on title and abstract. Of these, 68 were excluded for not meeting the eligibility criteria or being duplicates. The full texts of the remaining 15 articles were then assessed, resulting in the inclusion of 9 studies in the final review and meta-analysis.
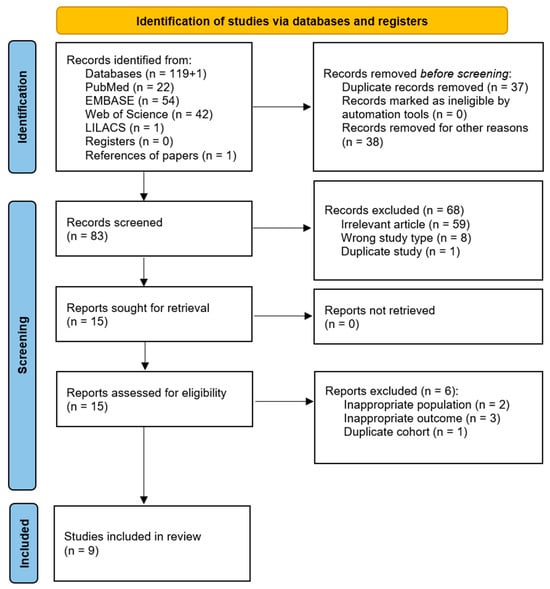
Figure 1.
Flow chart illustrating the identification, selection, and inclusion of articles in the review.
3.1. Study Characteristics
The characteristics of the included studies are detailed in Table 1 and Table 2. Five studies were conducted in Europe, and four in America. All studies employed a prospective cohort design. Acute kidney injury among marathon runners was diagnosed based on the RIFLE, AKIN, or KDIGO criteria. The mean age of participants varied across studies, with several reporting a mean age of around 40 years. However, one study included younger participants with a mean age of 23 years, while another reported a higher mean age of 50 years. Two studies included only male participants. In five studies, the proportions of male and female participants were approximately equal, while in two others, the percentage of female participants was 18% and 29%, respectively. Previous marathon experience, weekly running distance, and running history (in years) also varied across the studies.

Table 1.
Characteristics of the studies included in the review.

Table 2.
Characteristics of the studies included in the review (continued).
3.2. Most Frequently Reported Biomarkers
Serum creatinine was the most frequently reported biomarker. Its mean value before the marathon was 0.88 mg/dL (95% CI: 0.85–0.92), it increased immediately after the marathon to 1.38 mg/dL (95% CI: 1.04–1.71), and decreased at 24 h post-marathon to 1.12 mg/dL (95% CI: 0.64–1.61) (Figure 2). Heterogeneity of the results was substantial, with I2 inconsistency indices of 85%, 98%, and 98% for the three respective time points, all statistically significant.
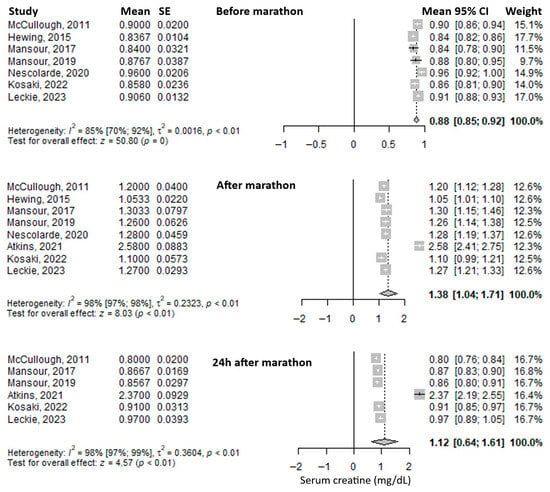
Figure 2.
Forest plots of the mean serum creatinine values before the marathon, immediately after the marathon, and 24 h after the marathon. Before marathon [7,8,20,21,23,24,26], after marathon [7,8,20,21,22,23,24,26], 24 h after marathon [7,8,20,21,22,24].
Another frequently reported biomarker was serum creatine kinase. The mean value before the marathon was 138.31 U/L (95% CI: 105.12–171.5), which increased threefold immediately after the marathon to 425.8 U/L (95% CI: 290.88–560.71), and rose even further at 24 h post-marathon—approximately an eightfold increase compared to baseline—to 1128.14 U/L (95% CI: 434.02–1822.26) (Figure 3). Heterogeneity was substantial, with I2 inconsistency values of 75%, 89%, and 84% for the three respective time points, all statistically significant.
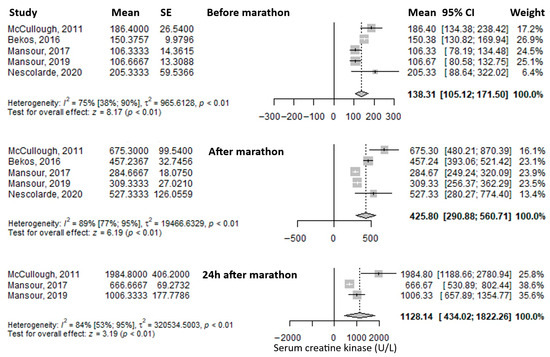
Figure 3.
Forest plots of the mean serum creatin kinase values before the marathon, immediately after the marathon, and 24 h after the marathon. Before marathon [7,8,23,24,25], after marathon [7,8,23,24,25], 24 h after marathon [7,8,24].
The most frequently reported urinary biomarker was urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL). The mean value before the marathon was 8.92 ng/mL (95% CI: 6.11–11.73), which increased substantially—approximately fourfold—immediately after the marathon to 41.30 ng/mL (95% CI: 31.74–50.87), and decreased to 29.57 ng/mL (95% CI: –0.81 to 59.94) at 24 h post-marathon, remaining approximately three times higher than baseline (Figure 4). Heterogeneity was moderate before and immediately after the marathon (I2 = 47% and 33%, respectively), and considerable at 24 h post-marathon (I2 = 99%), with only the latter being statistically significant.
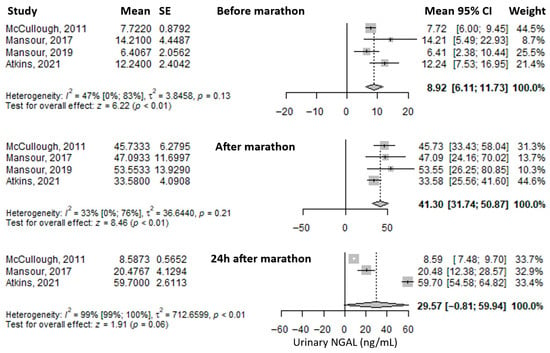
Figure 4.
Forest plots of the mean urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) values before the marathon, immediately after the marathon, and 24 h after the marathon. Before marathon [7,8,22,24,25], after marathon [7,8,22,24,25], 24 h after marathon [7,22,24].
Another reported urinary biomarker was urinary Kidney Injury Molecule-1 (uKIM-1). The mean value before the marathon was 755.02 pg/mL (95% CI: –474.24 to 1984.29), which increased significantly—approximately threefold—immediately after the marathon to 2255.64 pg/mL (95% CI: 827.69 to 3683.6), and then decreased at 24 h post-marathon to 1630.76 pg/mL (95% CI: –367.39 to 3628.9), remaining about twice as high as baseline (Figure 5). Heterogeneity of the results was considerable at all three time points, with I2 values of 97%, 93%, and 96%, respectively, all statistically significant.
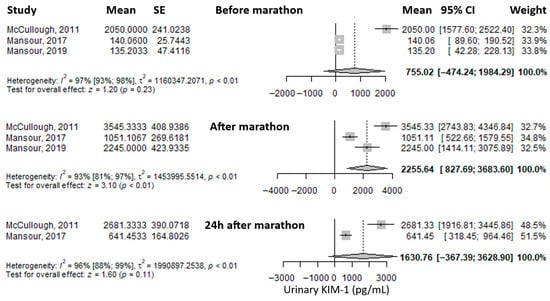
Figure 5.
Forest plots of the mean urinary Kidney Injury Molecule-1 (KIM-1) values before the marathon, immediately after the marathon, and 24 h post-marathon. Before marathon [7,8,24], after marathon [7,8,24], 24 h after marathon [7,24].
Another reported biomarker was serum cystatin C. The mean value before the marathon was 0.39 mg/L (95% CI: –0.21 to 0.99), followed by a slight decrease immediately after the marathon to 0.36 mg/L (95% CI: –0.11 to 0.83), and a further, more pronounced decrease at 24 h post-marathon to 0.10 mg/L (95% CI: 0.06–0.15), representing roughly one-quarter of the baseline level (Figure 6). Heterogeneity was considerable at all three time points, with I2 values of 100%, 100%, and 99%, respectively—all statistically significant.
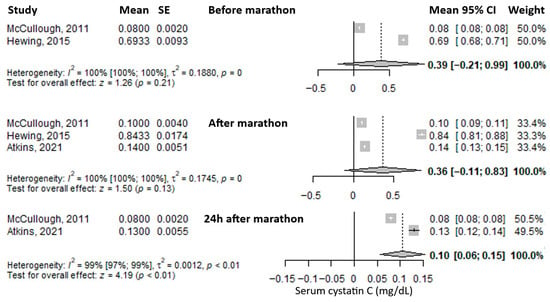
Figure 6.
Forest plots of the mean serum cystatin C values before the marathon, immediately after the marathon, and 24 h post-marathon. Before marathon [24,26], after marathon [22,24,26], 24 h after marathon [22,24].
Another reported biomarker was C-reactive protein (CRP). The mean value before the marathon was 0.93 mg/L (95% CI: 0.03–1.82), which decreased immediately after the marathon to 0.59 mg/L (95% CI: 0.08–1.10) (Figure 7). Heterogeneity of the results was considerable for both measurements, with I2 values of 96% and 95%, respectively, both statistically significant.
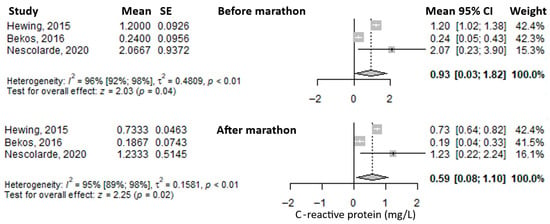
Figure 7.
Forest plots of the mean serum C-reactive protein values before the marathon and immediately after the marathon. Before marathon [23,25,26], after marathon [23,25,26].
3.3. Other Biomarkers
The mean urinary creatinine level before the marathon was 134.28 mg/dL (95% CI: 4.9–263.66) (Supplementary Table S2). It nearly doubled immediately after the marathon, reaching 283.09 mg/dL (95% CI: 127.91–438.27) (Supplementary Table S3), and then decreased at 24 h post-marathon to 103.74 mg/dL (95% CI: 79.5–127.97), falling below baseline levels (Supplementary Table S4).
The mean blood urea nitrogen-to-creatinine (BUN/Cr) ratio was 17.33 (95% CI: 15.68–18.98) before the marathon (Supplementary Table S2), decreased slightly to 15.33 (95% CI: 13.72–16.95) immediately after the marathon (Supplementary Table S3), and rose modestly above baseline to 19.33 (95% CI: 16.75–21.92) at 24 h post-marathon (Supplementary Table S4).
The mean serum urea level before the marathon was 35.2 mg/dL (95% CI: 31.2–39.2) (Supplementary Table S2), and it increased immediately after the marathon to 43.2 mg/dL (95% CI: 38.97–47.43) (Supplementary Table S3).
The mean value of TIMP-2 before the marathon was 2.4 ng/mL (95% CI: 2.15–2.65) (Supplementary Table S2). It rose markedly—by approximately fivefold—immediately after the marathon to 12.4 ng/mL (95% CI: 10.39–14.41) (Supplementary Table S3), and then decreased at 24 h post-marathon to 3.2 ng/mL (95% CI: 2.26–4.14), approaching baseline levels (Supplementary Table S4).
The mean value of IGFBP-7 before the marathon was 32.3 ng/mL (95% CI: 27.06–37.54) (Supplementary Table S2). It increased substantially—approximately sevenfold—immediately after the marathon to 236.6 ng/mL (95% CI: 188.33–284.87) (Supplementary Table S3), and decreased at 24 h post-marathon to 36.5 ng/mL (95% CI: 21.27–51.73), returning close to baseline levels (Supplementary Table S4).
The mean value of the TIMP-2*IGFBP-7 product before the marathon was 0.1 (95% CI: 0.08–0.12) (Supplementary Table S2). It increased dramatically—by 47 times—immediately after the marathon to 4.74 (95% CI: 2.92–6.56) (Supplementary Table S3), then decreased to 0.18 (95% CI: 0.07–0.29) at 24 h post-marathon, remaining nearly double the baseline value (Supplementary Table S4).
The mean urinary L-FABP (liver-type fatty acid binding protein) value before the marathon was 0.23 ng/mL (95% CI: 0.11–0.34) (Supplementary Table S2). It increased significantly—approximately twelve-fold—immediately after the marathon to 2.76 ng/mL (95% CI: 2.06–3.46) (Supplementary Table S3), then decreased at 24 h post-marathon to 0.63 ng/mL (95% CI: 0.38–0.88), roughly three times the baseline level (Supplementary Table S4).
The mean plasma NGAL value before the marathon was 43 ng/mL (95% CI: –25.75 to 111.76) (Supplementary Table S2). It doubled immediately after the marathon, reaching 100.68 ng/mL (95% CI: –5.57 to 206.94) (Supplementary Table S3), and then dropped substantially at 24 h post-marathon to 10.7 ng/mL (95% CI: 7.8–13.6), approximately one-quarter of the initial value (Supplementary Table S4).
The mean plasma KIM-1 value before the marathon was 1123.44 pg/mL (95% CI: –944.39 to 3191.26) (Supplementary Table S2). It increased immediately after the marathon to 1682.08 pg/mL (95% CI: –1453.4 to 4817.57) (Supplementary Table S3), and rose further at 24 h post-marathon to 2700 pg/mL (95% CI: 2072.81–3327.19), approximately double the baseline value (Supplementary Table S4).
The mean copeptin level before the marathon was 3.08 pmol/L (95% CI: 2.84–3.33) (Supplementary Table S2). It increased dramatically—about 15-fold—immediately after the marathon to 47.53 pmol/L (95% CI: 24.86–70.2) (Supplementary Table S3), and then decreased at 24 h post-marathon to 4.44 pmol/L (95% CI: 3.05–5.83), approaching the initial level (Supplementary Table S4).
The mean plasma TNF-alpha level before the marathon was 1.76 pg/mL (95% CI: 1.58–1.94) (Supplementary Table S2), and it increased after the marathon to 2.7 pg/mL (95% CI: 2.43–2.97) (Supplementary Table S3).
The mean urinary TNF-alpha value before the marathon was 0.02 pg/mL (95% CI: 0.01–0.03) (Supplementary Table S2). It increased fivefold immediately after the marathon to 0.1 pg/mL (95% CI: 0.05–0.15) (Supplementary Table S3), then decreased at 24 h post-marathon to 0.02 pg/mL (95% CI: 0.01–0.03), returning to baseline levels (Supplementary Table S4).
The mean plasma MCP-1 level before the marathon was 153.41 pg/mL (95% CI: 142.44–164.38) (Supplementary Table S2), and it doubled immediately after the marathon to 392.31 pg/mL (95% CI: 335.95–448.66) (Supplementary Table S3).
The mean urinary MCP-1 level before the marathon was 53.03 pg/mL (95% CI: 38.05–68.00) (Supplementary Table S2). It increased substantially—approximately tenfold—immediately after the marathon to 574.3 pg/mL (95% CI: 79.02–1069.59) (Supplementary Table S3), and then decreased at 24 h post-marathon to 202.98 pg/mL (95% CI: 100.04–305.91), remaining about four times higher than the baseline value (Supplementary Table S4).
The mean plasma YKL-40 level before the marathon was 29.33 ng/mL (95% CI: 23.04–35.63) (Supplementary Table S2), and it increased immediately after the marathon to 40.04 ng/mL (95% CI: 34.19–45.9) (Supplementary Table S3).
The mean urinary YKL-40 level before the marathon was 282.66 pg/mL (95% CI: –114.39 to 679.71) (Supplementary Table S2). It increased markedly—approximately fivefold—immediately after the marathon to 1530.34 pg/mL (95% CI: 361.32–2699.36) (Supplementary Table S3), and then decreased at 24 h post-marathon to 219.23 pg/mL (95% CI: 105.68–332.78), slightly below the initial value (Supplementary Table S4).
3.4. Identification of Biomarker Trends
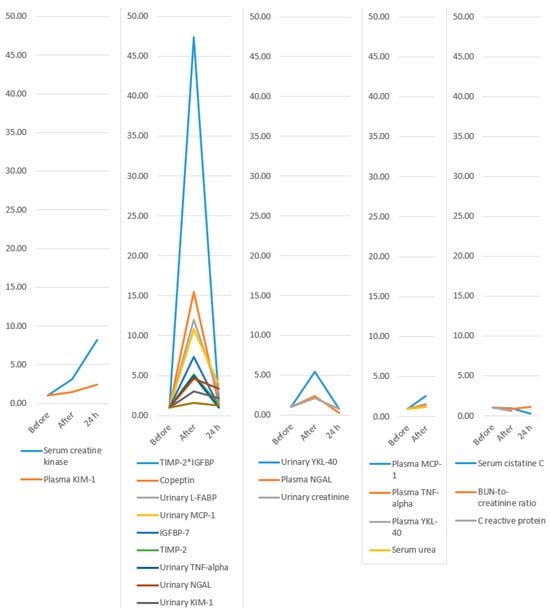
Figure 8.
Evolution of biomarkers: only increase; increase and decrease but remain above baseline; increase and decrease below baseline; increase with no 24 h values available; and decrease below baseline after the marathon. KIM-1, Kidney Injury Molecule-1; TIMP-2, Tissue Inhibitor of Metalloproteinases-2; IGFBP-7, Insulin-like Growth Factor Binding Protein-7; L-FABP, Liver-type fatty acid binding protein; MCP-1, Monocyte chemoattractant protein-1; TNF-alpha, Tumor necrosis factor alpha; NGAL, Neutrophil Gelatinase-Associated Lipocalin; YKL-40, Chitinase 3-like protein 1; BUN, Blood Urea Nitrogen; *, represents the multiplication sign.

Table 3.
Evolution of biomarkers expressed as a multiple of the pre-marathon value.
3.5. Risk of Bias Assessment
We assessed the methodological quality of the selected articles (Table 4) with the quality assessment tool for before and after (pre–post) studies with no control group from the National Heart, Lung, and Blood Institute [21]. All the studies presented a clear question or study objective, and had eligible participants for the intervention (marathon) who were all enrolled in the study. All the studies had the marathon as an intervention, and only participants who finished it were included. The outcome measures were prespecified, clearly defined, valid, reliable, and assessed consistently across all study participants in all studies since there were biomarker measurements from blood, serum, plasma, or urine samples. The blinding of outcome measurements was not reported in any study, but being a laboratory measurement, there is no risk of bias. Only one study reported the sample size calculation, and that it was sufficient to provide confidence in the results, but it was performed after the data collection. Nevertheless, statistically significant results indicate that the sample size was enough for the purpose. The loss to follow-up after baseline was 20% or less in six studies, in two studies it was above 20%, and one study did not report this information. All but one study (88.9%) presented correct statistical methods for pair-wise comparisons between repeated measurements. Nevertheless, our review could not include the comparison for repeated measurements since the data for this was not reported (e.g., stating only that the p-value was less than a limit, without reporting the actual p-value, or confidence interval, or standard deviation of the change), thus the choice of test did not impact our results. All the studies used multiple measurements: at baseline, immediately after the marathon, and 24 h, or later, after the marathon. The intervention was conducted at a group level, but the statistical analysis considered the use of individual-level data, which is appropriate. Seven (77.8%) clearly presented the selection criteria. Four supplementary questions were assessed in addition to those in the NHLBI assessment tool. Only one study used clear exclusion criteria for eating food or alcohol consumption before the baseline measurement. Seven (77.8%) studies clearly excluded comorbidities that could have altered the measurements. Four studies (44.4%) clearly indicated the exclusion of participants who took medication (e.g., anti-inflammatory drugs) that could have altered the measurements. Only two studies (22.2%) presented the use of water, electrolytes, and food during the marathon.

Table 4.
Risk of bias assessment using the quality assessment tool for before and after (pre–post) studies with no control group from the National Heart, Lung, and Blood Institute (NHLBI).
4. Discussion
Our systematic review and meta-analysis succeeded in achieving its objectives. Most biomarkers showed an increase in value immediately after the marathon compared to pre-marathon levels. The largest increases (as a multiple of the baseline value) were observed for TIMP-2*IGFBP (47-fold), copeptin (15-fold), urinary L-FABP (12-fold), urinary MCP-1 (10-fold), IGFBP-7 (7-fold), urinary YKL-40 (5-fold), and TIMP-2 (5-fold). These may represent the most promising biomarker candidates for future research and potential clinical application. Several patterns of biomarker evolution were observed: some increased without decreasing even at 24 h after the marathon (serum creatine kinase and plasma KIM-1); others increased post-marathon and decreased at 24 h while remaining above baseline (TIMP-2IGFBP, copeptin, urinary L-FABP, urinary MCP-1, IGFBP-7, TIMP-2, urinary TNF-alpha, urinary NGAL, urinary KIM-1, and serum creatinine); some increased after the marathon and then fell below baseline at 24 h (urinary YKL-40, plasma NGAL, and urinary creatinine); a few increased post-marathon with no available 24 h data (plasma MCP-1, plasma TNF-alpha, plasma YKL-40, and serum urea); and others decreased immediately post-marathon below baseline (serum cystatin C, which decreased further at 24 h; BUN-to-creatinine ratio, which increased above baseline at 24 h; and C-reactive protein, for which no 24 h values were available).
The data provided by the studies did not allow for statistical testing to determine whether there were significant differences before and after the marathon. Most studies reported statistical test results, which were statistically significant for most of the evaluated biomarkers. Even though change data were not available for meta-analysis, the magnitude of the observed changes from baseline suggests that a meta-analysis including such data would have likely yielded statistically significant results.
This is the first systematic review with meta-analysis focusing on AKI biomarkers. Thus, it adds a quantitative perspective on the topic, coupled with a bird’s-eye view comparison of the most important increases in biomarkers in AKI. We standardized the evolution of biomarkers to be able to make comparisons between them possible. We found that some of them had very important increases immediately after the marathon. Increases over tenfold were observed for TIMP-2*IGFBP, copeptin, uL-FABP, and uMCP-1. Increases over 5-fold were observed for IGFBP-7, uTNF-alpha, and uYKL-40. Increases over 2.5-fold were observed for sCrK, uNGAL, uKIM-1, and pMCP-1. The others had lower increases: pKIM-1, sCr, pNGAL, uCr, pTNF-alpha, pYKL-40, and serum urea. Lastly, there were some biomarkers that diminished after the marathon: sCys C, BUN/Creatinine ratio, and C-reactive protein.
During the marathon, the intense exercise has several effects. One is the reduction in blood flow in the kidney [27] that can induce ischemia, which comes with a plethora of modified biomarkers. Dehydration is another factor that can induce ischemia. Another effect is the increase in the core temperature during running [28]. A quasi-randomized controlled trial showed how increased core temperature and dehydration increase the risk of AKI [29]. ATP depletion is a hypothesized mechanism of AKI [30].
TIMP-2*IGFBP was the most sensitive biomarker of the ones observed. Tissue inhibitor of metal proteinase 2 is a member of a family of proteins, named the TIMP family [31]. These proteins include matrix metalloproteinases, which are peptidases. TIMP-2 is an important modulator of extracellular matrix turnover and contributes to several physiological and pathological processes, including kidney damage and healing [32]. Although IGFBP-7 is part of the insulin-like growth factor (IGF) binding protein family, it has roles in addition to not being related to IGF. It causes the inhibition of cell growth and cell apoptosis by activating a specific pathway (ERK1/2) [33]. The combination of the two biomarkers known as TIMP-2*IGFBP, and its assessment as a diagnostic utility was first presented by Kashani et al. 2013 [34].
Copeptin had the second most important change, and only one study assessed its evolution [8]. Copeptin is a peptide used as a marker of vasopressin secretion. It is used for diagnosis and prognosis of different diseases, including renal disease [35]
L-FABP had the third most important change immediately after the marathon. It is produced in renal proximal tubular cells and released into the urine in reaction to tubular hypoxia [36]. In addition to its renal secretion, L-FABP can be produced by the liver, too. Studies have shown that the urinary L-FABP can be used as a marker of kidney injury, specifically since it is independent of the serum values due to its liver production [37]
MCP-1 had the fourth most important change immediately after the marathon. Monocyte chemoattractant protein-1 belongs to the CC chemokine family. It plays an important role in the inflammatory process by drawing in or promoting the expression of other inflammatory cells and molecules [38]. MCP-1 has been shown to be associated with a rise in renal monocyte infiltration [39]. After renal ischemia–reperfusion damage, MCP-1 prevents the kidney from the immediate inflammatory response [40].
TNF alpha belongs to the TNF family that includes TNF alpha, beta, CD40 ligand, and others [41]. All these are cytokines with roles in inflammation, apoptosis, and tumor lysis. Macrophages are the main source of TNF alpha, though other cells can produce it too. TNF alpha is implicated in numerous functions: inducing fever, apoptosis, necrosis, tumor genesis, progression, and systemic inflammatory reactions [41]. Higher levels of urinary TNF alpha were observed in participants with acute interstitial nephritis [42]. Also, polymorphisms of TNF alpha were seen as predictive of acute kidney injury [43].
YKL-40 is a glycoprotein from the chitinase protein family [44], although it lacks chitinase activity [45]. It is secreted by many cell types, including macrophages, neutrophils, vascular smooth muscle cells, and chondrocytes [45]. It is implicated in many inflammatory diseases, including infections, cardiovascular, neurologic, respiratory, gastrointestinal, urinary, musculoskeletal, immunological, and endocrinological diseases [46]. YKL-40 levels increase after kidney injury (e.g., after transplantation) [47].
Two studies were performed only on male participants, and in two others, the percentage of female participants was 18% and 29%, respectively. In five (more than half) of the included studies, the proportions of male and female participants were approximately equal (with a slightly higher prevalence in females). Thus, some studies had male prevalence, and others were similarly represented. Nevertheless, we recognize that there are differences in the function of sex concerning physiology. These can influence the levels and the evolution in time of certain biomarkers based on sex. Women usually have lower serum creatinine levels [48] due to less muscle volume. Also, the sex hormones may influence the renal responses to exercise. Despite these differences, the mechanisms of exercise-induced kidney stress, such as transient hypoperfusion, dehydration, and oxidative stress, are likely present in both sexes.
4.1. Limitations
Statistical heterogeneity of the results was substantial, or considerable, and statistically significant in most of the analyses where it could be calculated. This can be explained by clinical heterogeneity among the included studies: differences in participants’ age, sex distribution, genetic predisposition, prior participation in marathon events, previous health condition, athlete type (professional or recreational), training volume and frequency (weekly running distance and duration), possible comorbidities that were not excluded, use of anti-inflammatory medications (e.g., NSAID), fluid (hydration status), electrolytes and food intake (before and during the marathon), environmental temperature during the event, and course difficulty (flat vs. uphill or downhill terrain).
None of the included studies performed adjusted analyses to account for potential confounding variables, such as those mentioned above as sources of variability. This may introduce a risk of confounding. Biomarker measurements were conducted using appropriate techniques, so measurement error at the sample level is unlikely. The laboratory measurements were not clearly reported, if they were blinded. Nevertheless, by being objective assessments, the risk of bias is avoided. However, the timing of measurement was inconsistent, particularly for the pre-marathon assessment. In some studies, baseline measurements were taken on the day of the marathon, while in others, they were obtained in the days, or even weeks, before. Of course, the participants were most likely healthy individuals, and we would expect limited variability in biomarker levels over time, reducing the likelihood of significant bias.
Some biomarkers, such as copeptin and IGFBP-7, were assessed in only one or two studies in our review. Thus, while their change post-marathon was important, the confidence in the results remains cautious. These findings should be viewed as exploratory and sources of hypotheses and not confirmatory.
4.2. Study Strengths
This study provides valuable insights into the early diagnosis of acute kidney injury in marathon runners using a wide range of biomarkers. These findings may support the role of the studied biomarkers in AKI. Moreover, it shows the comparative evolution of biomarkers during and after a marathon run. This can suggest which one of the biomarkers might be more sensitive to identifying AKI.
Currently, the use of kidney injury biomarkers in marathon runners is only an area of research. Future integration of these biomarkers for real-time or field screening of marathon runners for AKI would come with some challenges: costs of the analyses, the use of laboratories, the time to do the analysis and to obtain the result, and the availability of the marathon runners to be evaluated. For some biomarkers, such as urinary NGAL [49] or TIMP-2*IGFBP-7 [50], rapid tests were developed that are now used in some critical care units. These might be more feasible to be used in the framework of a marathon race at the end, and not in real-time, since the test is based on urine samples and requires 10–20 min to provide a result. Since most of the biomarkers return to their baseline values, the necessity of performing the tests on all marathon runners is limited. A pragmatic approach could be the identification of participants at high risk for AKI based on a questionnaire screening before the marathon, and performing these tests on this subgroup. If future portable tests were developed, then they could be used in real-time for high-risk participants.
Future research could focus on evaluating the long-term effects of repeated marathon participation. Expanding the study to include other types of endurance events and a larger sample size could offer a more comprehensive understanding of the impact of intense physical exertion on kidney function. Including other markers, such as oxidative stress and antioxidant protection enzymes, might be another venue of study. Animal studies can provide additional information in a controlled setting to complete the picture of evidence obtained in human studies.
5. Conclusions
The study showed significant increases in most biomarkers immediately after the marathon compared to baseline values. The largest increases were observed in TIMP-2*IGFBP, copeptin, urinary L-FABP, urinary MCP-1, IGFBP-7, urinary YKL-40, and TIMP-2, suggesting that these biomarkers are promising candidates for future research. Several patterns of biomarker evolution were observed: some increased without decreasing even at 24 h after the marathon (serum creatine kinase and plasma KIM-1); others increased post-marathon and decreased at 24 h while remaining above baseline (TIMP-2IGFBP, copeptin, urinary L-FABP, urinary MCP-1, IGFBP-7, TIMP-2, urinary TNF-alpha, urinary NGAL, urinary KIM-1, and serum creatinine); and some increased after the marathon and then fell below baseline at 24 h (urinary YKL-40, plasma NGAL, and urinary creatinine).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61101775/s1. Supplementary Table S1: Search strategy in bibliographic databases; Supplementary Table S2: Results of the meta-analyses of mean biomarker values before the marathon, including the inconsistency index (I2), associated p-value, Egger’s test for publication bias, and the number of studies included for each biomarker; Supplementary Table S3: Results of the meta-analyses of mean biomarker values immediately after the marathon, including the inconsistency index (I2), associated p-value, Egger’s test for publication bias, and the number of studies included for each biomarker; Supplementary Table S4: Results of the meta-analyses of mean biomarker values 24 h after the marathon, including the inconsistency index (I2), associated p-value, Egger’s test for publication bias, and the number of studies included for each biomarker.
Author Contributions
Conceptualization, L.-I.T., O.A., A.I. and D.-C.L.; methodology, D.-C.L., L.-I.T., O.A., Ș.L.P. and A.I.; software, D.-C.L.; validation, D.-C.L., L.-I.T., O.A., Ș.L.P. and A.I.; formal analysis, D.-C.L., L.-I.T., Ș.L.P. and O.A.; investigation, D.-C.L., L.-I.T., O.A., Ș.L.P. and A.I.; data curation, D.-C.L., L.-I.T., O.A., Ș.L.P. and A.I.; writing—original draft preparation, D.-C.L., L.-I.T., Ș.L.P., A.I. and O.A.; writing—review and editing, D.-C.L., L.-I.T., O.A., Ș.L.P. and A.I.; visualization, O.A., D.-C.L. and A.I.; supervision, A.I.; project administration, Ș.L.P. and A.I.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the paper.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT version 4.0 (OpenAI, San Francisco, CA, USA, accessed in June 2025) as a brainstorming tool to generate suggestions for perspectives to include in the Discussion section. Also, it was used to improve the scientific writing of the manuscript. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Explanation |
| AKI | Acute Kidney Injury |
| AKIN | Acute Kidney Injury Network |
| BUN | Blood Urea Nitrogen |
| BUN/Cr | Blood Urea Nitrogen/Creatinine Ratio |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CRP | C-Reactive Protein |
| IGFBP-7 | Insulin-like Growth Factor Binding Protein-7 |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KIM-1 | Kidney Injury Molecule-1 |
| L-FABP | Liver-type Fatty Acid Binding Protein |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| NA | Not Applicable |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NHLBI | National Heart, Lung, and Blood Institute |
| NR | Not Reported |
| pKIM-1 | Plasma Kidney Injury Molecule-1 |
| pNGAL | Plasma Neutrophil Gelatinase-Associated Lipocalin |
| RIFLE | Risk, Injury, Failure, Loss, and End-Stage Kidney Disease |
| sCK | Serum Creatine Kinase |
| sCRP | Serum C-Reactive Protein |
| sCr | Serum Creatinine |
| sCys C | Serum Cystatin C |
| TIMP-2 | Tissue Inhibitor of Metalloproteinases-2 |
| TNF-α | Tumor Necrosis Factor Alpha |
| uCr | Urinary Creatinine |
| uKIM-1 | Urinary Kidney Injury Molecule-1 |
| uNGAL | Urinary Neutrophil Gelatinase-Associated Lipocalin |
| YKL-40 | Chitinase 3-like Protein 1 |
References
- Zuo, Y.; Zou, L.; Zhang, M.; Smith, L.; Yang, L.; Loprinzi, P.D.; Ren, Z. The Temporal and Spatial Evolution of Marathons in China from 2010 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 5046. [Google Scholar] [CrossRef]
- Zhu, H.; Han, X.; Miao, G.; Yan, Q. A Preliminary Exploration of the Regression Equation for Performance in Amateur Half-Marathon Runners: A Perspective Based on Respiratory Muscle Function. Front. Physiol. 2024, 15, 1340513. [Google Scholar] [CrossRef] [PubMed]
- RunSignup. Annual Industry Report 2024 (RaceTrends 2024). Available online: https://info.runsignup.com/wp-content/uploads/sites/3/2025/01/24-Race-Trends-08-Online-compressed.pdf (accessed on 20 August 2025).
- Hespanhol Junior, L.C.; Pillay, J.D.; van Mechelen, W.; Verhagen, E. Meta-Analyses of the Effects of Habitual Running on Indices of Health in Physically Inactive Adults. Sports Med. 2015, 45, 1455–1468. [Google Scholar] [CrossRef]
- Braschler, L.; Nikolaidis, P.T.; Thuany, M.; Chlíbková, D.; Rosemann, T.; Weiss, K.; Wilhelm, M.; Knechtle, B. Physiology and Pathophysiology of Marathon Running: A Narrative Review. Sports Med. Open 2025, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sports Med. 2022, 52, 725–740. [Google Scholar] [CrossRef]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney Injury and Repair Biomarkers in Marathon Runners. Am. J. Kidney Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Martin, T.G.; Obeid, W.; Pata, R.W.; Myrick, K.M.; Kukova, L.; Jia, Y.; Bjornstad, P.; El-Khoury, J.M.; Parikh, C.R. The Role of Volume Regulation and Thermoregulation in AKI during Marathon Running. Clin. J. Am. Soc. Nephrol. 2019, 14, 1297–1305. [Google Scholar] [CrossRef]
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Bashir, K. Acute Kidney Injury. Crit. Care Nurse 2023, 36, 75–76. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pr. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Palant, C.E.; Amdur, R.L.; Chawla, L.S. The Acute Kidney Injury to Chronic Kidney Disease Transition: A Potential Opportunity to Improve Care in Acute Kidney Injury. Contrib. Nephrol. 2016, 187, 55–72. [Google Scholar] [CrossRef]
- Hodgson, L.; Walter, E.; Venn, R.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L. Acute Kidney Injury Associated with Endurance Events—Is It a Cause for Concern? A Systematic Review. BMJ Open Sport Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef]
- Oh, D.J. A Long Journey for Acute Kidney Injury Biomarkers. Ren. Fail. 2020, 42, 154–165. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Corporation for Digital Scholarship. Open-Source Reference Management Software, Zotero, Version [7.0.11]; Corporation for Digital Scholarship: Vienna, VA, USA. Available online: https://www.zotero.org/ (accessed on 29 May 2024).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 August 2025).
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R. News 2007, 7, 40–45. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, VA, USA, 2024. [Google Scholar]
- Leckie, T.; Fitzpatrick, D.; Richardson, A.J.; Hunter, A.; Bains, S.; Grimaldi, R.; Galloway, R.; Forni, L.G.; Hodgson, L.E. Marathon Running and Cell-Cycle Arrest Biomarkers of Acute Kidney Injury. J. Sci. Med. Sport 2023, 26, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, K.; Kumamoto, S.; Tokinoya, K.; Yoshida, Y.; Sugaya, T.; Murase, T.; Akari, S.; Nakamura, T.; Nabekura, Y.; Takekoshi, K.; et al. Xanthine Oxidoreductase Activity in Marathon Runners: Potential Implications for Marathon-Induced Acute Kidney Injury. J. Appl. Physiol. 2022, 133, 1–10. [Google Scholar] [CrossRef]
- Atkins, W.C.; Butts, C.L.; Kelly, M.R.; Troyanos, C.; Laursen, R.M.; Duckett, A.; Emerson, D.M.; Rosa-Caldwell, M.E.; McDermott, B.P. Acute Kidney Injury Biomarkers and Hydration Outcomes at the Boston Marathon. Front. Physiol. 2022, 12, 813554. [Google Scholar] [CrossRef]
- Nescolarde, L.; Roca, E.; Bogónez-Franco, P.; Hernández-Hermoso, J.; Bayes-Genis, A.; Ara, J. Relationship Be-tween Bioimpedance Vector Displacement and Renal Function After a Marathon in Non-Elite Runners. Front. Physiol. 2020, 11, 352. [Google Scholar] [CrossRef]
- Mccullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in Renal Markers and Acute Kidney Injury after Marathon Running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef]
- Bekos, C.; Zimmermann, M.; Unger, L.; Janik, S.; Hacker, P.; Mitterbauer, A.; Koller, M.; Fritz, R.; Gäbler, C.; Kessler, M.; et al. Non-Professional Marathon Running: RAGE Axis and ST2 Family Changes in Relation to Open-Window Effect, Inflammation and Renal Function. Sci. Rep. 2016, 6, 32315. [Google Scholar] [CrossRef]
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and Renal Function in a Large Cohort of Amateur Marathon Runners. Cardiovasc. Ultrasound 2015, 13, 13. [Google Scholar] [CrossRef][Green Version]
- Poortmans, J.R. Exercise and Renal Function. Sports Med. 1984, 1, 125–153. [Google Scholar] [CrossRef]
- Byrne, C.; Lee, J.K.; Chew, S.A.; Lim, C.L.; Tan, E.Y. Continuous Thermoregulatory Responses to Mass-Participation Distance Running in Heat. Med. Sci. Sports Exerc. 2006, 38, 803–810. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Vargas, N.T.; Hostler, D.; Parker, M.D.; Schlader, Z.J. Both Hyperthermia and Dehy-dration during Physical Work in the Heat Contribute to the Risk of Acute Kidney Injury. J. Appl. Physiol. 2020, 128, 715–728. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Hostler, D.; Parker, M.D.; Pryor, R.R.; Lohr, J.W.; Johnson, B.D.; Chapman, C.L. The Potential for Renal Injury Elicited by Physical Work in the Heat. Nutrients 2019, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The Role of TIMPs in Regulation of Extracellular Matrix Proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Martin, D.R.; Hammerman, M.R. Extracellular Matrix-Related Genes in Kidney after Ischemic Injury: Potential Role for TGF-β in Repair. Am. J. Physiol. 1998, 275, F894–F903. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, T.; Wan, X.; Meng, Y.; Zhao, Z.; Bian, J.; Bao, R.; Deng, X.; Yang, T. IGFBP7 Regulates Sepsis-Induced Acute Kidney Injury through ERK1/2 Signaling. J. Cell. Biochem. 2019, 120, 7602–7611. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and Validation of Cell Cycle Arrest Biomarkers in Human Acute Kidney Injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef]
- Jalleh, R.; Torpy, D.J. The Emerging Role of Copeptin. Clin. Biochem. Rev. 2021, 42, 17–25. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Kimura, K. Urinary Fatty Acid Binding Protein in Renal Disease. Clin. Chim. Acta 2006, 374, 1–7. [Google Scholar] [CrossRef]
- Kamijo, A.; Sugaya, T.; Hikawa, A.; Yamanouchi, M.; Hirata, Y.; Ishimitsu, T.; Numabe, A.; Takagi, M.; Hayakawa, H.; Tabei, F.; et al. Urinary Liver-Type Fatty Acid Binding Protein as a Useful Biomarker in Chronic Kidney Disease. Mol. Cell. Biochem. 2006, 284, 175–182. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Rice, J.C.; Spence, J.S.; Yetman, D.L.; Safirstein, R.L. Monocyte Chemoattractant Protein-1 Expression Correlates with Monocyte Infiltration in the Post-Ischemic Kidney. Ren. Fail. 2002, 24, 703–723. [Google Scholar] [CrossRef]
- Stroo, I.; Claessen, N.; Teske, G.J.; Butter, L.M.; Florquin, S.; Leemans, J.C. Deficiency for the Chemokine Monocyte Chem-oattractant Protein-1 Aggravates Tubular Damage after Renal Ischemia/Reperfusion Injury. PLoS ONE 2015, 10, e0123203. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.M. Tumor Necrosis Factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Wilson, F.P.; Pober, J.S.; Perazella, M.A.; Singh, N.; Luciano, R.L.; Obeid, W.; Lin, H.; Kuperman, M.; Moeckel, G.W.; et al. Urine TNF-α and IL-9 for Clinical Diagnosis of Acute Interstitial Nephritis. JCI Insight 2019, 4, e127456. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B.; Li, H.; Mai, Z.; Zhang, L.; Li, M.; Liu, L.; Chen, S.; Chen, J.; Liu, Y. Ethnicity-Stratified Analysis of the Association between TNF-α Genetic Polymorphisms and Acute Kidney Injury: A Systematic Review and Me-ta-Analysis. Biomed. Res. Int. 2020, 2020, 5262351. [Google Scholar] [CrossRef]
- Volck, B.; Price, P.A.; Johansen, J.S.; Sørensen, O.; Benfield, T.L.; Nielsen, H.J.; Calafat, J.; Borregaard, N. YKL 40, a Mammalian Member of the Chitinase Family, Is a Matrix Protein of Specific Granules in Human Neutrophils. Proc. Assoc. Am. Physicians 1998, 110, 351–360. Available online: https://pubmed.ncbi.nlm.nih.gov/9686683/ (accessed on 16 September 2025).
- Kazakova, M.H.; Sarafian, V.S. YKL 40—A Novel Biomarker in Clinical Practice? Folia Med. 2009, 51, 5–14. [Google Scholar]
- Blazevic, N.; Rogic, D.; Pelajic, S.; Miler, M.; Glavcic, G.; Ratkajec, V.; Vrkljan, N.; Bakula, D.; Hrabar, D.; Pavic, T. YKL-40 as a Biomarker in Various Inflammatory Diseases: A Review. Biochem. Med. 2024, 34, 010502. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Hall, I.E.; Kale, S.; Lee, S.; He, C.H.; Lee, Y.; Chupp, G.L.; Moeckel, G.W.; Lee, C.G.; Elias, J.A.; et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and pre-dicts delayed allograft function. J. Am. Soc. Nephrol. 2013, 24, 309–319. [Google Scholar] [CrossRef]
- Vikse, B.E.; Vollset, S.E.; Tell, G.S.; Refsum, H.; Iversen, B.M. Distribution and Determinants of Serum Creatinine in the General Population: The Hordaland Health Study. Scand. J. Clin. Lab. Investig. 2004, 64, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Bufkin, K.B.; Silva, J. The XpressCard Point-of-Care Test for Human Neutrophil Gelatinase-Associated Lipocalin Enhances the Predic-tion of Acute Kidney Injury. J. Clin. Med. 2024, 13, 7564. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Van Aken, H.; Martens, S.; Rossaint, J.; Singbartl, K.; Görlich, D.; Kellum, J.A.; Zarbock, A. Urinary TIMP-2 and IGFBP7 as Early Biomarkers of Acute Kidney Injury and Renal Recovery following Cardiac Surgery. PLoS ONE 2014, 9, e93460. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).