Cardiopulmonary Exercise Testing in the Prognostic Assessment of Heart Failure: From a Standardized Approach to Tailored Therapeutic Strategies
Abstract
1. Introduction
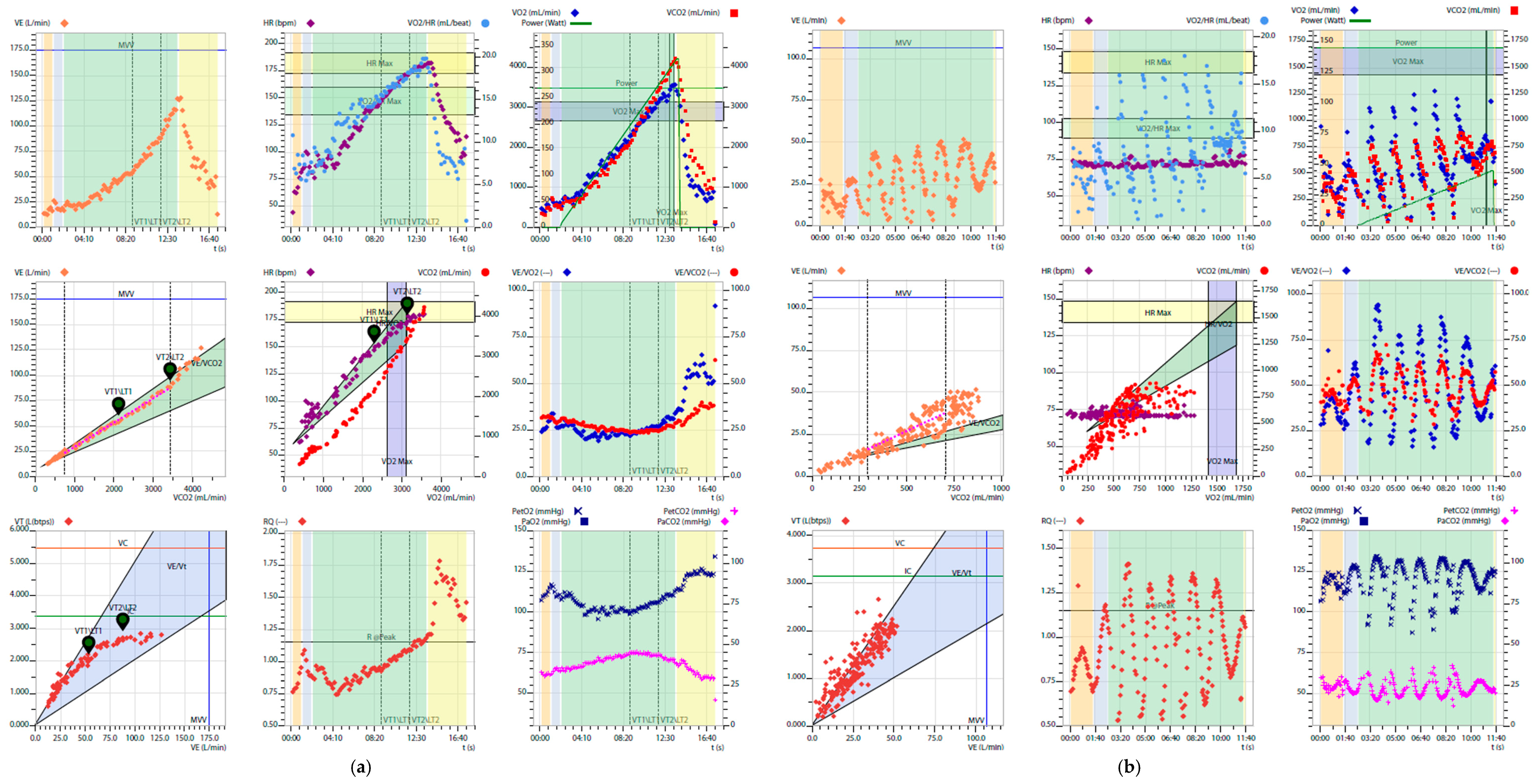
2. Prognostic Variables Derived from CPET
2.1. Peak VO2
2.2. VE/VCO2 Slope
2.3. Ventilation
- Carvedilol was associated with a reduction in alveolar-capillary diffusion capacity (DLCO), likely due to an adverse effect on the membrane component, but at the same time provided better ventilatory efficiency during exercise, probably through modulation of chemoreflex control.
- Nebivolol and Bisoprolol, both β1-selective, better preserved pulmonary diffusion capacity and peak exercise performance, although they showed a less favorable ventilatory profile compared with Carvedilol.
2.4. Anaerobic Threshold
2.5. Exercise Oscillatory Ventilation (EOV)
- Increased sensitivity to changes in arterial CO2 pressure (PaCO2),
- Reduced hemodynamic reserve,
- Elevated pulmonary capillary pressure,
- Stimulation of J receptors from pulmonary vascular congestion.
2.6. VO2/Work Rate (ΔVO2/ΔWR)
2.7. VO2/Heart Rate (O2 Pulse)
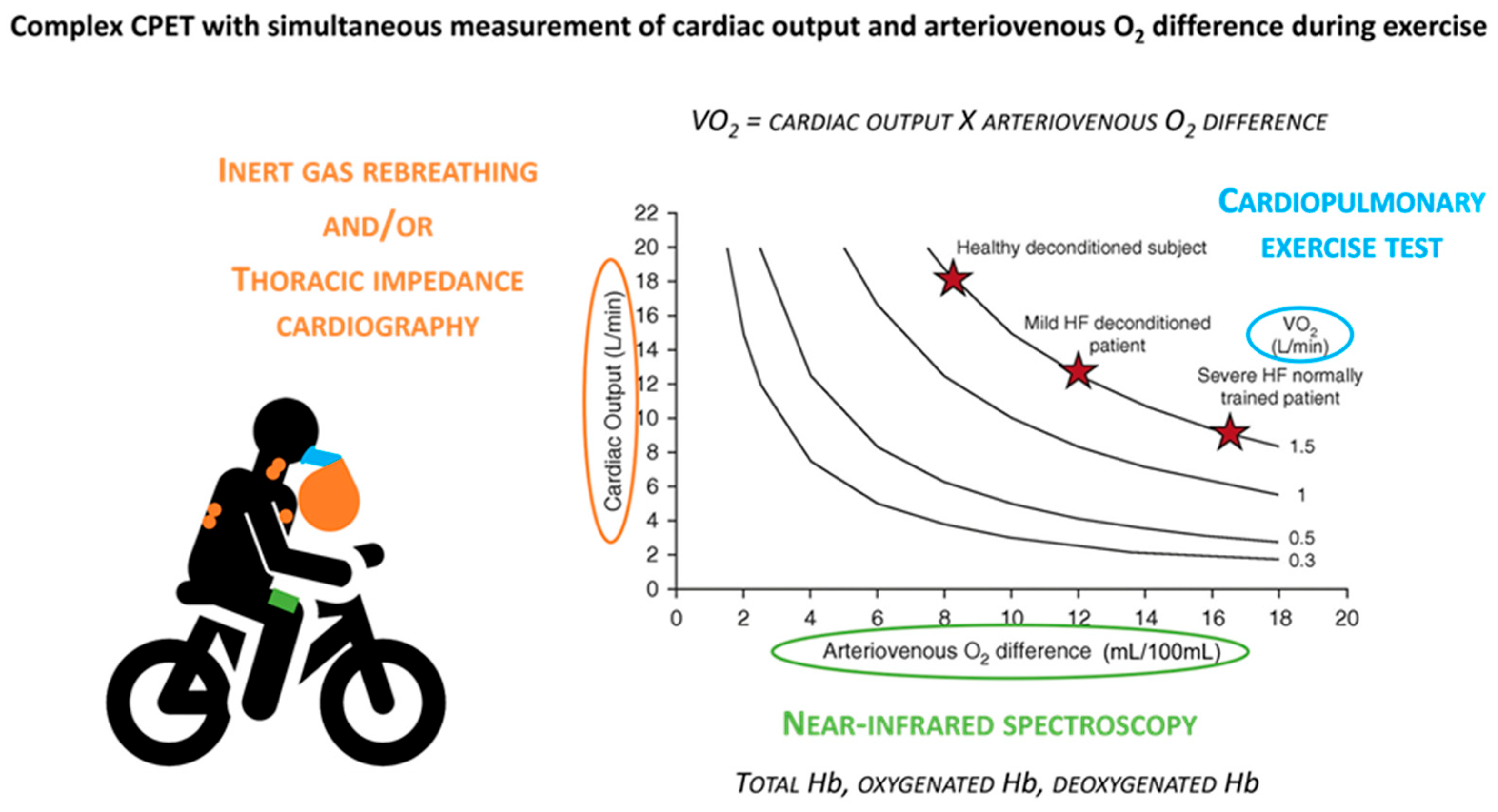
3. From Standard to Complex CPET: Methodological Insights
4. The Role of Multiparametric Scores Based on CPET
4.1. MECKI Score
- According to published data, the MECKI score stratifies patients into four categories:
- <5% (906 patients)
- 5–10% (449 patients)
- 10–15% (236 patients)
- >15% (418 patients)
4.2. HFSS (Heart Failure Survival Score)
- Low risk: HFSS > 8.1.
- Intermediate risk: HFSS 7.2–8.09.
- High risk: HFSS ≤ 7.19.
4.3. SHFM (Seattle Heart Failure Model)
4.4. ISHLT Listing Criteria
- Peak VO2 ≤ 14 mL/kg/min.
- Peak VO2 ≤ 12 mL/kg/min in patients on beta-blocker therapy.
- In patients with obesity (BMI ≥ 30 kg/m2): peak VO2 adjusted for lean body mass ≤ 19 mL/kg/min.
- For all patients—particularly when CPET is submaximal: VE/VCO2 slope > 35.
- In women or in patients aged ≤50 or ≥70 years: peak VO2 ≤ 50% of predicted.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin Converting Enzyme |
| ARNI | Angiotensin Receptor–Neprilysin Inhibitor |
| AT | Anaerobic Threshold |
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CO | Cardiac Output |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPET | Cardiopulmonary Exercise Testing |
| CRT | Cardiac Resynchronization Therapy |
| CSA | Central Sleep Apnea |
| DLCO | Diffusing Capacity of the Lungs for Carbon Monoxide |
| EOV | Exercise Oscillatory Ventilation |
| ESC | European Society of Cardiology |
| eGFR | estimated Glomerular Filtration Rate |
| HF | Heart Failure |
| HFimpEF | Heart Failure with improved Ejection Fraction |
| HFmrEF | Heart Failure with mildly reduced Ejection Fraction |
| HFpEF | Heart Failure with preserved Ejection Fraction |
| HFrEF | Heart Failure with reduced Ejection Fraction |
| HFSS | Heart Failure Survival Score |
| ICD | Implantable Cardioverter Defibrillator |
| ISHLT | International Society for Heart and Lung Transplantation |
| LVEF | Left Ventricular Ejection Fraction |
| LVAD | Left Ventricular Assist Device |
| MCS | Mechanical Circulatory Support |
| MECKI | Metabolic Exercise Cardiac Kidney Index (Score) |
| MRA | Mineralocorticoid Receptor Antagonist |
| NIRS | Near-Infrared Spectroscopy |
| NYHA | New York Heart Association (functional classification) |
| (VO2/HR) | Oxygen Pulse |
| PaCO2 | Arterial Partial Pressure of Carbon Dioxide |
| PetCO2 | End Tidal Partial Pressure of Carbon Dioxide |
| PetO2 | End Tidal Partial Pressure of Oxygen |
| ppVO2 | Peak Predicted Oxygen Uptake |
| RCP | Respiratory Compensation Point |
| ROC | Receiver Operating Characteristic |
| RER | Respiratory Exchange Ratio |
| SGLT2i | Sodium Glucose Co Transporter 2 inhibitor |
| SHFM | Seattle Heart Failure Model |
| VD | Dead Space Volume |
| VE | Minute Ventilation |
| VE/VCO2 slope | Ventilation/Carbon Dioxide Production slope |
| VO2 | Oxygen Uptake (Volume of Oxygen consumed) |
| VO2/HR | O2 Pulse Oxygen Pulse |
| VCO2 | Carbon Dioxide Output |
| VT | Tidal Volume |
| WR | Work Rate |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Agostoni, P.; Paolillo, S.; Mapelli, M.; Gentile, P.; Salvioni, E.; Veglia, F.; Bonomi, A.; Corrà, U.; Lagioia, R.; Limongelli, G.; et al. Multiparametric Prognostic Scores in Chronic Heart Failure with Reduced Ejection Fraction: A Long-term Comparison. Eur. J. Heart Fail. 2018, 20, 700–710. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.; Sue, D.; Whipp, B. Principles of Exercise Testing and Interpretation, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Corrà, U.; Agostoni, P.G.; Anker, S.D.; Coats, A.J.S.; Crespo Leiro, M.G.; De Boer, R.A.; Harjola, V.; Hill, L.; Lainscak, M.; Lund, L.H.; et al. Role of Cardiopulmonary Exercise Testing in Clinical Stratification in Heart Failure. A Position Paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 3–15. [Google Scholar] [CrossRef]
- ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [CrossRef] [PubMed]
- Vignati, C.; Campana, N.; Nava, A.; Ripamonti, R.; Montisci, R.; Cadeddu Dessalvi, C.; Pezzuto, B.; Agostoni, P. Feasibility of Cardiopulmonary Exercise Test in an Athlete Who Underwent Total Laringectomy. J. Sports Med. Phys. Fitness 2024, 64, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, K.D.; Schwartz, J.S.; Chen, T.-M.; Wong, K.-L.; Goin, J.E.; Mancini, D.M. Development and Prospective Validation of a Clinical Index to Predict Survival in Ambulatory Patients Referred for Cardiac Transplant Evaluation. Circulation 1997, 95, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of Survival in Heart Failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Agostoni, P.; Corrà, U.; Cattadori, G.; Veglia, F.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; Limongelli, G.; et al. Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes, the MECKI Score: A Multiparametric Approach to Heart Failure Prognosis. Int. J. Cardiol. 2013, 167, 2710–2718. [Google Scholar] [CrossRef]
- Bobbio, M.; Dogliani, S.; Giacomarra, G. Superiority of the Heart Failure Survival Score to Peak Exercise Oxygen Consumption in the Prediction of Outcomes in an Independent Population Referred for Heart Transplant Evaluation. Ital. Heart J. 2004, 5, 899–905. [Google Scholar]
- Peled, Y.; Ducharme, A.; Kittleson, M.; Bansal, N.; Stehlik, J.; Amdani, S.; Saeed, D.; Cheng, R.; Clarke, B.; Dobbels, F.; et al. International Society for Heart and Lung Transplantation Guidelines for the Evaluation and Care of Cardiac Transplant Candidates—2024. J. Heart Lung Transplant. 2024, 43, 1529–1628.e54. [Google Scholar] [CrossRef] [PubMed]
- Mattavelli, I.; Vignati, C.; Farina, S.; Apostolo, A.; Cattadori, G.; De Martino, F.; Pezzuto, B.; Zaffalon, D.; Agostoni, P. Beyond VO2: The Complex Cardiopulmonary Exercise Test. Eur. J. Prev. Cardiol. 2023, 30, ii34–ii39. [Google Scholar] [CrossRef] [PubMed]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary Exercise Testing and Its Application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the Italian Working Group on Cardiac Rehabilitation Prevention; Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology; Piepoli, M.F.; Corrà, U.; Agostoni, P.G.; Belardinelli, R.; Cohen-Solal, A.; Hambrecht, R.; Vanhees, L. Statement on Cardiopulmonary Exercise Testing in Chronic Heart Failure Due to Left Ventricular Dysfunction: Recommendations for Performance and Interpretation Part I: Definition of Cardiopulmonary Exercise Testing Parameters for Appropriate Use in Chronic Heart Failure. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 150–164. [Google Scholar] [CrossRef]
- Weber, K.T.; Janicki, J.S. Cardiopulmonary Exercise Testing for Evaluation of Chronic Cardiac Failure. Am. J. Cardiol. 1985, 55, A22–A31. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H.; Wilson, J.R. Value of Peak Exercise Oxygen Consumption for Optimal Timing of Cardiac Transplantation in Ambulatory Patients with Heart Failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Cattadori, G.; Di Marco, S.; Farina, S.; Limongelli, G.; Monda, E.; Badagliacca, R.; Papa, S.; Tricarico, L.; Correale, M. Beta-Blockers in Heart Failure Prognosis: Lessons Learned by MECKI Score Group Papers. Eur. J. Prev. Cardiol. 2020, 27, 65–71. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and Safety of Exercise Training in Patients with Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA 2009, 301, 1439. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Patel, M.; Kraus, W.E.; Brawner, C.A.; McConnell, T.R.; Piña, I.L.; Leifer, E.S.; Fleg, J.L.; Blackburn, G.; Fonarow, G.C.; et al. Variables Measured During Cardiopulmonary Exercise Testing as Predictors of Mortality in Chronic Systolic Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 780–789. [Google Scholar] [CrossRef]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.-C.; et al. Modest Increase in Peak VO2 Is Related to Better Clinical Outcomes in Chronic Heart Failure Patients: Results from Heart Failure and a Controlled Trial to Investigate Outcomes of Exercise Training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise Anaerobic Threshold and Ventilatory Efficiency Identify Heart Failure Patients for High Risk of Early Death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Palau, P.; Domínguez, E.; Núñez, E.; Ramón, J.M.; López, L.; Melero, J.; Sanchis, J.; Bellver, A.; Santas, E.; Bayes-Genis, A.; et al. Peak Exercise Oxygen Uptake Predicts Recurrent Admissions in Heart Failure with Preserved Ejection Fraction. Rev. Esp. Cardiol. Engl. Ed. 2018, 71, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, A.; Brawner, C.A.; Aldred, H.A.; Lewis, B.; Williams, C.T.; Tita, C.; Schairer, J.R.; Ehrman, J.K.; Velez, M.; Selektor, Y.; et al. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure with Preserved Ejection Fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT-CPX) Project. Am. Heart J. 2016, 174, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Neder, J.A.; Phillips, D.B.; Marillier, M.; Bernard, A.-C.; Berton, D.C.; O’Donnell, D.E. Clinical Interpretation of Cardiopulmonary Exercise Testing: Current Pitfalls and Limitations. Front. Physiol. 2021, 12, 552000. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing. J. Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Guazzi, M.; Myers, J.; Arena, R. Cardiopulmonary Exercise Testing in the Clinical and Prognostic Assessment of Diastolic Heart Failure. J. Am. Coll. Cardiol. 2005, 46, 1883–1890. [Google Scholar] [CrossRef]
- Lim, H.S.; Theodosiou, M. Exercise Ventilatory Parameters for the Diagnosis of Reactive Pulmonary Hypertension in Patients with Heart Failure. J. Card. Fail. 2014, 20, 650–657. [Google Scholar] [CrossRef]
- Nanas, S.N.; Nanas, J.N.; Sakellariou, D.C.; Dimopoulos, S.K.; Drakos, S.G.; Kapsimalakou, S.G.; Mpatziou, C.A.; Papazachou, O.G.; Dalianis, A.S.; Anastasiou-Nana, M.I.; et al. VE/VCO2 Slope Is Associated with Abnormal Resting Haemodynamics and Is a Predictor of Long-term Survival in Chronic Heart Failure. Eur. J. Heart Fail. 2006, 8, 420–427. [Google Scholar] [CrossRef]
- Reindl, I.; Wernecke, K.-D.; Opitz, C.; Wensel, R.; König, D.; Dengler, T.; Schimke, I.; Kleber, F.X. Impaired Ventilatory Efficiency in Chronic Heart Failure: Possible Role of Pulmonary Vasoconstriction. Am. Heart J. 1998, 136, 778–785. [Google Scholar] [CrossRef]
- Apostolo, A.; Vignati, C.; Cittar, M.; Baracchini, N.; Mushtaq, S.; Cattadori, G.; Sciomer, S.; Trombara, F.; Piepoli, M.; Agostoni, P. Determinants of Exercise Performance in Heart Failure Patients with Extremely Reduced Cardiac Output and Left Ventricular Assist Device. Eur. J. Prev. Cardiol. 2023, 30, ii63–ii69. [Google Scholar] [CrossRef]
- Pezzuto, B.; Contini, M.; Berna, G.; Galotta, A.; Cattaneo, G.; Maragna, R.; Gugliandolo, P.; Agostoni, P. Dynamic Trend of Lung Fluid Movement during Exercise in Heart Failure: From Lung Imaging to Alveolar-Capillary Membrane Function. Int. J. Cardiol. 2024, 407, 132041. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a Ventilatory Classification System in Patients with Heart Failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Corrà, U.; Mezzani, A.; Bosimini, E.; Scapellato, F.; Imparato, A.; Giannuzzi, P. Ventilatory Response to Exercise Improves Risk Stratification in Patients with Chronic Heart Failure and Intermediate Functional Capacity. Am. Heart J. 2002, 143, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Apostolo, A.; Paolillo, S.; Contini, M.; Vignati, C.; Tarzia, V.; Campodonico, J.; Mapelli, M.; Massetti, M.; Bejko, J.; Righini, F.; et al. Comprehensive Effects of Left Ventricular Assist Device Speed Changes on Alveolar Gas Exchange, Sleep Ventilatory Pattern, and Exercise Performance. J. Heart Lung Transplant. 2018, 37, 1361–1371. [Google Scholar] [CrossRef]
- Vignati, C.; Apostolo, A.; Cattadori, G.; Farina, S.; Del Torto, A.; Scuri, S.; Gerosa, G.; Bottio, T.; Tarzia, V.; Bejko, J.; et al. Lvad Pump Speed Increase Is Associated with Increased Peak Exercise Cardiac Output and VO2, Postponed Anaerobic Threshold and Improved Ventilatory Efficiency. Int. J. Cardiol. 2017, 230, 28–32. [Google Scholar] [CrossRef]
- Gargiulo, P.; Apostolo, A.; Perrone-Filardi, P.; Sciomer, S.; Palange, P.; Agostoni, P. A Non Invasive Estimate of Dead Space Ventilation from Exercise Measurements. PLoS ONE 2014, 9, e87395. [Google Scholar] [CrossRef][Green Version]
- Apostolo, A.; Laveneziana, P.; Palange, P.; Agalbato, C.; Molle, R.; Popovic, D.; Bussotti, M.; Internullo, M.; Sciomer, S.; Bonini, M.; et al. Impact of Chronic Obstructive Pulmonary Disease on Exercise Ventilatory Efficiency in Heart Failure. Int. J. Cardiol. 2015, 189, 134–140. [Google Scholar] [CrossRef]
- Gong, J.; Castro, R.R.T.; Caron, J.P.; Bay, C.P.; Hainer, J.; Opotowsky, A.R.; Mehra, M.R.; Maron, B.A.; Di Carli, M.F.; Groarke, J.D.; et al. Usefulness of Ventilatory Inefficiency in Predicting Prognosis across the Heart Failure Spectrum. ESC Heart Fail. 2022, 9, 293–302. [Google Scholar] [CrossRef]
- Klaassen, S.H.C.; Liu, L.C.Y.; Hummel, Y.M.; Damman, K.; van der Meer, P.; Voors, A.A.; Hoendermis, E.S.; van Veldhuisen, D.J. Clinical and Hemodynamic Correlates and Prognostic Value of VE/VCO2 Slope in Patients with Heart Failure with Preserved Ejection Fraction and Pulmonary Hypertension. J. Card. Fail. 2017, 23, 777–782. [Google Scholar] [CrossRef]
- Tsujinaga, S.; Iwano, H.; Chiba, Y.; Ishizaka, S.; Sarashina, M.; Murayama, M.; Nakabachi, M.; Nishino, H.; Yokoyama, S.; Okada, K.; et al. Heart Failure with Preserved Ejection Fraction vs. Reduced Ejection Fraction―Mechanisms of Ventilatory Inefficiency During Exercise in Heart Failure―. Circ. Rep. 2020, 2, 271–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nayor, M.; Houstis, N.E.; Namasivayam, M.; Rouvina, J.; Hardin, C.; Shah, R.V.; Ho, J.E.; Malhotra, R.; Lewis, G.D. Impaired Exercise Tolerance in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 605–617. [Google Scholar] [CrossRef] [PubMed]
- De Martino, F.; Agostoni, P. Insight Ventilation Perfusion Inefficiency in Patients with Heart Failure with Preserved Ejection Fraction. Chest 2022, 162, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Reina, G.; Tumminello, G.; Guazzi, M.D. Exercise Ventilation Inefficiency and Cardiovascular Mortality in Heart Failure: The Critical Independent Prognostic Value of the Arterial CO2 Partial Pressure. Eur. Heart J. 2005, 26, 472–480. [Google Scholar] [CrossRef]
- Kee, K.; Stuart-Andrews, C.; Ellis, M.J.; Wrobel, J.P.; Nilsen, K.; Sharma, M.; Thompson, B.R.; Naughton, M.T. Increased Dead Space Ventilation Mediates Reduced Exercise Capacity in Systolic Heart Failure. Am. J. Respir. Crit. Care Med. 2016, 193, 1292–1300. [Google Scholar] [CrossRef]
- Olson, T.P.; Joyner, M.J.; Johnson, B.D. Influence of Locomotor Muscle Metaboreceptor Stimulation on the Ventilatory Response to Exercise in Heart Failure. Circ. Heart Fail. 2010, 3, 212–219. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Brown, H.V.; Rubin, S.A. Respiratory and Circulatory Analysis of CO2 Output during Exercise in Chronic Heart Failure. Circulation 1991, 84, 605–612. [Google Scholar] [CrossRef]
- Wasserman, K.; Zhang, Y.-Y.; Gitt, A.; Belardinelli, R.; Koike, A.; Lubarsky, L.; Agostoni, P.G. Lung Function and Exercise Gas Exchange in Chronic Heart Failure. Circulation 1997, 96, 2221–2227. [Google Scholar] [CrossRef]
- Palermo, P.; Cattadori, G.; Bussotti, M.; Apostolo, A.; Contini, M.; Agostoni, P. Lateral Decubitus Position Generates Discomfort and Worsens Lung Function in Chronic Heart Failure. Chest 2005, 128, 1511–1516. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G.; Guazzi, M.; Palermo, P.; Bussotti, M.; Marenzi, G. Cardiomegaly as a Possible Cause of Lung Dysfunction in Patients with Heart Failure. Am. Heart J. 2000, 140, A17–A21. [Google Scholar] [CrossRef]
- Contini, M.; Apostolo, A.; Cattadori, G.; Paolillo, S.; Iorio, A.; Bertella, E.; Salvioni, E.; Alimento, M.; Farina, S.; Palermo, P.; et al. Multiparametric Comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in Moderate Heart Failure: The CARNEBI Trial. Int. J. Cardiol. 2013, 168, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, G.; Corrà, U.; Contini, M.; Magrì, D.; Paolillo, S.; Perrone Filardi, P.; Sciomer, S.; Badagliacca, R.; Agostoni, P. Choosing among β-Blockers in Heart Failure Patients According to β-Receptors’ Location and Functions in the Cardiopulmonary System. Pharmacol. Res. 2020, 156, 104785. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A New Method for Detecting Anaerobic Threshold by Gas Exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee; EACPR; Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; AHA; Arena, R.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Eur. Heart J. 2012, 33, 2917–2927. [Google Scholar] [CrossRef]
- Tomono, J.; Adachi, H.; Oshima, S.; Kurabayashi, M. Usefulness of Anaerobic Threshold to Peak Oxygen Uptake Ratio to Determine the Severity and Pathophysiological Condition of Chronic Heart Failure. J. Cardiol. 2016, 68, 373–378. [Google Scholar] [CrossRef]
- Agostoni, P.; Corrà, U.; Cattadori, G.; Veglia, F.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; et al. Prognostic Value of Indeterminable Anaerobic Threshold in Heart Failure. Circ. Heart Fail. 2013, 6, 977–987. [Google Scholar] [CrossRef]
- Carriere, C.; Corrà, U.; Piepoli, M.; Bonomi, A.; Merlo, M.; Barbieri, S.; Salvioni, E.; Binno, S.; Mapelli, M.; Righini, F.; et al. Anaerobic Threshold and Respiratory Compensation Point Identification During Cardiopulmonary Exercise Tests in Chronic Heart Failure. Chest 2019, 156, 338–347. [Google Scholar] [CrossRef]
- Corrà, U.; Pistono, M.; Mezzani, A.; Braghiroli, A.; Giordano, A.; Lanfranchi, P.; Bosimini, E.; Gnemmi, M.; Giannuzzi, P. Sleep and Exertional Periodic Breathing in Chronic Heart Failure: Prognostic Importance and Interdependence. Circulation 2006, 113, 44–50. [Google Scholar] [CrossRef]
- Amir, O.; Reisfeld, D.; Sberro, H.; Paz, H.; Mintz, S.; Lewis, B.S. Implications of Cheyne-Stokes Breathing in Advanced Systolic Heart Failure. Clin. Cardiol. 2010, 33, E8–E12. [Google Scholar] [CrossRef]
- Agostoni, P.; Salvioni, E. Exertional Periodic Breathing in Heart Failure. Clin. Chest Med. 2019, 40, 449–457. [Google Scholar] [CrossRef]
- Corrà, U.; Giordano, A.; Bosimini, E.; Mezzani, A.; Piepoli, M.; Coats, A.J.S.; Giannuzzi, P. Oscillatory Ventilation During Exercise in Patients with Chronic Heart Failure. Chest 2002, 121, 1572–1580. [Google Scholar] [CrossRef]
- Rovai, S.; Corrà, U.; Piepoli, M.; Vignati, C.; Salvioni, E.; Bonomi, A.; Mattavelli, I.; Arcari, L.; Scardovi, A.B.; Perrone Filardi, P.; et al. Exercise Oscillatory Ventilation and Prognosis in Heart Failure Patients with Reduced and Mid-range Ejection Fraction. Eur. J. Heart Fail. 2019, 21, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.J.; Mansur, A.J.; De Freitas, H.F.G.; Chizola, P.R.; Bocchi, E.A.; Terra-Filho, M.; Neder, J.A.; Lorenzi-Filho, G. Periodic Breathing during Incremental Exercise Predicts Mortality in Patients with Chronic Heart Failure Evaluated for Cardiac Transplantation. J. Am. Coll. Cardiol. 2003, 41, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Raimondo, R.; Vicenzi, M.; Arena, R.; Proserpio, C.; Sarzi Braga, S.; Pedretti, R. Exercise Oscillatory Ventilation May Predict Sudden Cardiac Death in Heart Failure Patients. J. Am. Coll. Cardiol. 2007, 50, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, J.; Taeymans, J.; Hens, W.; Beckers, P.; Vrints, C.; Vissers, D. Prognostic Respiratory Parameters in Heart Failure Patients with and without Exercise Oscillatory Ventilation—A Systematic Review and Descriptive Meta-Analysis. Int. J. Cardiol. 2015, 182, 476–486. [Google Scholar] [CrossRef]
- Guazzi, M.; Myers, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Arena, R. Exercise Oscillatory Breathing in Diastolic Heart Failure: Prevalence and Prognostic Insights. Eur. Heart J. 2008, 29, 2751–2759. [Google Scholar] [CrossRef]
- Magrì, D.; Palermo, P.; Salvioni, E.; Mapelli, M.; Gallo, G.; Vignati, C.; Mattavelli, I.; Gugliandolo, P.; Maruotti, A.; Di Loro, P.A.; et al. Influence of Exertional Oscillatory Breathing and Its Temporal Behavior in Patients with Heart Failure and Reduced Ejection Fraction. Int. J. Cardiol. 2023, 383, 50–56. [Google Scholar] [CrossRef]
- Belardinelli, R. Exercise-Induced Myocardial Ischaemia Detected by Cardiopulmonary Exercise Testing. Eur. Heart J. 2003, 24, 1304–1313. [Google Scholar] [CrossRef]
- Bussotti, M.; Apostolo, A.; Andreini, D.; Palermo, P.; Contini, M.; Agostoni, P. Cardiopulmonary Evidence of Exercise-Induced Silent Ischaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 249–253. [Google Scholar] [CrossRef]
- Mezzani, A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Ann. Am. Thorac. Soc. 2017, 14, S3–S11. [Google Scholar] [CrossRef]
- Accalai, E.; Vignati, C.; Salvioni, E.; Pezzuto, B.; Contini, M.; Cadeddu, C.; Meloni, L.; Agostoni, P. Non-Invasive Estimation of Stroke Volume during Exercise from Oxygen in Heart Failure Patients. Eur. J. Prev. Cardiol. 2021, 28, 280–286. [Google Scholar] [CrossRef]
- Munhoz, E.C.; Hollanda, R.; Vargas, J.P.; Silveira, C.W.; Lemos, A.L.; Hollanda, R.M.K.; Ribeiro, J.P. Flattening of Oxygen Pulse during Exercise May Detect Extensive Myocardial Ischemia. Med. Sci. Sports Exerc. 2007, 39, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Arena, R.; Wasserman, K.; Hansen, J.E.; Lewis, G.D.; Myers, J.; Chronos, N.; Boden, W.E. Exercise-Induced Myocardial Ischemia Detected by Cardiopulmonary Exercise Testing. Am. J. Cardiol. 2009, 103, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R. Peak Exercise Oxygen Pulse and Prognosis in Chronic Heart Failure. Am. J. Cardiol. 2004, 93, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.; Myers, J.; Araújo, C.G.S.; Arena, R.; Mandic, S.; Bensimhon, D.; Abella, J.; Chase, P.; Guazzi, M.; Brubaker, P.; et al. Does Peak Oxygen Pulse Complement Peak Oxygen Uptake in Risk Stratifying Patients with Heart Failure? Am. J. Cardiol. 2009, 104, 554–558. [Google Scholar] [CrossRef]
- Tashiro, M.; Goda, A.; Yanagisawa, Y.; Nakamaru, R.; Funabashi, S.; Takeuchi, S.; Soejima, K.; Kohno, T. Prognostic Value of Heart Rate and Oxygen Pulse Response in Heart Failure with Left Ventricular Ejection Fraction over 40%. Clin. Res. Cardiol. 2024. [Google Scholar] [CrossRef]
- Vignati, C.; Morosin, M.; Fusini, L.; Pezzuto, B.; Spadafora, E.; De Martino, F.; Salvioni, E.; Rovai, S.; Filardi, P.P.; Sinagra, G.; et al. Do Rebreathing Manoeuvres for Non-Invasive Measurement of Cardiac Output during Maximum Exercise Test Alter the Main Cardiopulmonary Parameters? Eur. J. Prev. Cardiol. 2019, 26, 1616–1622. [Google Scholar] [CrossRef]
- Mapelli, M.; Romani, S.; Magrì, D.; Merlo, M.; Cittar, M.; Masè, M.; Muratori, M.; Gallo, G.; Sclafani, M.; Carriere, C.; et al. Exercise Oxygen Pulse Kinetics in Patients with Hypertrophic Cardiomyopathy. Heart 2022, 108, 1629–1636. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G.; Vignati, C.; Apostolo, A.; Farina, S.; Salvioni, E.; Di Marco, S.; Sonaglioni, A.; Nodari, S.; Marenzi, G.; et al. Deceived by the Fick Principle: Blood Flow Distribution in Heart Failure. Eur. J. Prev. Cardiol. 2024, 31, 2001–2010. [Google Scholar] [CrossRef]
- Agostoni, P.; Cattadori, G.; Apostolo, A.; Contini, M.; Palermo, P.; Marenzi, G.; Wasserman, K. Noninvasive Measurement of Cardiac Output During Exercise by Inert Gas Rebreathing Technique: A New Tool for Heart Failure Evaluation. J. Am. Coll. Cardiol. 2005, 46, 1779–1781. [Google Scholar] [CrossRef]
- Richard, R.; Lonsdorfer-Wolf, E.; Charloux, A.; Doutreleau, S.; Buchheit, M.; Oswald-Mammosser, M.; Lampert, E.; Mettauer, B.; Geny, B.; Lonsdorfer, J. Non-Invasive Cardiac Output Evaluation during a Maximal Progressive Exercise Test, Using a New Impedance Cardiograph Device. Eur. J. Appl. Physiol. 2001, 85, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Bhambhani, Y.N. Muscle Oxygenation Trends During Dynamic Exercise Measured by Near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Vignati, C.; Cattadori, G. Measuring Cardiac Output during Cardiopulmonary Exercise Testing. Ann. Am. Thorac. Soc. 2017, 14, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Karsten, M.; Vignati, C.; Pezzuto, B.; Apostolo, A.; Teruzzi, G.; Baldi, G.S.; Gili, S.; Campodonico, J.; Grilli, G.; Galotta, A.; et al. Accurate Fick Cardiac Output Estimation: Direct and Simultaneous Oxygen Consumption Measurement Is Imperative in Heart Failure. Am. Heart J. 2025, 290, 249–257. [Google Scholar] [CrossRef]
- Wernhart, S.; Papathanasiou, M.; Rassaf, T.; Luedike, P. Heart Failure Classification Based on Resting Ejection Fraction Does Not Display a Unique Exercise Response Pattern. Int. J. Cardiol. 2023, 376, 157–164. [Google Scholar] [CrossRef]
- Bocchi, E.A.; Guimarães, G.V.; Moreira, L.F.P.; Bacal, F.; De Moraes, A.V.; Barreto, A.C.P.; Wajngarten, M.; Bellotti, G.; Stolf, N.; Jatene, A.; et al. Peak Oxygen Consumption and Resting Left Ventricular Ejection Fraction Changes After Cardiomyoplasty at 6-Month Follow-Up. Circulation 1995, 92, 216–222. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Henein, M.Y. Left Ventricular Ejection Fraction: Clinical, Pathophysiological, and Technical Limitations. Front. Cardiovasc. Med. 2024, 11, 1340708. [Google Scholar] [CrossRef]
- Mapelli, M.; Mattavelli, I.; Paolillo, S.; Salvioni, E.; Magrì, D.; Galotta, A.; De Martino, F.; Mantegazza, V.; Vignati, C.; Esposito, I.; et al. Effects of Sacubitril/Valsartan on Exercise Capacity: A Prognostic Improvement That Starts during Uptitration. Eur. J. Clin. Pharmacol. 2023, 79, 1173–1184. [Google Scholar] [CrossRef]
- Mapelli, M.; Mattavelli, I.; Salvioni, E.; Capra, N.; Mantegazza, V.; Garlaschè, A.; Campodonico, J.; Rubbo, F.M.; Paganin, C.; Capovilla, T.M.; et al. Dapagliflozin Effects on Exercise, Cardiac Remodeling, Biomarkers, and Renal and Pulmonary Function in Heart Failure Patients: Not as Good as Expected? Front. Cardiovasc. Med. 2025, 12, 1542870. [Google Scholar] [CrossRef]
- Corrà, U.; Agostoni, P.; Giordano, A.; Cattadori, G.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; et al. The Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes (MECKI) Score and Prognosis in Heart Failure. A Validation Study. Int. J. Cardiol. 2016, 203, 1067–1072. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Miliopoulos, D.; Piotrowicz, E.; Snoek, J.A.; Panagopoulou, N.; Nanas, S.; Niederseer, D.; Mazaheri, R.; Ma, J.; Chen, Y.; et al. International Validation of the Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes (MECKI) Score in Heart Failure. Eur. J. Prev. Cardiol. 2023, 30, 1371–1379. [Google Scholar] [CrossRef]
- Agostoni, P.; Pluchinotta, F.R.; Salvioni, E.; Mapelli, M.; Galotta, A.; Bonomi, A.; Magrì, D.; Perna, E.; Paolillo, S.; Corrà, U.; et al. Heart Failure Patients with Improved Ejection Fraction: Insights from the MECKI Score Database. Eur. J. Heart Fail. 2023, 25, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, S.; Veglia, F.; Salvioni, E.; Corrà, U.; Piepoli, M.; Lagioia, R.; Limongelli, G.; Sinagra, G.; Cattadori, G.; Scardovi, A.B.; et al. Heart Failure Prognosis over Time: How the Prognostic Role of Oxygen Consumption and Ventilatory Efficiency during Exercise Has Changed in the Last 20 Years. Eur. J. Heart Fail. 2019, 21, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, B.; Piepoli, M.; Galotta, A.; Sciomer, S.; Zaffalon, D.; Filomena, D.; Vignati, C.; Contini, M.; Alimento, M.; Baracchini, N.; et al. The Importance of Re-Evaluating the Risk Score in Heart Failure Patients: An Analysis from the Metabolic Exercise Cardiac Kidney Indexes (MECKI) Score Database. Int. J. Cardiol. 2023, 376, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Agarwal, S. Applying the Seattle Heart Failure Model in the Office Setting in the Era of Electronic Medical Records. Circ. J. 2018, 82, 724–731. [Google Scholar] [CrossRef]
- Ketchum, E.S.; Moorman, A.J.; Fishbein, D.P.; Mokadam, N.A.; Verrier, E.D.; Aldea, G.S.; Andrus, S.; Kenyon, K.W.; Levy, W.C. Predictive Value of the Seattle Heart Failure Model in Patients Undergoing Left Ventricular Assist Device Placement. J. Heart Lung Transplant. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Siedlecki, Ł.; Gąsior, M. Prognostic Scales in Advanced Heart Failure. Pol. J. Cardio-Thorac. Surg. 2018, 15, 183–187. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Giamouzis, G.; Smith, A.L.; Agha, S.A.; Waheed, S.; Laskar, S.; Puskas, J.; Dunbar, S.; Vega, D.; et al. Utility of the Seattle Heart Failure Model in Patients with Advanced Heart Failure. J. Am. Coll. Cardiol. 2009, 53, 334–342. [Google Scholar] [CrossRef]
- Li, S.; Marcus, P.; Núñez, J.; Núñez, E.; Sanchis, J.; Levy, W.C. Validity of the Seattle Heart Failure Model after Heart Failure Hospitalization. ESC Heart Fail. 2019, 6, 509–515. [Google Scholar] [CrossRef]
- Levy, W.C.; Aaronson, K.D.; Dardas, T.F.; Williams, P.; Haythe, J.; Mancini, D. Prognostic Impact of the Addition of Peak Oxygen Consumption to the Seattle Heart Failure Model in a Transplant Referral Population. J. Heart Lung Transplant. 2012, 31, 817–824. [Google Scholar] [CrossRef]
- Dardas, T.; Li, Y.; Reed, S.D.; O’Connor, C.M.; Whellan, D.J.; Ellis, S.J.; Schulman, K.A.; Kraus, W.E.; Forman, D.E.; Levy, W.C. Incremental and Independent Value of Cardiopulmonary Exercise Test Measures and the Seattle Heart Failure Model for Prediction of Risk in Patients with Heart Failure. J. Heart Lung Transplant. 2015, 34, 1017–1023. [Google Scholar] [CrossRef]
- Gama, F.; Rocha, B.; Aguiar, C.; Strong, C.; Freitas, P.; Brízido, C.; Tralhão, A.; Durazzo, A.; Mendes, M. Exercise Oscillatory Ventilation Improves Heart Failure Prognostic Scores. Heart Lung Circ. 2023, 32, 949–957. [Google Scholar] [CrossRef]
- Kirsch, M.; Iliou, M.-C.; Vitiello, D. Hemodynamic Response to Exercise Training in Heart Failure with Reduced Ejection Fraction Patients. Cardiol. Res. 2024, 15, 18–28. [Google Scholar] [CrossRef]
- Chwiedź, A.; Minarowski, Ł.; Mróz, R.M.; Razak Hady, H. Non-Invasive Cardiac Output Measurement Using Inert Gas Rebreathing Method during Cardiopulmonary Exercise Testing—A Systematic Review. J. Clin. Med. 2023, 12, 7154. [Google Scholar] [CrossRef]
- Reis, M.S.; Nasser, I.; Barroco, A.; Berton, D.C.; Neder, J.A.; Arena, R.; Borghi-Silva, A. Ventilatory and Near-Infrared Spectroscopy Responses Similarly Determine Anaerobic Threshold in Patients with Heart Failure. J. Cardiopulm. Rehabil. Prev. 2020, 40, E18–E21. [Google Scholar] [CrossRef]
- Agostoni, P.; Chiesa, M.; Salvioni, E.; Emdin, M.; Piepoli, M.; Sinagra, G.; Senni, M.; Bonomi, A.; Adamopoulos, S.; Miliopoulos, D.; et al. The Chronic Heart Failure Evolutions: Different Fates and Routes. ESC Heart Fail. 2025, 12, 418–433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttini, F.; Pezzuto, B.; Vignati, C. Cardiopulmonary Exercise Testing in the Prognostic Assessment of Heart Failure: From a Standardized Approach to Tailored Therapeutic Strategies. Medicina 2025, 61, 1770. https://doi.org/10.3390/medicina61101770
Puttini F, Pezzuto B, Vignati C. Cardiopulmonary Exercise Testing in the Prognostic Assessment of Heart Failure: From a Standardized Approach to Tailored Therapeutic Strategies. Medicina. 2025; 61(10):1770. https://doi.org/10.3390/medicina61101770
Chicago/Turabian StylePuttini, Fiorella, Beatrice Pezzuto, and Carlo Vignati. 2025. "Cardiopulmonary Exercise Testing in the Prognostic Assessment of Heart Failure: From a Standardized Approach to Tailored Therapeutic Strategies" Medicina 61, no. 10: 1770. https://doi.org/10.3390/medicina61101770
APA StylePuttini, F., Pezzuto, B., & Vignati, C. (2025). Cardiopulmonary Exercise Testing in the Prognostic Assessment of Heart Failure: From a Standardized Approach to Tailored Therapeutic Strategies. Medicina, 61(10), 1770. https://doi.org/10.3390/medicina61101770






