Abstract
Background and Objectives: Sepsis-induced cardiac dysfunction (SICD) is a transient cardiac disfunction, with variable described prevalence and uncertain prognostic. This study aimed to characterize the laboratory and microbiological findings in critically ill patients with sepsis who developed left ventricular (LV) or biventricular systolic dysfunction. Materials and Methods: Patients who required intensive care unit hospitalization for sepsis were screened retrospectively. Only patients with positive cultures and echocardiography performed within 24 h from admission were included. The exclusion criteria were infective endocarditis, acute coronary syndrome, history of cardiomyopathy, severe valve disease, end-stage organ or oncological disease. Cardiac function was appreciated on transthoracic echocardiography, using LV ejection fraction for the left ventricle and tricuspid annular plane systolic excursion (TAPSE) for the right ventricle. SICD was confirmed if the systolic dysfunction found upon admission was reversible within 7–10 days. Results: A total of 100 patients with positive cultures were included. The median age was 73 and 55% were male. SICD was diagnosed in 14% of patients. Patients with SICD were more likely to develop septic shock and had longer hospital and intensive care unit stay. In-hospital mortality was 44% with no significant difference between SICD and non-SICD patients. Laboratory markers upon hospital admission showed that SICD patients had significantly higher values of lactate and transaminases. Cardiac (troponin and NT-proBNP) and inflammation markers (leukocytes, neutrophils, NLR, C-reactive protein, procalcitonin) had higher values in patients with SICD but the difference did not reach statistical significance. Streptococcal infections and polymicrobial cultures were risk factors for developing SICD. Higher rates of infections with Enterobacterales were seen in the SICD group but the difference was not significant. Conclusions: SICD patients had higher lactate, inflammation, and cardiac biomarkers levels upon admission and significantly higher rates of streptococcal infections and polymicrobial cultures.
1. Introduction
Sepsis is a severe, life-threatening organ dysfunction triggered by an anomalous response to a bacterial, fungal, viral or parasitic infection [1]. Despite recent advances in hospital care and medication, sepsis remains a clinical condition with significant rates of mortality and morbidity worldwide. It is estimated that 19.7% of all deaths worldwide are due to sepsis [2]. Mortality occurs in more than one-third of patients hospitalized for sepsis and is greater when patients require intensive care unit (ICU) stays [3]. The most common origin of infection leading to sepsis is the respiratory tract, followed by the genitourinary tract, abdominal, and wound and soft tissue [4,5,6]. Bacterial infections are the most common causes of sepsis. Approximately half of the patients hospitalized with sepsis have positive cultures [7,8]. In septic patients with positive cultures, Gram-negative bacteria are more prevalent [5,9].
Sepsis can lead to a wide range of complications and organ dysfunction, including but not limited to cardiac dysfunction. Sepsis-induced cardiac dysfunction (SICD) is a type of acute, reversible cardiac dysfunction that occurs in a significant proportion of patients with sepsis [10]. The incidence varies across studies due to differences in cohort sizes and definitions used. A recent meta-analysis reported a 20% occurrence of SICD in patients with sepsis [11]. There are no universally accepted diagnostic criteria for SICD, but it is usually defined as a transient decline in left ventricular ejection fraction (LVEF) of less than 50% and at least a 10% decrease from baseline, which is reversible in 7–10 days [12].
The pathophysiology of SICD has not been fully elucidated, with ongoing research investigating a complex combination of several factors, such as pathogen and damage-associated molecular patterns, inflammation mediators, mitochondrial dysfunction, and calcium metabolism dysregulation, all resulting in myocardial cell injury [13]. In vitro and mouse studies have revealed the molecular mechanisms associated with different bacterial types and their role in causing cardiac dysfunction associated with sepsis [14,15,16,17].
The primary objective of this study was to describe the incidence and outcome implications of SICD in a cohort of culture-positive septic patients in an emergency hospital setting. The secondary purpose was to analyze laboratory markers, especially inflammation and cardiac markers, and microbiological findings in patients with SICD.
2. Methods
2.1. Study Design and Population
A retrospective, observational analysis was conducted in patients over 18 years old who presented to the Emergency Department and were admitted to the ICU with sepsis as the main diagnosis in an emergency hospital between January 2023 and October 2024. The local ethics committee of Elias University Emergency Hospital approved this study (Protocol number 45/4 January 2023). Sepsis-3 criteria were used to define sepsis and septic shock [1]. All patients with positive cultures who also had laboratory markers and transthoracic echocardiography performed within 24 h of admission were included in the analysis. The exclusion criteria were infective endocarditis, acute coronary syndrome, Takotsubo syndrome, history of heart failure with mildly reduced or reduced ejection fraction, history of cardiomyopathy, history of severe valve disease, history of inflammatory disease, end stage organ disease (liver, kidney, lung), advanced or end-stage oncological or hematological disease.
2.2. Definitions and Data Collection
The sepsis diagnosis was characterized by clinical, laboratory, microbiological, and imaging data and categorized based on the source origin of infection (community-acquired or healthcare-associated infections), the primary site of infection, and the causative microorganism. All cultures were collected upon admission. The source of infection was determined based on medical history, symptoms, and clinical examinations. Relevant cultures were collected upon admission in all patients. The types of cultures analyzed included blood, respiratory, urine, intra-abdominal, skin or soft tissue, and bone or joint samples, and they were collected according to the suspected sepsis origin. We collected demographic data (age, gender), data about comorbidities and associated conditions (lung disease, chronic kidney disease, chronic liver disease), and hematological and biochemical markers upon admission (leukocyte count, neutrophil–lymphocyte ratio (NLR), neutrophil count, lymphocyte count, platelet count, C-reactive protein (CRP), procalcitonin, alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin, lactate, troponin, NT-proBNP).
Cardiac function was assessed on transthoracic echocardiography. All exams were performed by experienced cardiologists. Left ventricular systolic dysfunction was considered in patients with a new-onset LVEF less than or equal to 45% and a decrease of at least 10% from baseline, with or without right ventricular systolic dysfunction. LVEF was assessed using the biplane Simpson method whenever possible. In patients with poor acoustic window, the LVEF was visually estimated. Right ventricular systolic dysfunction was considered in patients with a tricuspid annular systolic excursion (TAPSE) of under 17 mm. In patients with poor acoustic window, the right ventricular function was visually estimated. In surviving patients with cardiac dysfunction, follow-up TTEs were reviewed. SICD was confirmed if the systolic dysfunction was reversible within 7–10 days.
2.3. Outcomes
The following outcomes were defined: in-hospital mortality, prolonged hospital stay (over 28 days hospitalization), and prolonged ICU stay (over 7-day hospitalization in the ICU).
2.4. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). The distributions of continuous variables were assessed via the Shapiro–Wilk test. Variables with a normal distribution are reported as the means with standard deviations (SD) and non-normally distributed variables are reported as medians with interquartile ranges (IQR). Categorical variables are presented as frequencies and percentages. Comparisons between groups for normally distributed continuous variables were conducted via independent sample t tests. For non-normally distributed data, the Mann-Whitney U test was used. The chi-square test was used to compare categorical variables (or Fisher’s exact test, when frequencies were less than 5). A p-value of <0.05 was considered statistically significant. To identify risk factors associated with developing SICD, univariate logistic regression analyses were performed. Odds ratios (OR) with 95% confidence intervals (CI) were reported, and a p-value < 0.05 was considered statistically significant.
3. Results
A total of 100 patients with positive cultures were identified, with a median age 73 (IQR 64, 81), and 55% of them were male. All patients underwent transthoracic echocardiography within 24 h of admission (Table 1). SICD was diagnosed in 14% of patients, with a median age of 73 years (IQR 54, 82), and 71% of them were male. In SICD patients, the median LVEF improved from 40% (IQR 30, 44) upon admission to 58% (IQR 42.5, 63) at 7–10 days follow-up, and the median TAPSE improved from 16.5 mm (IQR 14, 19) to 19 mm (17, 20.5) (Figure 1).

Table 1.
Baseline characteristics. Statistically significant values are shown in bold. Underlined are the 2 wider categories of variables. ICU = intensive care unit; IQR = interquartile range; LVEF = left ventricular ejection fraction; OTI = orotracheal intubation; TAPSE = Tricuspid Annular Plane Systolic Excursion; SICD = sepsis-induced cardiac dysfunction; SOFA = The Sequential Organ Failure Assessment Score.
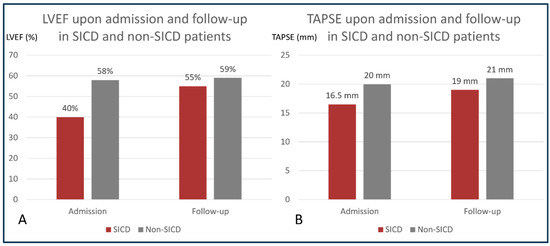
Figure 1.
Progression of left and right ventricular function in SICD and non-SICD patients. Panel (A): comparison between LVEF upon admission and LVEF at follow-up in patients with and without SICD; (B): comparison between TAPSE upon admission and TAPSE at follow-up in patients with and without SICD. LVEF = left ventricular ejection fraction; SICD = sepsis-induced cardiac dysfunction; TAPSE = tricuspid annular systolic excursion.
Patients with SICD were more likely to develop septic shock than patients without SICD. Compared with non-SICD patients, SICD patients had slightly longer hospital and intensive care unit stays (Table 1). The in-hospital mortality rate in this study cohort was 44%, and it did not differ between SICD patients and non-SICD patients.
The respiratory tract was the most prevalent infection site leading to sepsis (37%), followed by the urinary tract (29%), abdominal (14%), wound and soft tissue (12%), catheter-related (5%), bone and joint (1%), and ear, nose, and throat (1%).
Laboratory markers upon hospital admission revealed that SICD patients had significantly higher lactate, AST, and ALT levels upon admission (Table 2). Lactate levels upon admission were a predictive marker for developing SICD even after adjusting for age and sex (OR 1.5, 95% CI: 1.2, 1.96; p = 0.001) (Figure 2). “Cardiac” biomarkers, troponin, and NT-proBNP had higher values upon admission in SICD patients but the difference did not reach statistical significance. Similarly, inflammation markers (leukocytes, neutrophils, NLR, CRP, and procalcitonin) were more elevated in patients with SICD than in those without SICD, but the difference did not reach statistical significance (Table 2).

Table 2.
Microbiological and laboratory findings.
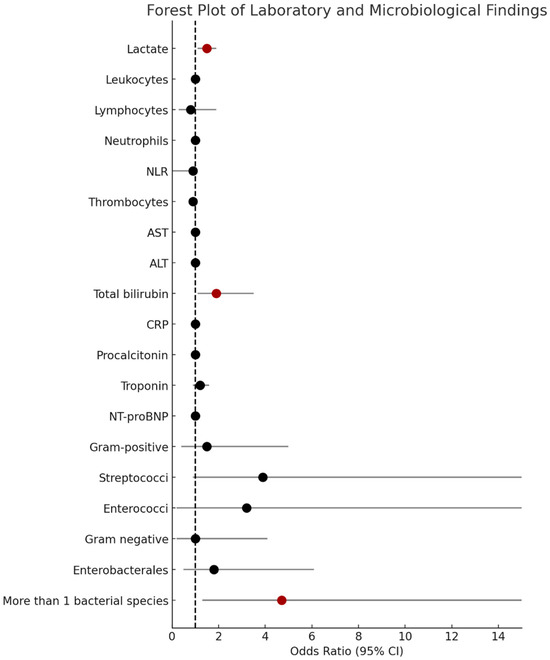
Figure 2.
Forest plot of laboratory and microbiological findings associated with SICD. This forest plot illustrates the odds ratios (OR) and the corresponding 95% confidence intervals (CI) for various laboratory and microbiological variables associated with the development of SICD. Each marker represents an individual OR, with statistically significant associations (p < 0.05) shown in dark red. ALT = alanine transaminase; AST = aspartate transaminase; NLR = neutrophil–lymphocyte ratio; CRP = C-reactive protein; SICD = sepsis-induced cardiac dysfunction.
A total of 80% of patients had blood cultures drawn, and 37% were positive. In patients with negative blood cultures, microbiological diagnosis was established based on respiratory cultures (26%), urinary cultures (24%), skin and soft tissue cultures (9%), abdominal surgical intervention (3%), and stool culture (1%). Polymicrobial cultures were identified in five (35%) SICD patients and in nine (10%) non-SICD patients (p = 0.025) and were a risk factor for developing SICD (OR 4.7, 95% CI: 1.3,17; p = 0.018) (Supplementary File). Patients with SICD had significantly higher rates of streptococcal infections: Streptococcus spp. positive cultures were obtained from 29% of the patients with SICD versus 9% of the patients without SICD (p = 0.040). The distribution of streptococcal infections in SICD patients were as follows: two patients had Streptococcus pneumoniae; one patient had Streptococcus viridans; and one patient had Streptococcus pyogenes. Streptococcal infections were associated with higher risk of developing SICD (OR 3.9, 95% CI 1–15) and a value of p = 0.051 suggesting a trend towards statistical significance. Furthermore, patients with SICD had higher rates of infections with Enterobacterales, which was mainly explained by higher rates of Escherichia coli (E. coli) and Proteus mirabilis, but the difference did not reach statistical significance (Table 2).
There were no patients in the SICD group with positive Staphylococcus spp. cultures (either methicillin-sensitive Staphylococcus aureus—MSSA, or methicillin-resistant Staphylococcus aureus—MRSA).
4. Discussion
The aim of this retrospective analysis was to identify patients with cardiac dysfunction associated with sepsis and to discuss their outcomes, laboratory, and microbiological findings.
Age did not differ between the two groups, and the majority of patients who developed SICD were male (71%). Previous reports showed that males seem to be more susceptible to bacterial infection and that there is also a male predominance in patients who develop SICD [18,19].
Mortality was significant—44% of patients during their hospital stay—but it did not differ between SICD and non-SICD patients. Previous studies have shown variable results regarding the prognostic implications for patients who develop SICD. Many indicated that patients who develop SICD have worse outcomes [11]. In this case, the similar mortality rates between patients with SICD vs. without SICD can be explained by the limited sample size but also by the significant mortality rates in sepsis, even in the absence of cardiac dysfunction. Poor outcomes in sepsis have been documented in previous studies, with in-hospital mortality rates of almost 42% in patients with sepsis treated in the ICU [3]. In the current study, patients who developed cardiac dysfunction had longer hospital and ICU stays and were more likely to develop septic shock, which suggests a more severe disease course. An important proportion of the study group developed septic shock (68%). Patients with cardiac dysfunction had higher rates of septic shock (93%), which suggests increased infection severity and higher grade of multiorgan dysfunction.
Patients with SICD had significantly higher lactate levels upon admission than the non-SICD patients, and lactate was found to be a significant predictor of SICD, similar to earlier studies [18,20,21]. Elevated lactate was associated with higher rates of organ dysfunction and worse outcome in septic patients, and measuring lactate levels upon hospital admission is currently recommended in all septic patients [1,22].
Previous studies proposed that SICD patients presented higher inflammation levels. In a study by Sato et al., C-reactive protein levels upon admission were higher in SID patients [20]. In the present study, even though it did not reach statistical significance, SICD patients had clearly higher leukocytes, neutrophils, NLR, CRP, and procalcitonin levels (Table 2). Infection leading to higher inflammatory response and multiple inflammation system activation can lead to cardiac dysfunction by multiple mechanisms. Bacterial infections induce an exaggerated activation of multiple inflammatory pathways which may contribute to myocardial injury and cardiac dysfunction.
Cardiac biomarkers, troponin and NTproBNP, have been analyzed in multiple sepsis cohorts. Even though troponin has an essential and well-established role in diagnosing acute myocardial infarction, positive circulating levels have been reported in other clinical instances. Positive troponin levels in SICD are not considered diagnosis criteria, as cardiac dysfunction can occur even in the absence of troponin elevation. However, measuring troponin upon hospital admission or in the first 24 h does give prognostic information in septic patients and mildly elevated values suggestive of myocardial injury raises the suspicion of cardiac dysfunction [20,23,24,25]. Although NT-proBNP is a key maker in diagnosing heart failure, high values have also been described in septic patients, triggered by myocardial stretch, and have been associated with short- and long-term outcome [20,26]. Septic patients who developed systolic dysfunction had significantly elevated natriuretic peptide levels [20,23,27].
Even though the differences did not reach statistical significance in this study, significant observation can still be noted. There is a clear trend: patients who develop SICD tend to have higher routine laboratory inflammation markers, slightly elevated troponin, and high NT-proBNP levels upon admission. These findings might help identify patients who would benefit from early cardiac imaging.
In a retrospective septic patient cohort analysis, Hanumanthu et al. described culture positivity as an independent risk factor for developing SICD [28]. Based on the assumption that SICD rates might vary depending on the bacterial etiology of sepsis, this study explored potential associations between specific microorganisms and cardiac dysfunction. The current analysis reveals that patients who developed SICD had higher rates of streptococcal and Enterobacterales infections. Likewise, patients with cardiac dysfunction had higher rates of positive cultures with more than one bacterial species.
Overall, data on SICD in streptococcal sepsis patients are limited. One important thing to consider in patients with group A streptococcal infections is the possibility of them developing streptococcal toxic shock syndrome. In STSS, bacterial toxins act as “superantigens”, which leads to massive inflammation through excessive T cell activation and cytokine release, resulting in tissue and vascular damage, clinical shock, and organ dysfunction [29]. Streptolysin O seems to be the major streptococcal toxin responsible for cardiac dysfunction in streptococcal infections [17]. In a study comprising 13 patients with Streptococcus pyogenes respiratory and skin/soft tissue infections, 10 patients developed cardiac dysfunction [30]. Alhamdi et al. reported that circulating pneumolysin, a virulence factor expressed by Streptococcus pneumoninae, induces cardiac contractile depression in cultured cardiomyocytes and leads to elevated troponin levels in mice [31]. In the current study, patients who developed SICD had significantly higher rates of streptococcal infections than patients without SICD (29% versus 9%, respectively).
Gram-negative bacteria are significant causes of sepsis, often leading to severe infections, difficult management due to antibiotic resistance, and contributing overall to poor outcomes. Endotoxins are Gram-negative bacteria membrane components released upon bacterial lysis. They were shown to exhibit a depressor effect on the cardiovascular system and ventricular contractility in several in vitro and in vivo studies [15,32]. Suffredini et al. showed that administrating endotoxins in healthy subjects leads to impaired LV systolic function [33]. There were few cases of reported cardiac dysfunction induced by sepsis in patients with E. coli sepsis [34,35]. In our study, the proportion of Gram-negative bacteria was similar in SICD and non-SICD patients (79% vs. 78%, respectively) but patients with SICD had higher incidence of Enterobacterales infections (71% vs. 58%, respectively), with higher incidence of E. coli and Proteus mirabilis.
Interestingly, no patient with SICD had positive Staphylococcus spp. cultures (neither MRSA nor MSSA), which highlights the pathogen-specific mechanisms implicated in developing cardiac dysfunction. Even though animal studies showed the myocardial depressant effect of Staphylococcus aureus, there is currently no clearly established association between Staphylococcus infections and SICD in humans [36,37,38]. Furthermore, patients who developed cardiac dysfunction had higher rates of positive cultures with more than one microorganism, which may be attributed to higher virulence, multiple pathogenic mechanisms, and higher inflammatory response. Research regarding the association between patients who develop SICD and specific bacterial species remains scarce. Further prospective studies are needed to clarify microbiological-specific mechanisms and their contribution to developing cardiac dysfunction in septic patients.
5. Conclusions
In the present study, LV or biventricular systolic dysfunction associated with sepsis occurred in 14% of patients. Although overall mortality did not differ between SICD and non-SICD groups, patients who developed cardiac dysfunction tended to have higher rates of septic shock, and longer hospital and ICU stays, contributing to important long-term outcome implications. Patients who developed SICD had higher lactate, transaminase, inflammation, and cardiac markers levels upon hospital admission, with lactate emerging as a predictive marker even after adjusting for age and sex. The novelty of the study consists of the conjoined rise in inflammatory and cardiac biomarkers, alongside higher lactate, transaminase, and bilirubin levels. These findings point out that this profile of septic patients might be more at risk for developing cardiac involvement and early cardiac imaging should be considered in these cases. Furthermore, SICD patients had significantly higher rates of streptococcal infections and polymicrobial cultures, highlighting possible specific mechanisms implicated in the development of systolic dysfunction during sepsis.
Limitations
This study has several limitations. The relatively small sample size and retrospective nature of the analysis limit the generalizability of these findings. Obtaining good quality echographic images in critically ill patients can be difficult. The acoustic windows are operator-dependent and can be influenced by several factors, such as patient positioning, and invasive and non-invasive ventilation. Microbiological data from previous hospitalizations in other institutions were not always available, limiting the assessment of the virulence of microorganisms involved in healthcare-associated infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61101765/s1, Supplementary File: Microbiology findings in patients with polymicrobial cultures.
Author Contributions
All authors (C.P., D.O.N., M.R.P., C.C.V., E.M., S.I.N. and S.M.B.) contributed to the study conception and design. Material preparation, data collection, and analysis were performed by C.P., D.O.N. and M.R.P. The first draft of the manuscript was written by C.P., M.R.P. and E.M. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no relevant financial or non-financial interests to disclose. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the Local Ethics Committee of Elias University Emergency Hospital approved this study (Protocol number 45/4 January 2023).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study and the use of anonymized data without identifiable personal information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the Institutional Open Access Program.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction in the Abstract. This change does not affect the scientific content of the article.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA—J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Ljungström, L.; Andersson, R.; Jacobsson, G. Incidences of community onset severe sepsis, Sepsis-3 sepsis, and bacteremia in Sweden—A prospective population-based study. PLoS ONE 2019, 14, e0225700. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Mayr, F.B.; Yende, S.; Linde-Zwirble, W.T.; Peck-Palmer, O.M.; Barnato, A.E.; Weissfeld, L.A.; Angus, D.C. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA 2010, 303, 2495–2503. [Google Scholar] [CrossRef]
- Panday, R.S.N.; Lammers, E.M.J.; Alam, N.; Nanayakkara, P.W.B. An overview of positive cultures and clinical outcomes in septic patients: A sub-analysis of the Prehospital Antibiotics Against Sepsis (PHANTASi) trial. Crit. Care 2019, 23, 182. [Google Scholar] [CrossRef]
- Gupta, S.; Sakhuja, A.; Kumar, G.; McGrath, E.; Nanchal, R.S.; Kashani, K.B. Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest 2016, 150, 1251–1259. [Google Scholar] [CrossRef]
- Ohnuma, T.; Chihara, S.; Costin, B.; Treggiari, M.; Bartz, R.R.M.; Raghunathan, K.M.; Krishnamoorthy, V. Epidemiology, Resistance Profiles, and Outcomes of Bloodstream Infections in Community-Onset Sepsis in the United States. Crit. Care Med. 2023, 51, 1148–1158. [Google Scholar] [CrossRef]
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019, 155, 427–437. [Google Scholar] [CrossRef]
- Hasegawa, D.; Ishisaka, Y.; Maeda, T.; Prasitlumkum, N.; Nishida, K.; Dugar, S.; Sato, R. Prevalence and Prognosis of Sepsis-Induced Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2023, 38, 797–808. [Google Scholar] [PubMed]
- Paraschiv, C.; Moraru, M.R.P.; Paduraru, L.F.; Palcau, C.A.; Popescu, A.C.; Balanescu, S.M. Current challenges in understanding, diagnosing and managing sepsis-induced cardiac dysfunction. J. Crit. Care 2026, 91, 155250. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yu, M.-M.; Shou, S.-T.; Chai, Y.-F. Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef]
- Brown, A.O.; Singh, K.V.; Cruz, M.R.; Kaval, K.G.; Francisco, L.E.; Murray, B.E.; Garsin, D.A. Cardiac microlesions form during severe bacteremic enterococcus faecalis infection. J. Infect. Dis. 2021, 223, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, K.; Park, K.-S.; Wikström, J.; Lässer, C.; Crescitelli, R.; Shelke, G.V.; Jang, S.C.; Suzuki, S.; Bandeira, E.; Olofsson, C.S.; et al. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci. Rep. 2017, 7, 17434. [Google Scholar] [CrossRef]
- Li, Z.; Bryant, A.E.; Parimon, T.; Stevens, D.L. Cardiac dysfunction in StrepTSS: Group A streptococcus disrupts the directional cardiomyocyte-to-macrophage crosstalk that maintains macrophage quiescence. Cytokine 2012, 59, 191–194. [Google Scholar] [CrossRef]
- Bolz, D.D.; Li, Z.; McIndoo, E.R.; Tweten, R.K.; Bryant, A.E.; Stevens, D.L. Cardiac myocyte dysfunction induced by streptolysin O is membrane pore and calcium dependent. Shock 2015, 43, 178–184. [Google Scholar] [CrossRef]
- Sato, R.; Kuriyama, A.; Takada, T.; Nasu, M.; Luthe, S.K. Prevalence and risk factors of sepsis-induced cardiomyopathy A retrospective cohort study. Medicine 2016, 95, e5031. [Google Scholar] [CrossRef]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef]
- Klouche, K.; Pommet, S.; Amigues, L.; Bargnoux, A.S.; Dupuy, A.M.; Machado, S.; Serveaux-Delous, M.; Morena, M.; Jonquet, O.; Cristol, J.P. Plasma brain natriuretic peptide and troponin levels in severe sepsis and septic shock: Relationships with systolic myocardial dysfunction and intensive care unit mortality. J. Intensive Care Med. 2014, 29, 229–237. [Google Scholar] [CrossRef]
- Liang, Y.-W.; Zhu, Y.-F.; Zhang, R.; Zhang, M.; Ye, X.-L.; Wei, J.-R. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J. Clin. Cases 2021, 9, 9452–9468. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the surviving sepsis campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, G.; Gilon, D.; Meroz, Y.; Georgieva, M.; Levin, P.D.; Goodman, S.; Avidan, A.; Beeri, R.; Weissman, C.; Jaffe, A.S.; et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur. Heart J. 2012, 33, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wang, X.; Guo, T.; Gao, W.; Huang, Q.; Yang, J.; Gao, H.; Liu, Q. Cardiac troponin as a prognosticator of mortality in patients with sepsis: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e1014. [Google Scholar] [CrossRef] [PubMed]
- Gajardo, A.I.J.; Ferrière-Steinert, S.; Valenzuela Jiménez, J. Early high-sensitivity troponin elevation and short-term mortality in sepsis: A systematic review with meta-analysis. Crit. Care 2025, 29, 76. [Google Scholar] [CrossRef]
- Wang, Z.; Murad, M.H.; Sundaragiri, P.R.; Kashani, K.; Miller, W.L.; Jaffe, A.S.; Vallabhajosyula, S. Natriuretic Peptides to Predict Short-Term Mortality in Patients with Sepsis: A Systematic Review and Meta-analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 50–64. [Google Scholar]
- Landesberg, G.; Levin, P.D.; Gilon, D.; Goodman, S.; Georgieva, M.; Weissman, C.; Jaffe, A.S.; Sprung, C.L.; Barak, V. Myocardial dysfunction in severe sepsis and septic shock: No correlation with inflammatory cytokines in real-life clinical setting. Chest 2015, 148, 93–102. [Google Scholar] [CrossRef]
- Hanumanthu, B.K.J.; Nair, A.S.; Katamreddy, A.; Gilbert, J.S.; You, J.Y.; Offor, O.L.; Kushwaha, A.; Krishnan, A.; Napolitano, M.; Palaidimos, L.; et al. Sepsis-induced cardiomyopathy is associated with higher mortality rates in patients with sepsis. Acute Crit. Care 2021, 36, 215–222. [Google Scholar] [CrossRef]
- Lappin, E.; Ferguson, A.J. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 2009, 9, 281–290. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, J.C.; Chiscano-Camón, L.; Maldonado, C.; Ruiz-Sanmartin, A.; Martin, L.; Bajaña, I.; Bastidas, J.; Lopez-Martinez, R.; Franco-Jarava, C.; González-López, J.J.; et al. Catastrophic Streptococcus pyogenes Disease: A Personalized Approach Based on Phenotypes and Treatable Traits. Antibiotics 2024, 13, 187. [Google Scholar] [CrossRef]
- Alhamdi, Y.; Neill, D.R.; Abrams, S.T.; Malak, H.A.; Yahya, R.; Barrett-Jolley, R.; Wang, G.; Kadioglu, A.; Toh, C.-H. Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection. PLoS Pathog. 2015, 11, e1004836. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Vallejo, J.G.; Knuefermann, P.; Misra, A.; Defreitas, G.; Carabello, B.A.; Mann, D.L. Escherichia coli LPS-induced LV dysfunction: Role of toll-like receptor-4 in the adult heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2316–H2323. [Google Scholar] [CrossRef]
- Suffredini, A.F.; Fromm, R.E.; Parker, M.M.; Brenner, M.; Kovacs, J.A.; Wesley, R.A.; Parrillo, J.E. The Cardiovascular Response of Normal Humans to the Administration of Endotoxin. New Engl. J. Med. 1989, 321, 280–287. [Google Scholar] [CrossRef]
- Sato, K.; Naito, A.; Shiratori, T.; Yamamoto, M.; Shimane, K.; Mikami, M.; Senda, M.; Kume, H.; Suzuki, M. A case of sepsis-induced cardiomyopathy successfully treated with venoarterial extracorporeal membrane oxygenation. IJU Case Rep. 2023, 6, 26–29. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, S.; Nag, D.S.; Diwakar, K.; Singh, N. Sepsis-Induced Cardiomyopathy Secondary to Escherichia coli Sepsis and Intestinal Perforation: A Diagnostic Challenge in a Paediatric Patient. Cureus 2024, 16, e76552. [Google Scholar] [CrossRef]
- Mutig, N.; Geers-Knoerr, C.; Piep, B.; Pahuja, A.; Vogt, P.M.; Brenner, B.; Niederbichler, A.D.; Kraft, T. Lipoteichoic acid from Staphylococcus aureus directly affects cardiomyocyte contractility and calcium transients. Mol. Immunol. 2013, 56, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Knuefermann, P.; Sakata, Y.; Baker, J.S.; Huang, C.-H.; Sekiguchi, K.; Hardarson, H.S.; Takeuchi, O.; Akira, S.; Vallejo, J.G. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 2004, 110, 3693–3698. [Google Scholar] [CrossRef] [PubMed]
- Grandel, U.; Hopf, M.; Buerke, M.; Hattar, K.; Heep, M.; Fink, L.; Bohle, R.M.; Morath, S.; Hartung, T.; Pullamsetti, S.; et al. Mechanisms of cardiac depression caused by lipoteichoic acids from Staphylococcus aureus in isolated rat hearts. Circulation 2005, 112, 691–698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).