Systematic Review of Post-Viral Delayed Inflammation Associated with Hyaluronic Acid Dermal Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. The Protocol for the Systematic Review
2.2. Types of Publications
2.3. Types of Studies
2.4. Information Sources
2.5. Article Search Strategy
2.6. Inclusion and Exclusion Criteria
2.7. Selection of Studies
2.8. Population
2.9. Sequential Search Strategy
2.10. Risk-of-Bias Assessment
3. Results
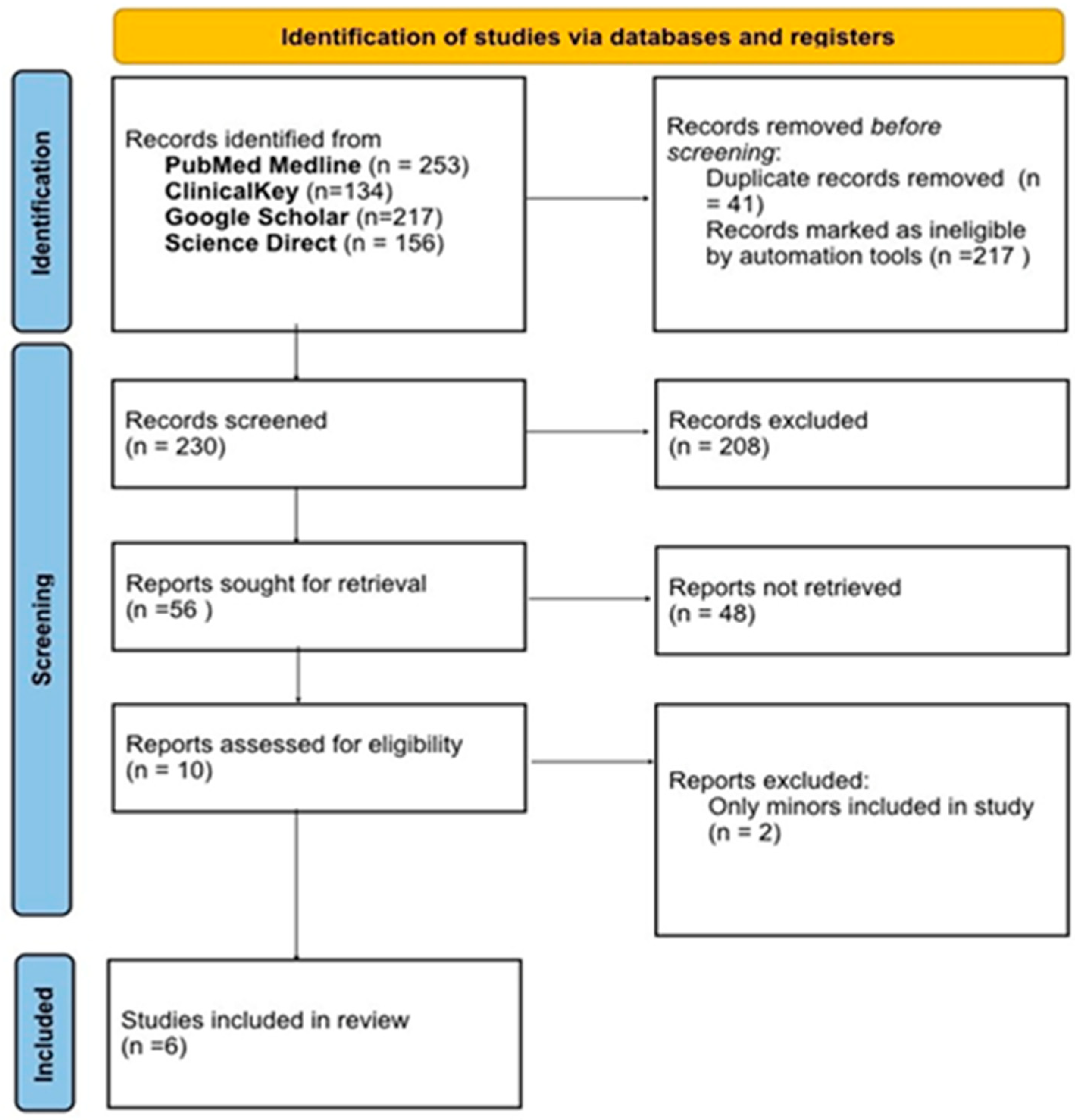
3.1. Study Selection
3.2. Quality Assessment of the Included Studies
3.3. Characteristics of Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| HA | Hyaluronic Acid |
| DIR | Delayed Inflammatory Reactions |
| SD | Standard Deviation |
References
- The American Society for Aesthetic Plastic Surgery’s Cosmetic Surgery National Data Bank: Statistics 2018. Aesthet Surg. J. 2019, 39 (Suppl. 4), 1–27. [CrossRef] [PubMed]
- Market Research Future. Hyaluronic Acid-Based Dermal Filler Market Size, Share & Trends Analysis Report by Application (Wrinkle Removal, Lip Augmentation, Rhinoplasty), Product, Region, and Segment Forecasts, 2024–2030. Published 2024. Available online: https://www.marketresearchfuture.com/reports/hyaluronic-acid-based-dermal-filler-market-42755 (accessed on 9 April 2025).
- Wongprasert, P.; Dreiss, C.A.; Murray, G. Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol Ther. 2022, 35, e15453. [Google Scholar] [CrossRef] [PubMed]
- Guinot, C.; Malvy, D.J.-M.; Ambroisine, L.; Latreille, J.; Mauger, E.; Tenenhaus, M.; Morizot, F.; Lopez, S.; Le Fur, I.; Tschachler, E. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch. Dermatol. 2002, 138, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast. Surg. 2003, 27, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H. Use of hyaluronic acid fillers for the treatment of the aging face. Clin. Interv. Aging 2007, 2, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.N.; Gupta, A. Hyaluronic Acid Fillers for Midface Augmentation: A Systematic Review. Facial Plast. Surg. 2021, 37, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Attenello, N.H.; Maas, C.S. Injectable fillers: Review of material and properties. Facial Plast. Surg. 2015, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.A.; Merrill, E.W.; Smith, K.A.; Balazs, E.A. Rheology of hyaluronic acid. Biopolymers 1968, 6, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, S.L. Understanding and using hyaluronic acid. Aesthet Surg. J. 2004, 24, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Arron, S.T.; Neuhaus, I.M. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J. Cosmet. Dermatol. 2007, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.G.; Guthridge, R.; Knutsen-Larson, S.; Brodsky, A.; Matthew, E.; Landau, M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment. Arch. Dermatol. Res. 2022, 314, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Turkmani, M.G.; De Boulle, K.; Philipp-Dormston, W.G. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin. Cosmet. Investig. Dermatol. 2019, 12, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Maxwell, C.A.; Patnaik, R. Adverse reactions to dermal fillers: Review. Dermatol. Surg. 2005, 31 Pt 2, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- De Boulle, K. Management of complications after implantation of fillers. J. Cosmet. Dermatol. 2004, 3, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Wiest, L.G.; Stolz, W.; Schroeder, J.A. Electron microscopic documentation of late changes in permanent fillers and clinical management of granulomas in affected patients. Dermatol. Surg. 2009, 35 (Suppl. 2), 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, Y.J. Foreign body granulomas after the use of dermal fillers: Pathophysiology, clinical appearance, histologic features, and treatment. Arch. Plast. Surg. 2015, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Michon, A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination—A case report. J. Cosmet. Dermatol. 2021, 20, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, L. Hyaluronic acid delayed inflammatory reaction after third dose of SARS-CoV-2 vaccine. J. Cosmet. Dermatol. 2022, 21, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Savva, D.; Battineni, G.; Amenta, F.; Nittari, G. Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS-CoV-2. Int. J. Infect. Dis. 2021, 113, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Beamish, I.V.; Bogoch, I.I.; Carr, D. Delayed inflammatory reaction to dermal fillers after COVID-19 vaccination: A case report. Can. J. Emerg. Med. 2022, 24, 444–446. [Google Scholar] [CrossRef] [PubMed]
| Component | Description |
|---|---|
| Population (P) | Patients who developed DIRs to hyaluronic acid dermal fillers following virus infection |
| Intervention (I) | Hyaluronic acid dermal filler injection |
| Comparison (C) | None |
| Outcome (O) | Adverse reactions |
| Focus question | Delayed inflammatory reactions to hyaluronic acid dermal fillers following virus infection |
| Date | Database | Keywords Used | Number of Articles Found |
|---|---|---|---|
| 19 October 2024 | Pubmed Medline | Hyaluronic Acid, Dermal Filler, Delayed inflammatory reactions following HA, DIR after Dermal Fillers following virus infection, Vaccination, COVID-19 vaccine, SARS-CoV-2 vaccine | 253 |
| 19 October 2024 | Science Direct | Hyaluronic Acid, Dermal Filler, Delayed inflammatory reactions following HA, DIR after Dermal Fillers following virus infection, Vaccination, COVID-19 vaccine, SARS-CoV-2 vaccine | 156 |
| 19 October 2024 | ClincalKey | Hyaluronic Acid, Dermal Filler, Delayed inflammatory reactions following HA, DIR after Dermal Fillers following virus infection, Vaccination, COVID-19 vaccine, SARS-CoV-2 vaccine | 134 |
| 19 October 2024 | Google Scholar | Hyaluronic Acid, Dermal Filler, Delayed inflammatory reactions following HA, DIR after Dermal Fillers following virus infection, Vaccination, COVID-19 vaccine, SARS-CoV-2 vaccine | 217 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Case reports, case series. | Studies conducted on animals or in vitro models |
| Full access to the article is available. Studies in English language. | Any other type of study: Prospective or Retrospective clinical trials, cohort studies, retrospective analyses, randomized controlled clinical trials, meta-analysis. |
| Studies in English language. | Full access to the article is not available. |
| Studies published less than 10 years ago. | Articles published in languages other than English. |
| Study | Selection of Cases | Outcome | Bias and Follow-Up | Total Score |
|---|---|---|---|---|
| Munavalli, GG et al. (2021) [12] | *** | ** | ** | 7 stars |
| Michon A. et al. (2021) [18] | ** | ** | * | 5 stars |
| Calvisi L. et al. (2022) [19] | *** | ** | ** | 7 stars |
| Savva D et al. (2021) [20] | ** | ** | * | 5 stars |
| Turkmani MG et al. (2019) [13] | * | * | * | 3 stars |
| Beamish IV et al. (2022) [21] | ** | * | * | 4 stars |
| Study | Study Type | Sample Size (Patients, (n)) | Age Mean SD | Age Range | Gender |
|---|---|---|---|---|---|
| Munavalli, GG et al. (2021) [12] | Case Series | 4 | 45 | 36–51 | Females |
| Michon A. et al. (2021) [18] | Case Series | 2 | 50 | 39–61 | Females |
| Calvisi L. et al. (2022) [19] | Case Series | 3 | 48.33 | 40–60 | Females |
| Savva D et al. (2021) [20] | Case Report | 1 | 38 | 38 | Female |
| Turkmani MG et al. (2019) [13] | Case Series | 14 | 43.5 | 22–65 | Females |
| Beamish IV et al. (2022) [21] | Case Report | 1 | 23 | 23 | Female |
| Study | Patients (n) | Type of HA Dermal Filler | Virus Infection/COVID-19 Vaccination | Post Symptoms | Time of Onset of Symptoms | Common Treatment Used | Conclusion |
|---|---|---|---|---|---|---|---|
| Munavalli, GG et al. (2021) [12] | 4 | Restylane, Voluma, Juvederm Voluma |
| Burning, facial swelling, erythema, periobital edema, body ache |
| Hylenex injection, Prednisone, doxycycline and oral corticosteroid | All cases except for one experienced DIR, treatment included corticosteroids, hyaluronidase leading to improvement. |
| Michon A. et al. (2021) [18] | 2 | Juvederm Volite, Juvederm Voluma |
| Facial swelling, erythematous swelling at tear through area |
|
| DIRs developed in both cases, hyaluronidase was administered resulting in complete resolution. |
| Calvisi L. et al. (2022) [19] | 3 | Juvederm Ultra, Juvederm Volift |
| Swelling in upper lip, angioedema, erythema and edema. | 5 days | Prednisolone | All patients experienced DIRs, 2 of them resolved spontaneously and 1 used Prednisolone. |
| Savva D et al. (2021) [20] | 1 | HA |
| Erythematous edema on both lips | 1–2 days | Methylprednisonole | Course of Methylprednisonole was used after DIRs, resulting in resolution within 5 days. |
| Turkmani MG et al. (2019) [13] | 14 | Juvederm Volbella, Juvederm Voluma | All patients (+) | Influenza-like illness. (Fever, headache, sore throat, fatigue) | Average 3–5 days | Prednisolone, Hyaluronidase | DIRs developed in all cases, corticosteroids and hyaluronidase was administered resulting in complete resolution. |
| Beamish IV et al. (2022) [21] | 1 | HA |
| Acute painful asymmetric swelling in maxilla, mandible and lips | 6 weeks | Intravenous diphenhydramine, and dexamethasone | DIRs developed post COVID-19 vaccine, antihistamine and dexamethasone were used, leading to improvement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, L.; Al Rekabi, S.; Janovskienė, A.; Stonkutė, I.; Razukevičius, D.; Stučinskaitė-Maračinskienė, J. Systematic Review of Post-Viral Delayed Inflammation Associated with Hyaluronic Acid Dermal Fillers. Medicina 2025, 61, 1764. https://doi.org/10.3390/medicina61101764
Bhatia L, Al Rekabi S, Janovskienė A, Stonkutė I, Razukevičius D, Stučinskaitė-Maračinskienė J. Systematic Review of Post-Viral Delayed Inflammation Associated with Hyaluronic Acid Dermal Fillers. Medicina. 2025; 61(10):1764. https://doi.org/10.3390/medicina61101764
Chicago/Turabian StyleBhatia, Lorena, Saja Al Rekabi, Audra Janovskienė, Inesa Stonkutė, Dainius Razukevičius, and Justina Stučinskaitė-Maračinskienė. 2025. "Systematic Review of Post-Viral Delayed Inflammation Associated with Hyaluronic Acid Dermal Fillers" Medicina 61, no. 10: 1764. https://doi.org/10.3390/medicina61101764
APA StyleBhatia, L., Al Rekabi, S., Janovskienė, A., Stonkutė, I., Razukevičius, D., & Stučinskaitė-Maračinskienė, J. (2025). Systematic Review of Post-Viral Delayed Inflammation Associated with Hyaluronic Acid Dermal Fillers. Medicina, 61(10), 1764. https://doi.org/10.3390/medicina61101764






