Efficacy and Safety of Intraosseous Versus Intravenous Antibiotic in Primary and Revision Total Joint Arthroplasty: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk-of-Bias Assessment
2.6. Statistics
3. Results
3.1. Study Identification
3.2. Study Characteristics
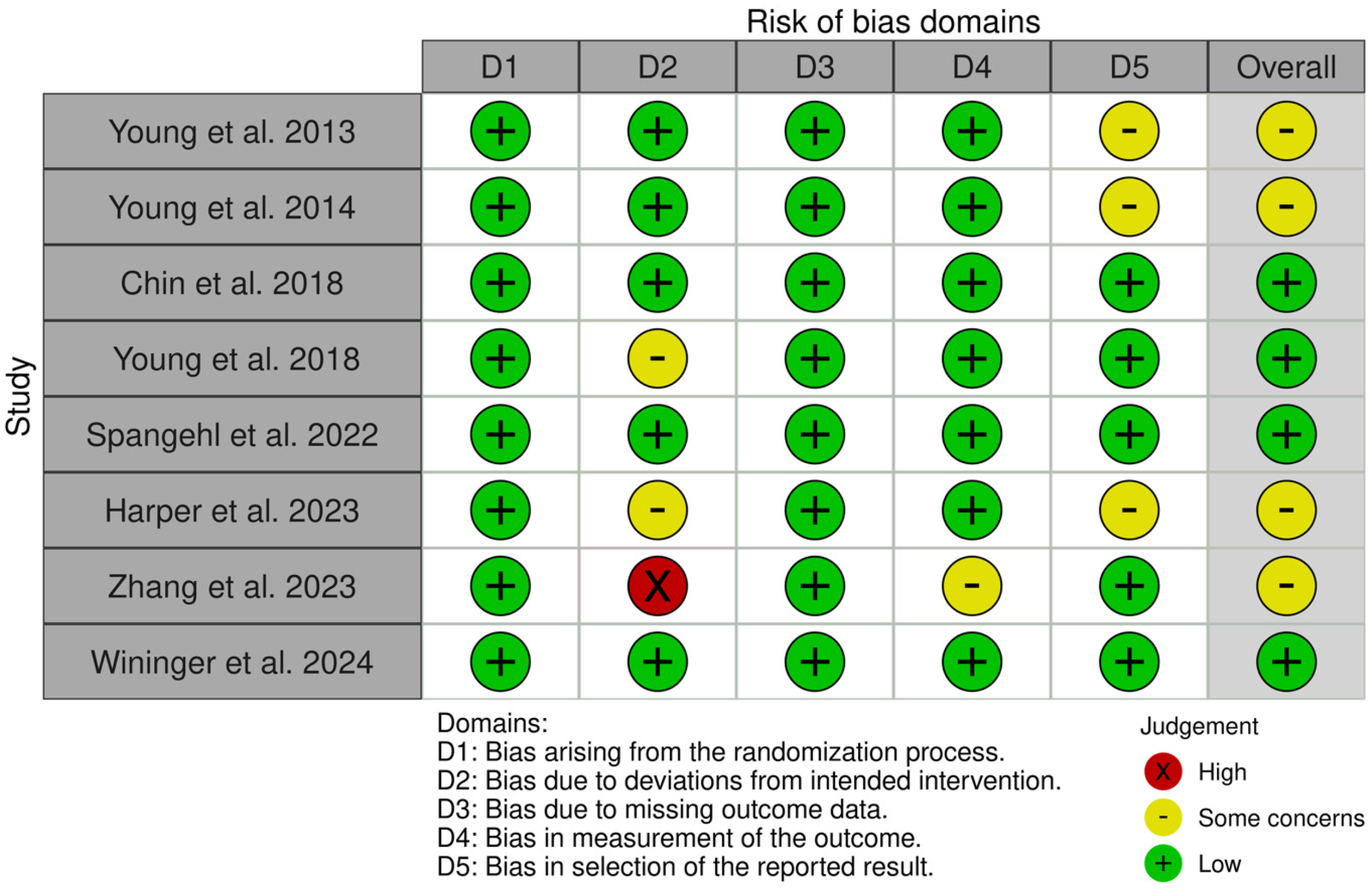
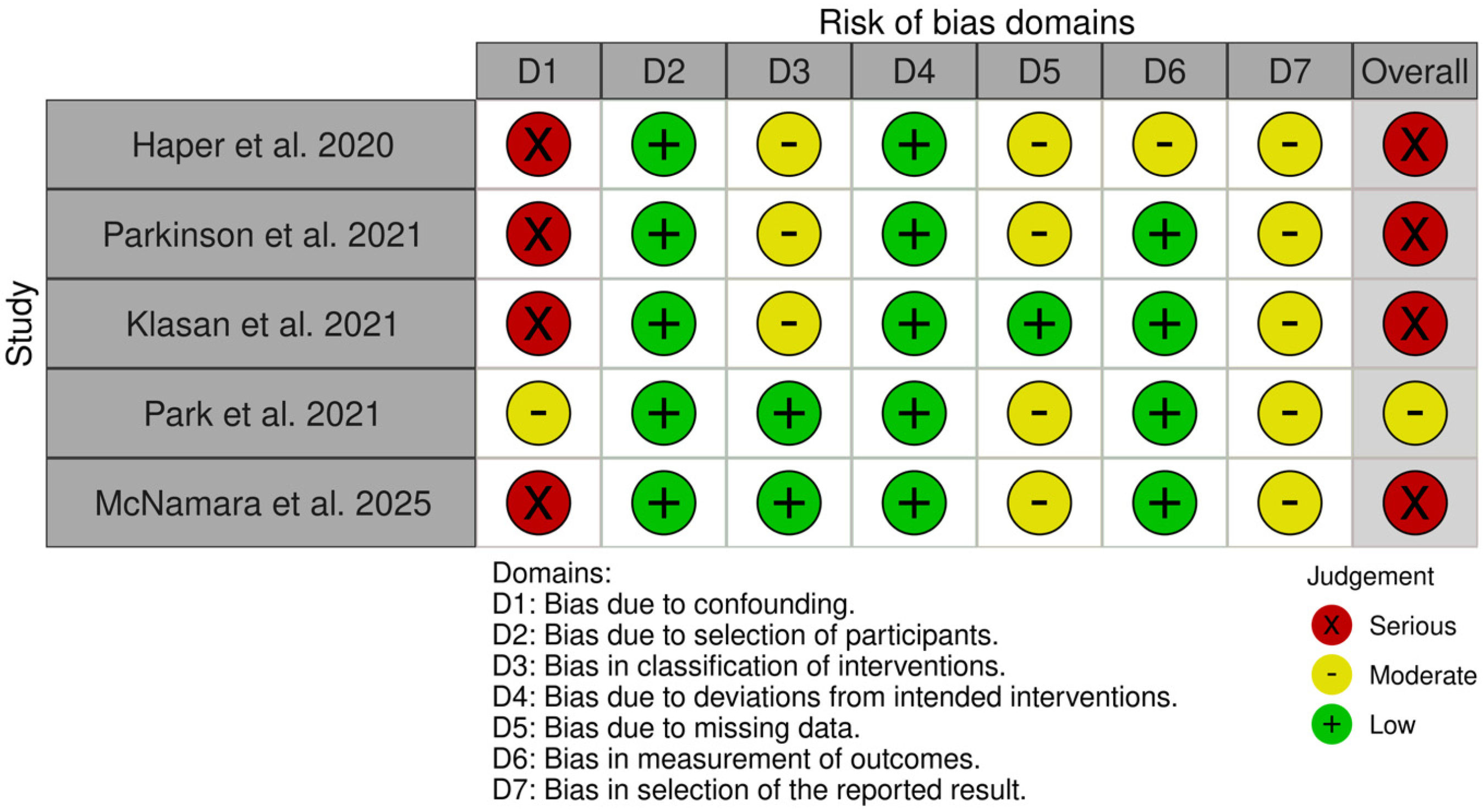
3.3. Risk of Bias
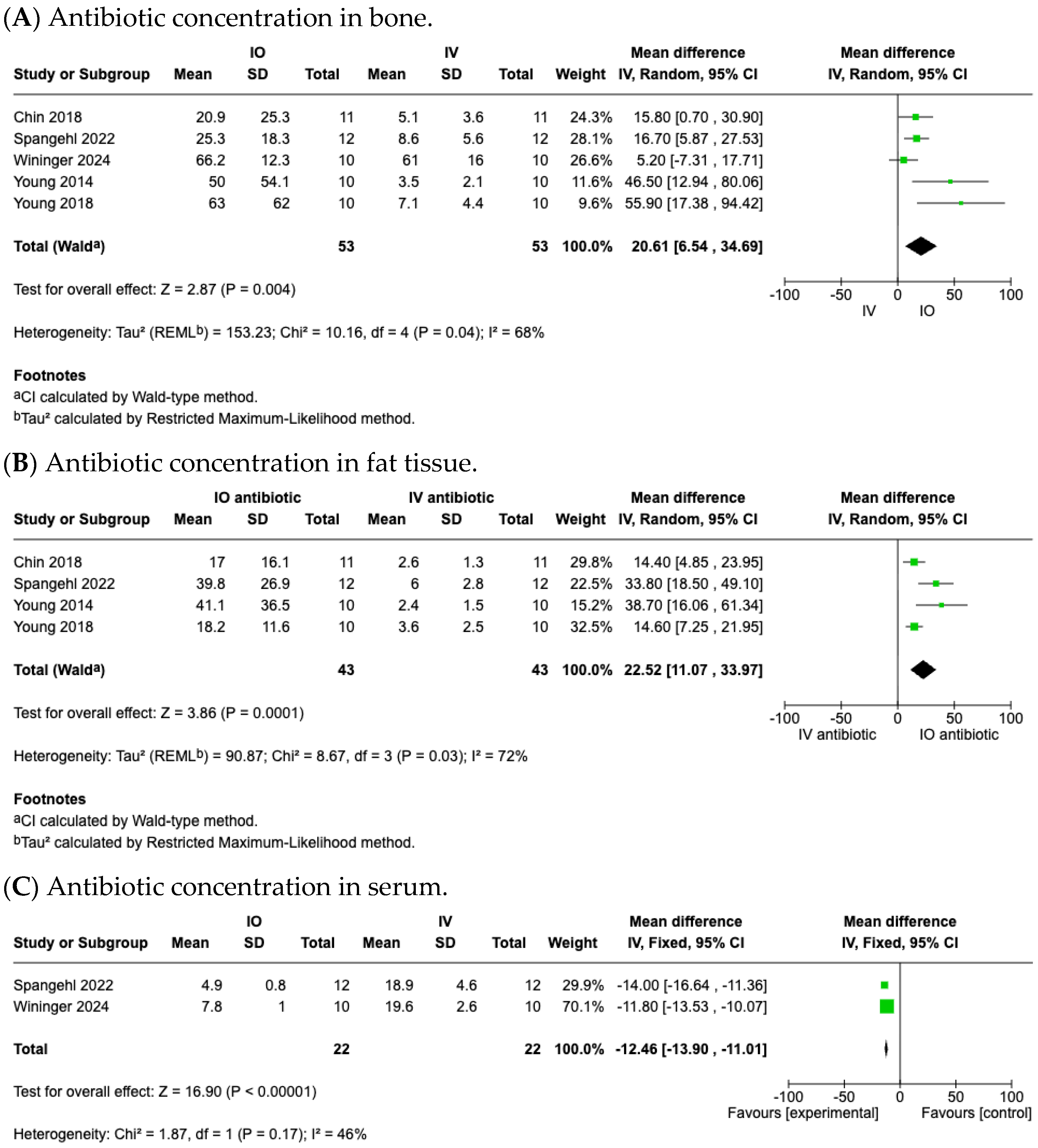
3.4. Efficacy of IO Antibiotic Prophylaxis Achieving Higher Antibiotic Concentrations
3.4.1. Antibiotic Concentration in Local Bone Tissues
3.4.2. Antibiotic Concentration in Local Fat Tissues
3.4.3. Antibiotic Concentration in Serum
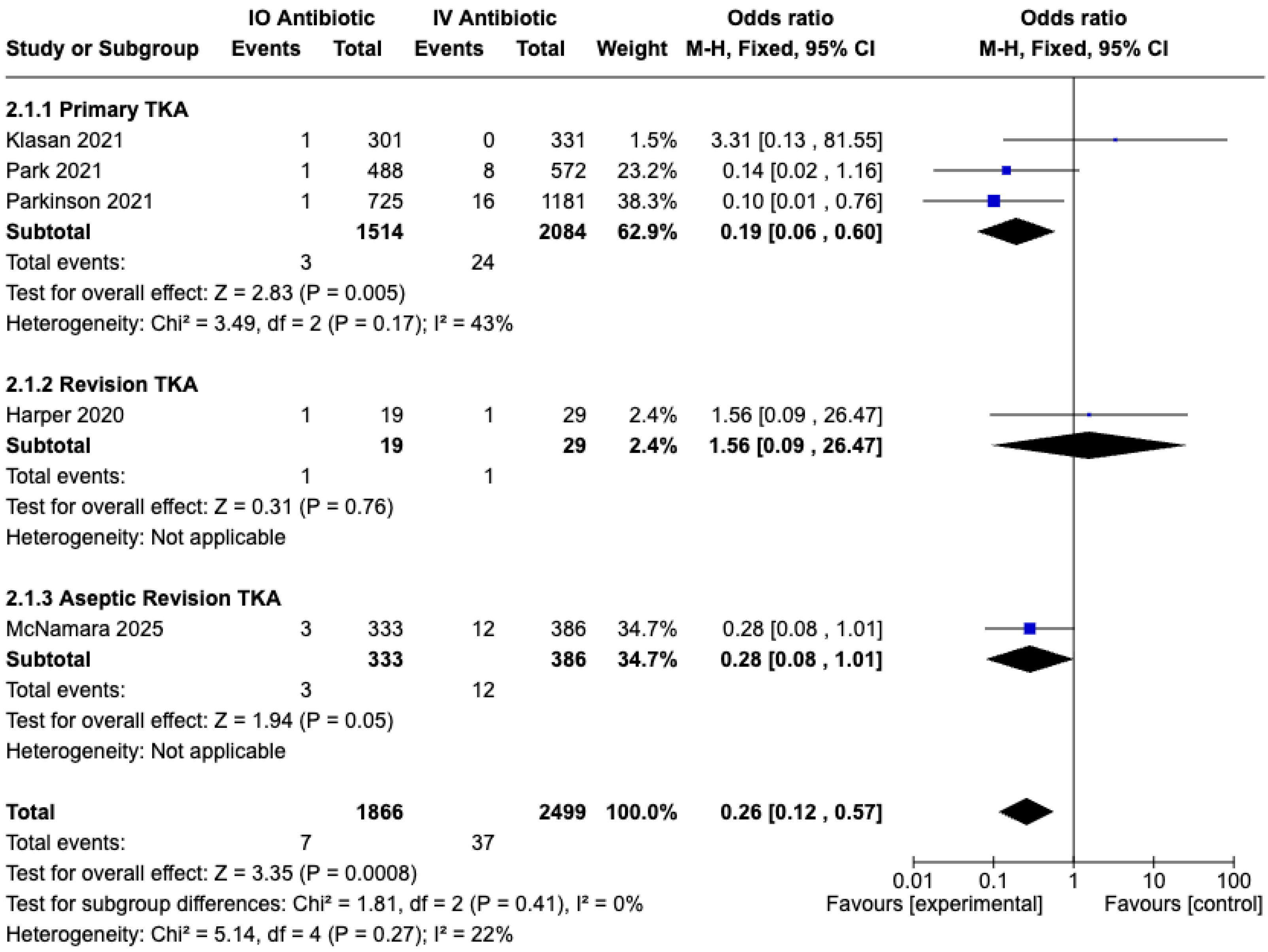
3.5. Safety of IO Antibiotic Prophylaxis in Preventing PJI and Complications
3.5.1. Prosthesis Joint Infection (PJI) Rate
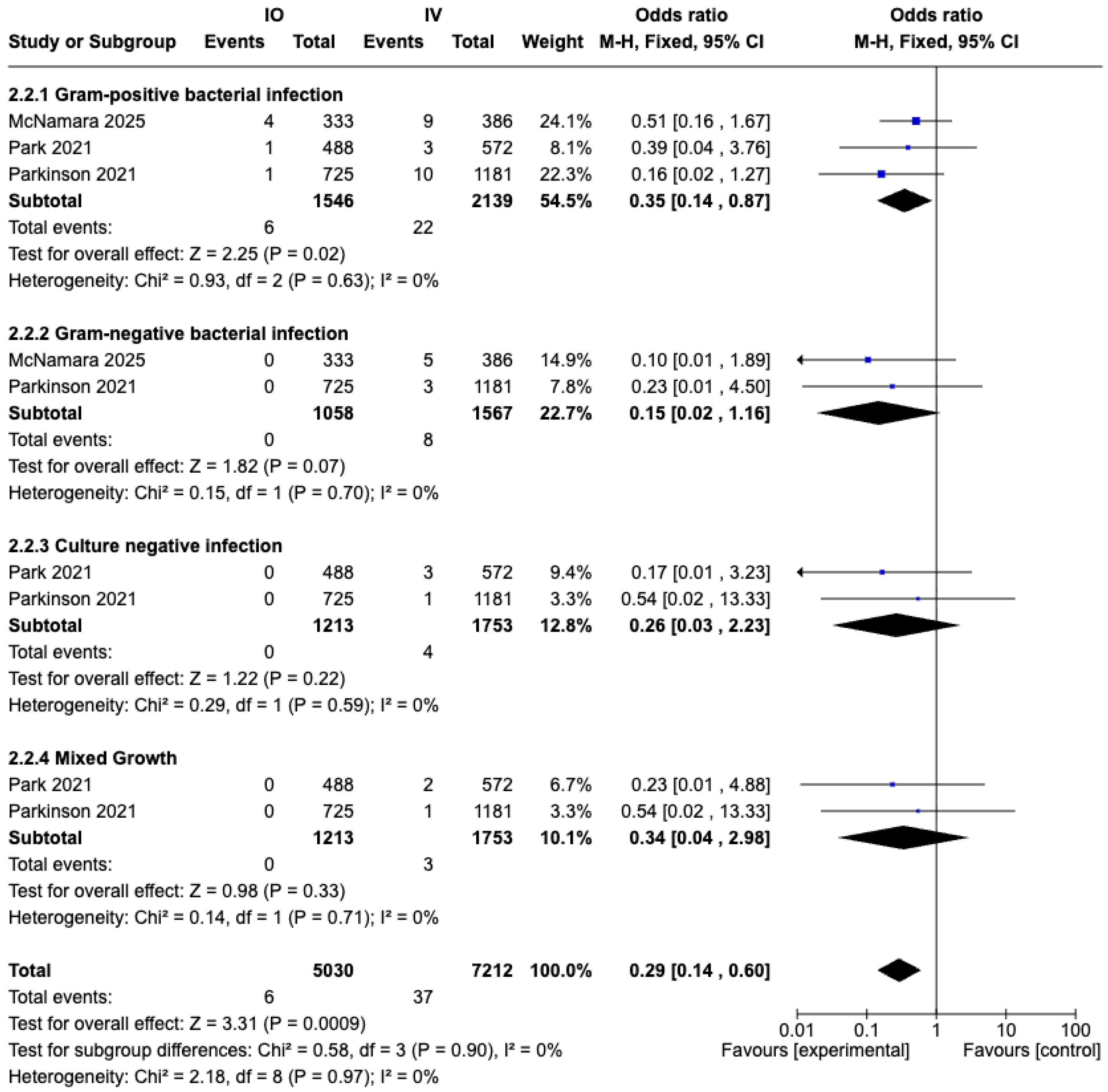
3.5.2. Prosthesis Joint Infection (PJI) Stratified by Causative Pathogens
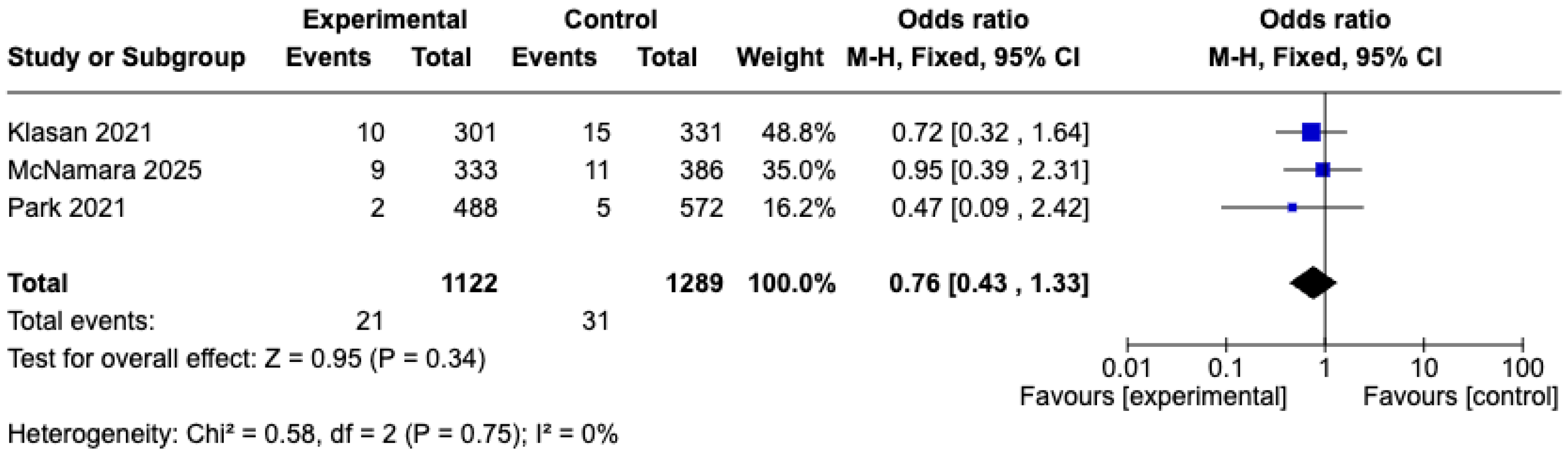
3.5.3. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THA | Total hip arthroplasty |
| TKA | Total knee arthroplasty |
| TJA | Total joint arthroplasty |
| RCT | Randomized controlled trial |
| IO | Intraosseous |
| IV | Intravenous |
| BMI | Body mass index |
| PJI | Prosthesis joint infection |
| AKI | Acute kidney injury |
| PE | Pulmonary embolism |
| DVT | Deep vein thrombosis |
| RMS | Red man syndrome |
References
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. 2018, 32, 843–859. [Google Scholar] [CrossRef]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i5–i10. [Google Scholar] [CrossRef]
- Alt, V.; Szymski, D.; Rupp, M.; Fontalis, A.; Vaznaisiene, D.; Marais, L.C.; Wagner, C.; Walter, N.; Clauss, M.; Ferrari, M.C.; et al. The health-economic burden of hip and knee periprosthetic joint infections in Europe: A comprehensive analysis following primary arthroplasty. Bone Jt. Open 2025, 6, 298–311. [Google Scholar] [CrossRef]
- DeBiase, J.M. A Clinical Review of Prosthetic Joint Infections. Med. Clin. 2025, 109, 615–623. [Google Scholar] [CrossRef]
- Liu, J.; Daher, M.; Gilreath, N.; Barrett, C.; Cohen, E.; Antoci, V. A Comparison of In-vitro Staphylococcus aureus Growth on Rough and Smooth Titanium Surfaces. Hip Pelvis 2025, 37, 197–204. [Google Scholar] [CrossRef]
- Carlson, Ä.S.; Lidgren, L.; Lindberg, L. Prophylactic antibiotics against early and late deep infections after total hip replacements. Acta Orthop. Scand. 1977, 48, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Tyllianakis, M.E.; Karageorgos, A.C.; Marangos, M.N.; Saridis, A.G.; Lambiris, E.E. Antibiotic prophylaxis in primary hip and knee arthroplasty: Comparison between cefuroxime and two specific antistaphylococcal agents. J. Arthroplast. 2010, 25, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.; Rodvold, K.A.; Solomkin, J.S. Vancomycin for surgical prophylaxis? Clin. Infect. Dis. 2012, 54, 1474–1479. [Google Scholar] [CrossRef]
- Symonds, T.; Parkinson, B.; Hazratwala, K.; McEwen, P.; Wilkinson, M.; Grant, A. Use of regional administration of prophylactic antibiotics in total knee arthroplasty. ANZ J. Surg. 2018, 88, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Feder, O.I.; Yeroushalmi, D.; Lin, C.C.; Galetta, M.S.; Meftah, M.; Lajam, C.M.; Slover, J.D.; Schwarzkopf, R.; Bosco, J.A.; Macaulay, W.B. Incomplete Administration of Intravenous Vancomycin Prophylaxis is Common and Associated with Increased Infectious Complications After Primary Total Hip and Knee Arthroplasty. J. Arthroplast. 2021, 36, 2951–2956. [Google Scholar] [CrossRef]
- Young, S.W.; Zhang, M.; Freeman, J.T.; Vince, K.G.; Coleman, B. Higher Cefazolin Concentrations with Intraosseous Regional Prophylaxis in TKA. Clin. Orthop. Relat. Res. 2013, 471, 244–249. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Patralekh, M.K.; Iyengar, K.P.; Jain, V.K. Intraosseous regional antibiotic prophylaxis in total joint arthroplasty (TJA): Systematic review and meta-analysis. J. Clin. Orthop. Trauma 2024, 57, 102553. [Google Scholar] [CrossRef]
- Yu, M.; Wei, Z.; Yang, X.; Xu, Y.; Zhu, W.; Weng, X.; Feng, B. Safety and effectiveness of intraosseous regional prophylactic antibiotics in total knee arthroplasty: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2024, 144, 4233–4245. [Google Scholar] [CrossRef]
- Lei, X.; Xiang, J.; Yang, H.; Bao, H.; Zhu, Z.; Luo, H. Intraosseous regional prophylactic antibiotics decrease the risk of infection in total knee arthroplasty compared with intravenous antibiotics: A systematic review and meta-analysis. EFORT Open Rev. 2023, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Miltenberg, B.; Ludwick, L.; Masood, R.; Menendez, M.E.; Moverman, M.A.; Pagani, N.R.; Puzzitiello, R.N.; Smith, E.L. Intraosseous Regional Administration of Antibiotic Prophylaxis for Total Knee Arthroplasty: A Systematic Review. J. Arthroplast. 2023, 38, 769–774. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Seo, H.J.; Lee, Y.J.; Jang, B.H.; Son, H.J.; Suh, H.S.; Shin, C.M. NECA’s Guidance for Undertaking Systematic Reviews and Meta-Analyses for Intervention; National Evidence-based Healthcare Collaborating Agency: Seoul, Republic of Korea, 2011. [Google Scholar]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.; Lin, L.; Chu, H.; Tong, T. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, D.; Wan, X.; Liu, Y.; Liu, J.; Bian, Z.; Tong, T. Detecting the skewness of data from the five-number summary and its application in meta-analysis. Stat. Methods Med. Res. 2023, 32, 1338–1360. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Young, S.W.; Zhang, M.; Freeman, J.T.; Mutu-Grigg, J.; Pavlou, P.; Moore, G.A. The Mark Coventry Award: Higher Tissue Concentrations of Vancomycin with Low-dose Intraosseous Regional Versus Systemic Prophylaxis in TKA: A Randomized Trial. Clin. Orthop. Relat. Res. 2014, 472, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.J.; Moore, G.A.; Zhang, M.; Clarke, H.D.; Spangehl, M.J.; Young, S.W. The AAHKS Clinical Research Award: Intraosseous Regional Prophylaxis Provides Higher Tissue Concentrations in High BMI Patients in Total Knee Arthroplasty: A Randomized Trial. J. Arthroplast. 2018, 33, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Young, S.W.; Zhang, M.; Moore, G.A.; Pitto, R.P.; Clarke, H.D.; Spangehl, M.J. The John, N. Insall Award: Higher Tissue Concentrations of Vancomycin Achieved with Intraosseous Regional Prophylaxis in Revision TKA: A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2018, 476, 66–74. [Google Scholar] [CrossRef]
- Spangehl, M.J.; Clarke, H.D.; Moore, G.A.; Zhang, M.; Probst, N.E.; Young, S.W. Higher Tissue Concentrations of Vancomycin Achieved with Low-Dose Intraosseous Injection Versus Intravenous Despite Limited Tourniquet Duration in Primary Total Knee Arthroplasty: A Randomized Trial. J. Arthroplast. 2022, 37, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.D.; Park, K.J.; Brozovich, A.A.; Sullivan, T.C.; Serpelloni, S.; Taraballi, F.; Incavo, S.J.; Clyburn, T.A. Otto Aufranc Award: Intraosseous Vancomycin in Total Hip Arthroplasty—Superior Tissue Concentrations and Improved Efficiency. J. Arthroplast. 2023, 38, S11–S15. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Yu, X.; Liu, Y.; Li, Z.; Zhang, X.; Zhong, Q.; Xu, R. Higher cefazolin concentrations in synovial fluid with intraosseous regional prophylaxis in knee arthroplasty: A randomized controlled trial. Arch. Orthop. Trauma Surg. 2024, 144, 4069–4075. [Google Scholar] [CrossRef]
- Wininger, A.E.; Gurusamy, P.; Sullivan, T.C.; Serpelloni, S.; Taraballi, F.; Park, K.J.; Brown, T.S. Intraosseous Versus Intravenous Vancomycin in Tourniquetless Primary Total Knee Arthroplasty: A Randomized Trial. J. Arthroplast. 2024, 39, S224–S228. [Google Scholar] [CrossRef]
- Harper, K.D.; Lambert, B.S.; O’dOwd, J.; Sullivan, T.; Incavo, S.J. Clinical outcome evaluation of intraosseous vancomycin in total knee arthroplasty. Arthroplast. Today 2020, 6, 220–223. [Google Scholar] [CrossRef]
- Parkinson, B.M.; McEwen, P.M.; Wilkinson, M.M.; Hazratwala, K.M.; Hellman, J.B.; Kan, H.; McLean, A.M.; Panwar, Y.M.; Doma, K.P.; Grant, A.B.; et al. Intraosseous Regional Prophylactic Antibiotics Decrease the Risk of Prosthetic Joint Infection in Primary TKA: A Multicenter Study. Clin. Orthop. Relat. Res. 2021, 479, 2504–2512. [Google Scholar] [CrossRef]
- Klasan, A.; Patel, C.K.; Young, S.W. Intraosseous Regional Administration of Vancomycin in Primary Total Knee Arthroplasty Does Not Increase the Risk of Vancomycin-Associated Complications. J. Arthroplast. 2021, 36, 1633–1637. [Google Scholar] [CrossRef]
- Park, K.J.; Chapleau, J.; Sullivan, T.C.; Clyburn, T.A.; Incavo, S.J. 2021 Chitranjan, S. Ranawat Award: Intraosseous vancomycin reduces periprosthetic joint infection in primary total knee arthroplasty at 90-day follow-up. Bone Jt. J. 2021, 103-B, 13–17. [Google Scholar] [CrossRef]
- McNamara, C.A.; Wininger, A.E.; Sullivan, T.C.; Brown, T.S.; Clyburn, T.A.; Incavo, S.J.; Park, K.J. The AAHKS Best Podium Presentation Research Award: Intraosseous Vancomycin Reduces the Rate of Periprosthetic Joint Infection Following Aseptic Revision Total Knee Arthroplasty. J. Arthroplast. 2025, 40, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Lachiewicz, P.F. Intraosseous Vancomycin May Not Be Helpful in Aseptic Revision Knee Arthroplasty: A Single-Surgeon Consecutive Case Series. J. Arthroplast. 2023, 38, S281–S283. [Google Scholar] [CrossRef] [PubMed]
- Christopher, Z.K.; Pulicherla, N.; Iturregui, J.M.; Brinkman, J.C.; Spangehl, M.J.; Clarke, H.D.; Bingham, J.S. Low Risk of Periprosthetic Joint Infection After Aseptic Revision Total Knee Arthroplasty with Intraosseous Vancomycin. J. Arthroplast. 2024, 39, S305–S309. [Google Scholar] [CrossRef]
- Hill, C.; Mazas, F.; Flamant, R.; Evrard, J. Prophylactic cefazolin versus placebo in total hip replacement. Report of a multicentre double-blind randomised trial. Lancet 1981, 317, 795–797. [Google Scholar] [CrossRef]
- Doyon, F.; Evrard, J.; Mazas, F.; Hill, C. Long-term results of prophylactic cefazolin versus placebo in total hip replacement. Lancet 1987, 329, 860. [Google Scholar] [CrossRef]
- Nickinson, R.S.J.; Board, T.N.; Gambhir, A.K.; Porter, M.L.; Kay, P.R. The microbiology of the infected knee arthroplasty. Int. Orthop. 2010, 34, 505–510. [Google Scholar] [CrossRef]
- McNamara, D.R.; Steckelberg, J.M. Vancomycin. J. Am. Acad. Orthop. Surg. 2005, 13, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, A.; Phillips, M.; Dubrovskaya, Y.; Hutzler, L.; Bosco, J. The standard one gram dose of vancomycin is not adequate prophylaxis for MRSA. Iowa Orthop. J. 2014, 34, 111–117. [Google Scholar]

| Study | Year | Design | IO | IV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Mean Age | Female (%) | Average BMI | Tourniquet Time (min) | Minimum Follow-Up (Months) | Number | Mean Age | Female (%) | Average BMI | Tourniquet Time (min) | Minimum Follow-Up (Months) | |||
| Young et al. [11] | 2013 | RCT | 11 | 71.8 | 45.5% | 27.7 | 84 | 12 | 11 | 65.3 | 63.6% | 29.1 | 82 | 12 |
| (A) Young et al. [23] | 2014 | RCT | 10 | 70.8 | 50% | 32.2 | 105 | - | 10 | 71.4 | 90% | 34.8 | 99 | - |
| (B) Young et al. [23] | 2014 | RCT | 10 | 71.7 | 60% | 30 | 102 | - | ||||||
| Chin et al. [24] | 2018 | RCT | 11 | 66 | 36.4% | 41.1 | For entire procedure | 6 | 11 | 63 | 45.5% | 40.1 | For entire procedure | 6 |
| Young et al. [25] | 2018 | RCT | 10 | 68 | 50% | 33 | 129 | - | 10 | 69 | 70% | 32 | 126 | - |
| (A) Harper et al. [30] | 2020 | Cohort R | 100 | 67 | 53% | 32 | +1.87 min than IV | 3 | 100 | 67 | 60% | 32 | - | 3 |
| (B) Harper et al. [30] | 2020 | Cohort R | 19 | 66 | 53% | 32 | - | 3 | 29 | 69 | 59% | 32 | - | 3 |
| Parkinson et al. [31] | 2021 | Cohort R | 725 | 67 | 52% | 31.5 | At least 30 min | 12 | 1181 | 67 | 49% | 31.3 | At least 30 min | 12 |
| Klasan et al. [32] | 2021 | Cohort R | 301 | 67.7 | 58.5% | 31.8 | - | 12 | 331 | 68.7 | 57.1% | 31.4 | - | 12 |
| Park et al. [33] | 2021 | Cohort R | 448 | 67.4 | 58.6% | 32.0 | +2 min than IV | 3 | 572 | 66.7 | 57.7% | 32.5 | - | 3 |
| Spangehl et al. [26] | 2022 | RCT | 12 | 69 | 41.7% | 32 | 25 | - | 12 | 68 | 58.3% | 33 | 15 | - |
| Harper et al. [27] | 2023 | RCT | 10 | 67.9 | 30% | 27.9 | - | 3 | 10 | 67.3 | 20% | 27.4 | - | 3 |
| Lachiewicz et al. [35] | 2023 | Cohort P | 20 | 67 | 10% | 34.4 | - | 3 | - | - | - | - | - | - |
| Zhang et al. [28] | 2024 | RCT | 30 | 68.9 | 80% | 25,9 | 75 | - | 30 | 67.8 | 76.7% | 25.6 | - | - |
| Wininger et al. [29] | 2024 | RCT | 10 | 69 | 70% | 29.4 | 0 | 3 | 10 | 67 | 50% | 31 | 0 | 3 |
| Christopher et al. [36] | 2024 | Cohort R | 117 | 68 | 53% | 30.2 | - | 12 | - | - | - | - | - | - |
| McNamara et al. [34] | 2025 | Cohort R | 333 | 67 | 66.4% | 32.7 | 66.3 | 3 | 386 | 66 | 58.8 | 33.2 | 66.2 | 3 |
| Study | Year | Operation | IO | IV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosage | Bone (μg/g) | Fat (μg/g) | Serum (μg/mL) | Complication | Dosage | Bone (μg/g) | Fat (μg/g) | Serum (μg/mL) | Complication | |||
| Young et al. [11] | 2013 | TKA | 1 g Cefazolin | 130 | 186 | - | No | 1 g Cefazolin | 11.4 | 10.6 | - | No |
| (A) Young et al. [23] | 2014 | TKA | 250 mg Vancomycin | 16 | 14 | - | No | 1 g Vancomycin | 4.0 | 3.2 | - | No |
| (B) Young et al. [23] | 2014 | TKA | 500 mg Vancomycin | 38 | 44 | - | 1 DVT | |||||
| Chin et al. [24] | 2018 | TKA | 500 mg Vancomycin | 34.3 | 39.3 | 1.8 | 1 PE | 15 mg/kg Vancomycin | 6.1 | 4.4 | 16.6 | No |
| Young et al. [25] | 2018 | Aseptic rTKA | 500 mg Vancomycin | 63 | 18 | - | No | 1 g Vancomycin | 7.1 | 3.6 | - | No |
| Spangehl et al. [26] | 2022 | TKA | 500 mg Vancomycin | 25.3 | 40.3 | 4.9 | No | 15 mg/kg Vancomycin | 8.6 | 6 | 18.9 | No |
| Harper et al. [27] | 2023 | THA | 500 mg Vancomycin | 130.9 | - | 5.8 | No | 15 mg/kg Vancomycin | 68 | - | 21 | No |
| Zhang et al. [28] | 2024 | TKA | 2 g Cefazolin | - | 247.9 | - | No | 2 g Cefazolin | - | 11.4 | - | No |
| Wininger et al. [29] | 2024 | TKA | 500 mg Vancomycin | 66.2 | - | 7.8 | 0 | 15 mg/kg Vancomycin | 61 | - | 19.6 | 0 |
| Study | Year | Operation | IO | IV | ||||
|---|---|---|---|---|---|---|---|---|
| Dosage | PJI Rate (%) | Complication | Dosage | PJI Rate (%) | Complication | |||
| (A) Harper et al. [30] | 2020 | TKA | 500 mg Vancomycin | 0 | 0 | 15 mg/kg Vancomycin | 0 | 1 DVT |
| (B) Harper et al. [30] | 2020 | rTKA | 500 mg Vancomycin | 5.3 | No | 15 mg/kg Vancomycin | 3.4 | No |
| Parkinson et al. [31] | 2021 | TKA | 1 g Cefazolin(46%) or 500 mg Vancomycin (54%) | 0.1 | No | 2 g Cefazolin | 1.4 | No |
| Klasan et al. [32] | 2021 | TKA | 500 mg Vancomycin | 0.3 | AKI: 3.0% | 3 × 1 g Cefazolin | 0 | AKI: 5.0% |
| Park et al. [33] | 2021 | TKA | 500 mg Vancomycin | 0.22 | No | 15 mg/kg Vancomycin | 1.5 | No |
| Lachiewicz et al. [35] | 2023 | Aseptic rTKA | 500 mg Vancomycin | 15% | No | - | - | - |
| Christopher et al. [36] | 2024 | Aseptic rTKA | 500 mg Vancomycin | 0.88% | No | - | - | - |
| McNamara et al. [34] | 2025 | Aseptic rTKA | 500 mg Vancomycin | 0.9 | DVT: 1.2% PE: 0% AKI: 2.7% | 15 mg/kg Vancomycin | 3.1 | DVT: 1.8% PE: 0.3% AKI: 2.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kang, J.; Moon, Y.; Hong, J.; Kim, H.; Jo, S. Efficacy and Safety of Intraosseous Versus Intravenous Antibiotic in Primary and Revision Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1750. https://doi.org/10.3390/medicina61101750
Lee S, Kang J, Moon Y, Hong J, Kim H, Jo S. Efficacy and Safety of Intraosseous Versus Intravenous Antibiotic in Primary and Revision Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(10):1750. https://doi.org/10.3390/medicina61101750
Chicago/Turabian StyleLee, Sunwoo, Jiyun Kang, Yonggyun Moon, Jaeyoung Hong, Hyoungtae Kim, and Suenghwan Jo. 2025. "Efficacy and Safety of Intraosseous Versus Intravenous Antibiotic in Primary and Revision Total Joint Arthroplasty: A Systematic Review and Meta-Analysis" Medicina 61, no. 10: 1750. https://doi.org/10.3390/medicina61101750
APA StyleLee, S., Kang, J., Moon, Y., Hong, J., Kim, H., & Jo, S. (2025). Efficacy and Safety of Intraosseous Versus Intravenous Antibiotic in Primary and Revision Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Medicina, 61(10), 1750. https://doi.org/10.3390/medicina61101750