Effect of Myofascial Release on Pain and Uterine Artery Hemodynamic Indices in Women with Primary Dysmenorrhea: A Randomized Controlled Trial
Abstract
1. Introduction
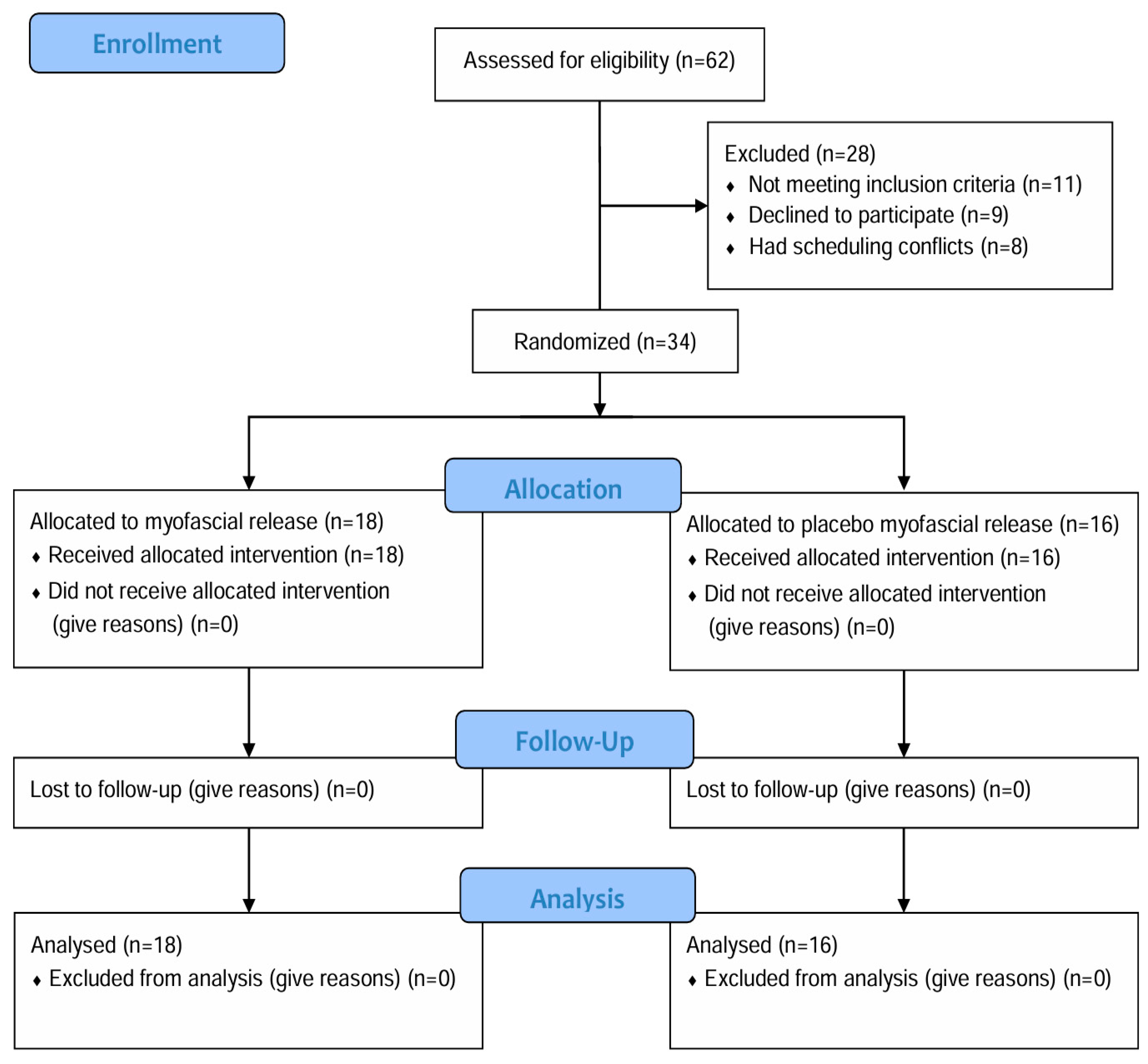
2. Methods
2.1. Study Design and Ethical Approval
2.2. Participants
2.3. Procedure
2.4. Outcome Measures
2.4.1. Primary Outcome-Menstrual Pain
2.4.2. Secondary Outcomes
Pressure Pain Threshold at Myofascial Trigger Points
Menstrual Symptom
Uterine Artery Hemodynamic Indices
2.5. Intervention
2.5.1. Thermotherapy
2.5.2. Myofascial Release
- (1)
- Pelvic diaphragm release was applied in the supine position using bilateral hand contact, with one hand placed on the sacral region and the other on the lower abdomen between the ASIS. Anteroposterior compression was applied targeting superficial and deep abdominal fasciae.
- (2)
- Anterior hip release was applied in the supine position using a cross-hand technique, with one hand on the rectus femoris and the other between the ASIS and umbilicus. Gentle pressure was followed by longitudinal traction toward the inguinal region and sustained tissue twisting until a release was perceived.
- (3)
- Abdominal fascia release was applied in the supine position using elbow contact on the anterolateral abdominal wall. Laterally directed pressure was applied in three regions (upper, middle, and lower abdomen), followed by deep fascial mobilization within the pelvic cavity until a release was perceived.
- (4)
- Quadratus lumborum fascia release was applied in the side-lying position using flat elbow pressure. Anteroposterior mobilization was followed by gentle oscillation in the same direction until a release was perceived.
- (5)
- Thoracolumbar fascia release was applied in the seated, forward-flexed position using bilateral hand contact. Sustained downward pressure was applied to the thoracolumbar junction, followed by gradual tension release until a release was perceived.
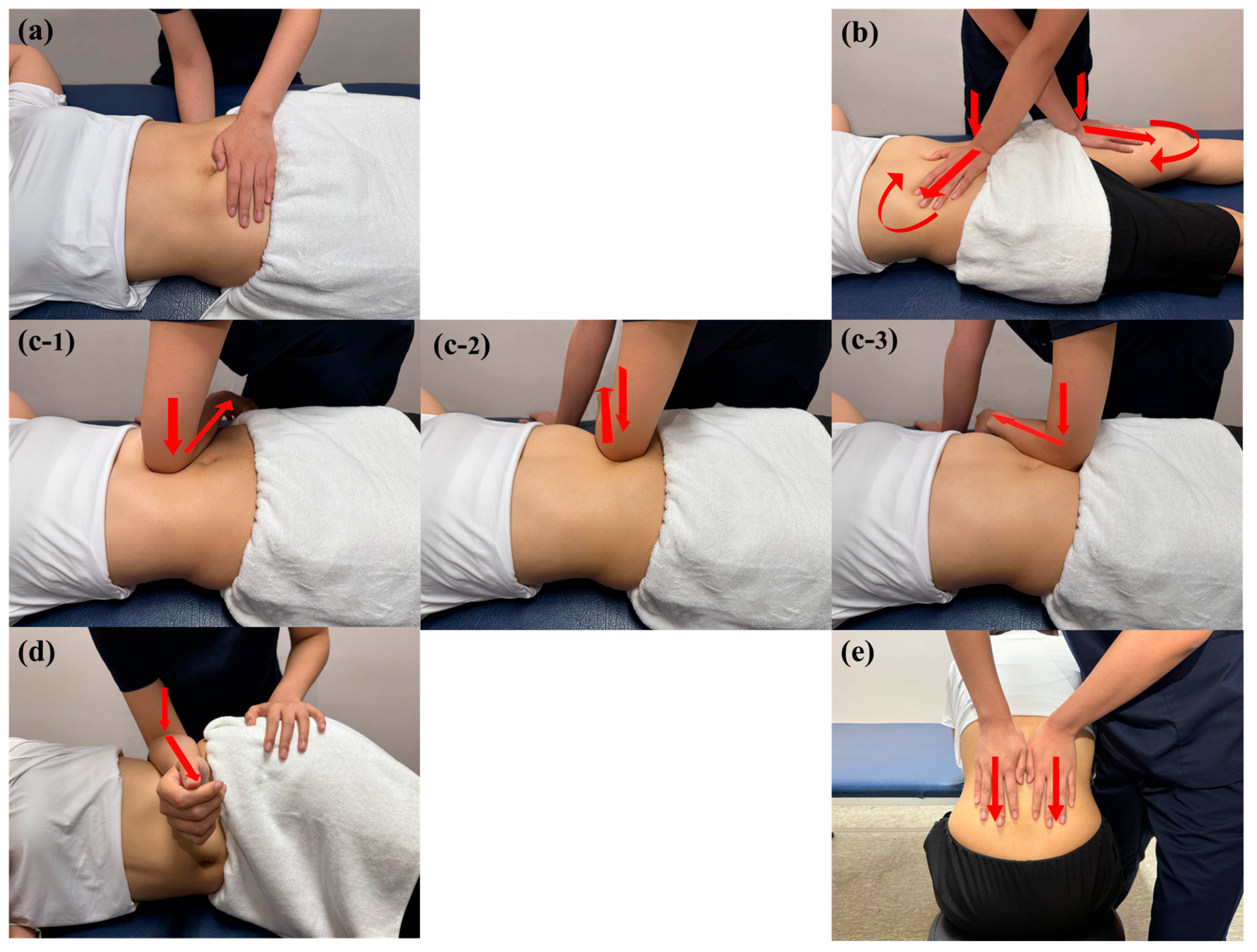
2.5.3. Placebo Myofascial Release
2.6. Statistical Analysis
3. Results
3.1. Menstrual Pain
3.2. Pressure Pain Threshold at Myofascial Trigger Points
3.3. Menstrual Symptoms
3.4. Uterine Artery Hemodynamic Indices
4. Discussion
4.1. Analgesic and Symptom-Related Effects of MFR
4.2. Vascular Responses to MFR
4.3. Clinical Implications
4.4. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horvat, M.; Pavan Jukić, D.; Marinović, L.; Bursać, D.; Ribić, R.; Neuberg, M.; Bursać, D. Prevalence of primary dysmenorrhoea and its impact on academic performance among Croatian students during the COVID-19 pandemic. Obstet. Gynecol. Int. 2023, 2023, 2953762. [Google Scholar] [CrossRef]
- Esan, D.T.; Ariyo, S.A.; Akinlolu, E.F.; Akingbade, O.; Olabisi, O.I.; Olawade, D.B.; Bamigboye, T.O.; Ogunfowokan, A.A. Prevalence of dysmenorrhea and its effect on the quality of life of female undergraduate students in Nigeria. J. Endometr. Uterine Disord. 2024, 5, 100059. [Google Scholar] [CrossRef]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M. Primary dysmenorrhea: Pathophysiology, diagnosis, and treatment updates. Korean J. Fam. Med. 2022, 43, 101. [Google Scholar] [CrossRef]
- Nagy, H.; Carlson, K.; Khan, M.A. Dysmenorrhea. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dawood, M.Y. Primary dysmenorrhea: Advances in pathogenesis and management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Elboim-Gabyzon, M.; Kalichman, L. Transcutaneous electrical nerve stimulation (TENS) for primary dysmenorrhea: An overview. Int. J. Women’s Health 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Yacubovich, Y.; Cohen, N.; Tene, L.; Kalichman, L. The prevalence of primary dysmenorrhea among students and its association with musculoskeletal and myofascial pain. J. Bodyw. Mov. Ther. 2019, 23, 785–791. [Google Scholar] [CrossRef]
- Hoyos-Calderon, Y.-T.; Martínez-Merinero, P.; Nunez-Nagy, S.; Pecos-Martín, D.; Calvo-Lobo, C.; Romero-Morales, C.; Abuín-Porras, V.; Serrano-Imedio, A. Myofascial trigger points and central sensitization signs, but no anxiety, are shown in women with dysmenorrhea: A case-control study. Biology 2022, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Özbay, K.; Semiz, A. Assessment of Uterine Blood Flow in Mild Primary Dysmenorrhea. J. Pain Res. 2024, 17, 2071–2077. [Google Scholar] [CrossRef]
- Kirsch, E.; Rahman, S.; Kerolus, K.; Hasan, R.; Kowalska, D.B.; Desai, A.; Bergese, S.D. Dysmenorrhea, a narrative review of therapeutic options. J. Pain Res. 2024, 17, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Immediate effects of myofascial release treatment on lumbar microcirculation: A randomized, placebo-controlled trial. J. Clin. Med. 2023, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef]
- Kasturi, J.; Palla, P.R.; Bakshi, V.; Bogg, N. Non-steroidal anti-inflammatory drugs: An overview. J. Drug Deliv. Ther. 2019, 9, 442–448. [Google Scholar]
- González-Mena, Á.; Leirós-Rodríguez, R.; Hernandez-Lucas, P. Treatment of women with primary dysmenorrhea with manual therapy and electrotherapy techniques: A systematic review and meta-analysis. Phys. Ther. 2024, 104, pzae019. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Wang, Y.; Wang, X.; Yu, C. Manual therapy in primary dysmenorrhea: A systematic review and meta-analysis. J. Pain Res. 2024, 17, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of physiotherapy treatment in primary dysmenorrhea: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef] [PubMed]
- Yena, K.; Eun, Y.P.; Haneul, L. The effect of myofascial release in patients with breast cancer-related lymphedema: A cross-over randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 85. [Google Scholar]
- Ajimsha, M.; Al-Mudahka, N.R.; Al-Madzhar, J. Effectiveness of myofascial release: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef]
- Moraska, A.F.; Hickner, R.C.; Kohrt, W.M.; Brewer, A. Changes in blood flow and cellular metabolism at a myofascial trigger point with trigger point release (ischemic compression): A proof-of-principle pilot study. Arch. Phys. Med. Rehabil. 2013, 94, 196–200. [Google Scholar] [CrossRef]
- Fanuscu, A.; Talu, B. Evaluation of the effect of myofascial release techniques on pain and general health status in primary dysmenorrhea. J. Hacet. Univ. Phys. Ther. Rehabil. Fac. 2023, 1, 1–8. [Google Scholar]
- Yılmaz, H.M.; Biçki, D.; Ağar, E. Comparison of the effectiveness of connective tissue massage and myofascial release technique in young adult women with primary dysmenorrhea: Comparison of connective tissue massage and myofascial release. J. Surg. Med. 2023, 7, 48–53. [Google Scholar] [CrossRef]
- Ovgun, C.D.; Tuzun, E.H. The effect of progressive muscle relaxation technique and myofascial release technique on premenstrual symptoms, blood circulation, and quality of life in women with premenstrual syndrome: A single-blind randomized controlled study. Medicine 2023, 102, e34223. [Google Scholar] [CrossRef]
- Lv, Y.; Yin, Y. A Review of the Application of Myofascial Release Therapy in the Treatment of Diseases. J. Multidiscip. Healthc. 2024, 17, 4507–4517. [Google Scholar] [CrossRef]
- Laimi, K.; Mäkilä, A.; Bärlund, E.; Katajapuu, N.; Oksanen, A.; Seikkula, V.; Karppinen, J.; Saltychev, M. Effectiveness of myofascial release in treatment of chronic musculoskeletal pain: A systematic review. Clin. Rehabil. 2018, 32, 440–450. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.C.; Vieira, L.S.; Santos, L.V.; Gaudreault, N.; Cruvinel-Júnior, R.H.; Santos, G.M. Effectiveness of visceral fascial therapy targeting visceral dysfunctions outcome: Systematic review of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, G.T.; Driusso, P.; Rodrigues, J.C.; de Godoy, A.G.; Avila, M.A. Numerical rating scale for dysmenorrhea-related pain: A clinimetric study. Gynecol. Endocrinol. 2022, 38, 661–665. [Google Scholar] [CrossRef]
- Bahreini, M.; Safaie, A.; Mirfazaelian, H.; Jalili, M. How much change in pain score does really matter to patients? Am. J. Emerg. Med. 2020, 38, 1641–1646. [Google Scholar] [CrossRef]
- van Essen, D.; Aufdemkampe, G.; Houvast, M.; Nijeboer, I. Reliability study of the microFET for pressure pain thresholds of myofascial trigger points in patients who have been operated on for herniated nucleus pulposi. Physiother. Theory Pract. 1995, 11, 81–88. [Google Scholar] [CrossRef]
- Lee, Y.; Sohng, K.-Y. Translation and Cross-Cultural Validation of Korean Version of the Menstrual Distress Questionnaire. Sage Open 2020, 10, 2158244020951550. [Google Scholar] [CrossRef]
- Markum, R.A. Assessment of the reliability of and the effect of neutral instructions on the symptom ratings on the Moos Menstrual Distress Questionnaire. Biopsychosoc. Sci. Med. 1976, 38, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; Lim, Y.; Lee, S.; Lee, H. The correlation between transperineal shear-wave elastography and transabdominal ultrasound when assessing pelvic floor function in nulliparous women. Diagnostics 2023, 13, 3002. [Google Scholar] [CrossRef]
- de Ganzo Suárez, T.; de Paco Matallana, C.; Plasencia, W. Spiral, uterine artery doppler and placental ultrasound in relation to preeclampsia. Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 92, 102426. [Google Scholar] [CrossRef]
- Drouin, O.; Johnson, J.A.; Chaemsaithong, P.; Metcalfe, A.; Huber, J.; Schwarzenberger, J.; Winters, E.; Stavness, L.; Tse, A.W.; Lu, J. Transverse technique: Complementary approach to measurement of first-trimester uterine artery Doppler. Ultrasound Obstet. Gynecol. 2018, 52, 639–647. [Google Scholar] [CrossRef]
- Hollis, B.; Mavrides, E.; Campbell, S.; Tekay, A.; Thilaganathan, B. Reproducibility and repeatability of transabdominal uterine artery Doppler velocimetry between 10 and 14 weeks of gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2001, 18, 593–597. [Google Scholar] [CrossRef]
- Zieliński, G. Effect Size Guidelines for Individual and Group Differences in Physiotherapy. Arch. Phys. Med. Rehabil. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Imedio, A.; Calvo-Lobo, C.; Casañas-Martin, C.; Garrido-Marin, A.; Pecos-Martin, D. Myofascial pain syndrome in women with primary dysmenorrhea: A case-control study. Diagnostics 2022, 12, 2723. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; David, C.B. Effect of self-myofascial release on myofascial pain, muscle flexibility, and strength: A narrative review. J. Bodyw. Mov. Ther. 2017, 21, 446–451. [Google Scholar] [CrossRef]
- Joshi, R.; Pachpute, S. Immediate Effect of Hot Pack versus Kinesiotape and Hot Pack on Pain in Primary Dysmenorrhea. Int. J. Health Sci. Res. 2021, 11, 11–16. [Google Scholar] [CrossRef]
- Jo, J.; Lee, S.H. Heat therapy for primary dysmenorrhea: A systematic review and meta-analysis of its effects on pain relief and quality of life. Sci. Rep. 2018, 8, 16252. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Clair, C.; Pirri, C.; Petrelli, L.; Zhao, X.; Sun, Y.; Macchi, V.; Stecco, C. The Human Superficial Fascia: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1289. [Google Scholar] [CrossRef]
- van Lennep, J.P.A.; Trossèl, F.; Perez, R.S.G.M.; Otten, R.H.J.; van Middendorp, H.; Evers, A.W.M.; Szadek, K.M. Placebo effects in low back pain: A systematic review and meta-analysis of the literature. Eur. J. Pain 2021, 25, 1876–1897. [Google Scholar] [CrossRef]
- Meissner, K. The placebo effect and the autonomic nervous system: Evidence for an intimate relationship. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Kuruc, R.; Szórádová, A.; Kristová, J.; Solárová, M.; Šidlo, J.; Matejčík, V. Morphological Peculiarities of the Pelvic Autonomic Nervous System and Their Impact on Clinical Interventions in the Lesser Pelvic Region. Medicina 2022, 59, 72. [Google Scholar] [CrossRef] [PubMed]

| MFR (n = 18) | Placebo MFR (n = 16) | p | |
|---|---|---|---|
| Age (years) | 22.56 ± 4.64 | 20.50 ± 1.67 | 0.093 a |
| Height (cm) | 162.11 ± 5.03 | 164.04 ± 7.14 | 0.364 a |
| Weight (kg) | 57.89 ± 6.74 | 61.38 ± 10.75 | 0.260 a |
| BMI (kg/m2) | 22.04 ± 2.55 | 22.72 ± 3.06 | 0.485 a |
| Menstrual cycle (days) | 30.28 ± 4.97 | 31.25 ± 3.22 | 0.509 a |
| Regular exercise | 0.642 b | ||
| Yes | 7 (38.9) | 5 (31.3) | |
| No | 11 (61.1) | 11 (68.8) | |
| Menstrual duration | 0.479 b | ||
| 1–3 days | 0 | 1 (6.3) | |
| 4–5 days | 10 (55.6) | 6 (37.5) | |
| 6–7 days | 8 (44.4) | 8 (50.0) | |
| 8–9 days | 0 | 1 (6.3) | |
| Menstrual blood volume (Number of pads per day) | 0.549 b | ||
| Little bleeding (<5) | 4 (22.2) | 5 (31.3) | |
| Moderate bleeding (5–7) | 12 (66.7) | 11 (68.8) | |
| Large bleeding (>7) | 2 (11.1) | 0 | |
| Pain duration | 0.604 b | ||
| <24 h | 5 (27.8) | 3 (18.8) | |
| 1–3 days | 10 (55.6) | 12 (75.0) | |
| >3 days | 3 (16.7) | 1 (6.3) | |
| Taking analgesics | 0.173 b | ||
| Every month | 13 (72.2) | 6 (37.5) | |
| Occasionally | 4 (22.2) | 7 (43.8) | |
| No | 1 (5.6) | 3 (18.8) | |
| SF-MPQ | |||
| PRI (score) | 16.56 ± 6.42 | 12.50 ± 6.54 | 0.078 a |
| Pain over the past week (score) | 4.20 ± 1.73 | 4.18 ± 2.07 | 0.977 a |
| PPI | 0.818 b | ||
| 1 | 3 (16.7) | 3 (18.8) | |
| 2 | 15 (83.3) | 12 (75.0) | |
| 3 | 0 | 1 (6.3) |
| Outcome | Groups | Within-Group Difference | Between-Group Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post 1 | Post 2 | Post 1 Minus Pre | Post 2 Minus Pre | Post 1 Minus Pre | Post 2 Minus Pre | ||||||
| MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR Minus Placebo MFR | MFR Minus Placebo MFR | |
| NRS | 3.86 (0.81) | 3.47 (0.80) | 1.52 (0.81) | 1.79 (0.80) | 1.86 (1.10) | 1.35 (1.12) | −2.34 (1.14) | −1.69 (1.13) | −2.00 (1.37) | −2.13 (1.38) | −0.65 [−1.32, 0.03] | 0.13 [−0.59, 0.84] |
| Outcome | Groups | Within-Group Difference | Between-Group Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post 1 | Post 2 | Post 1 Minus Pre | Post 2 Minus Pre | Post 1 Minus Pre | Post 2 Minus Pre | ||||||
| MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR | Placebo MFR | MFR Minus Placebo MFR | MFR Minus Placebo MFR | |
| MDQ-T | 36.94 (16.04) | 29.07 (16.08) | 10.71 (8.82) | 14.45 (8.88) | 11.16 (10.06) | 12.45 (10.08) | −26.23 (18.30) | −14.63 (18.37) | −25.78 (18.93) | −16.63 (18.98) | −11.60 [−22.37, −0.83] | −9.15 [−19.35, 1.05] |
| UA_L | ||||||||||||
| PI | 3.66 (0.72) | 3.47 (0.72) | 3.72 (0.89) | 3.30 (0.92) | 3.14 (0.85) | 3.44 (0.84) | 0.07 (1.15) | −0.17 (1.17) | −0.51 (1.11) | −0.02 (1.11) | 0.24 [−0.29, 0.76] | −0.49 [−1.06, 0.08] |
| RI | 0.98 (0.04) | 0.96 (0.04) | 0.98 (0.08) | 0.93 (0.08) | 0.95 (0.08) | 0.95 (0.08) | −0.01 (0.09) | −0.03 (0.09) | −0.04 (0.09) | −0.01 (0.09) | 0.02 [−0.02, 0.06] | −0.03 [−0.09, 0.03] |
| UA_R | ||||||||||||
| PI | 3.71 (0.72) | 3.16 (0.72) | 3.50 (0.93) | 3.04 (0.96) | 3.01 (0.76) | 3.44 (0.76) | −0.21 (1.18) | −0.12 (1.20) | −0.69 (1.05) | 0.28 (1.05) | −0.09 [−0.69, 0.51] | −0.97 [−1.54, −0.39] |
| RI | 0.98 (0.04) | 0.94 (0.08) | 0.95 (0.08) | 0.91 (0.08) | 0.93 (0.08) | 0.96 (0.08) | −0.03 (0.09) | −0.03 (0.11) | −0.05 (0.09) | 0.02 (0.11) | −0.002 [−0.05, 0.05] | −0.07 [−0.11, −0.02] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Choi, J.; Lee, H. Effect of Myofascial Release on Pain and Uterine Artery Hemodynamic Indices in Women with Primary Dysmenorrhea: A Randomized Controlled Trial. Medicina 2025, 61, 1736. https://doi.org/10.3390/medicina61101736
Jin S, Choi J, Lee H. Effect of Myofascial Release on Pain and Uterine Artery Hemodynamic Indices in Women with Primary Dysmenorrhea: A Randomized Controlled Trial. Medicina. 2025; 61(10):1736. https://doi.org/10.3390/medicina61101736
Chicago/Turabian StyleJin, Shiyu, Jongwon Choi, and Haneul Lee. 2025. "Effect of Myofascial Release on Pain and Uterine Artery Hemodynamic Indices in Women with Primary Dysmenorrhea: A Randomized Controlled Trial" Medicina 61, no. 10: 1736. https://doi.org/10.3390/medicina61101736
APA StyleJin, S., Choi, J., & Lee, H. (2025). Effect of Myofascial Release on Pain and Uterine Artery Hemodynamic Indices in Women with Primary Dysmenorrhea: A Randomized Controlled Trial. Medicina, 61(10), 1736. https://doi.org/10.3390/medicina61101736







