Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Participants
2.3. Measurement of Parameters
2.4. Statistical Analysis
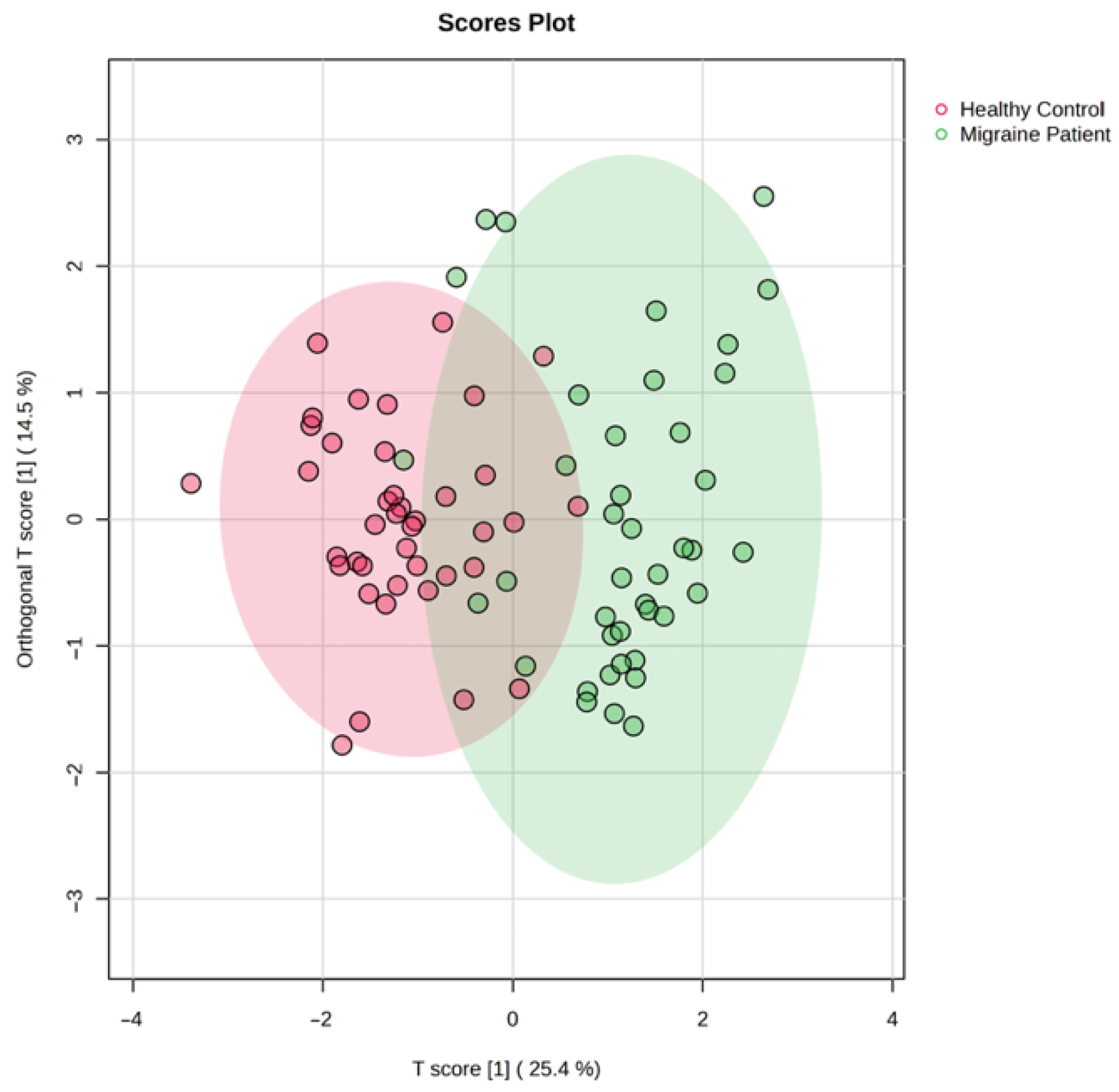
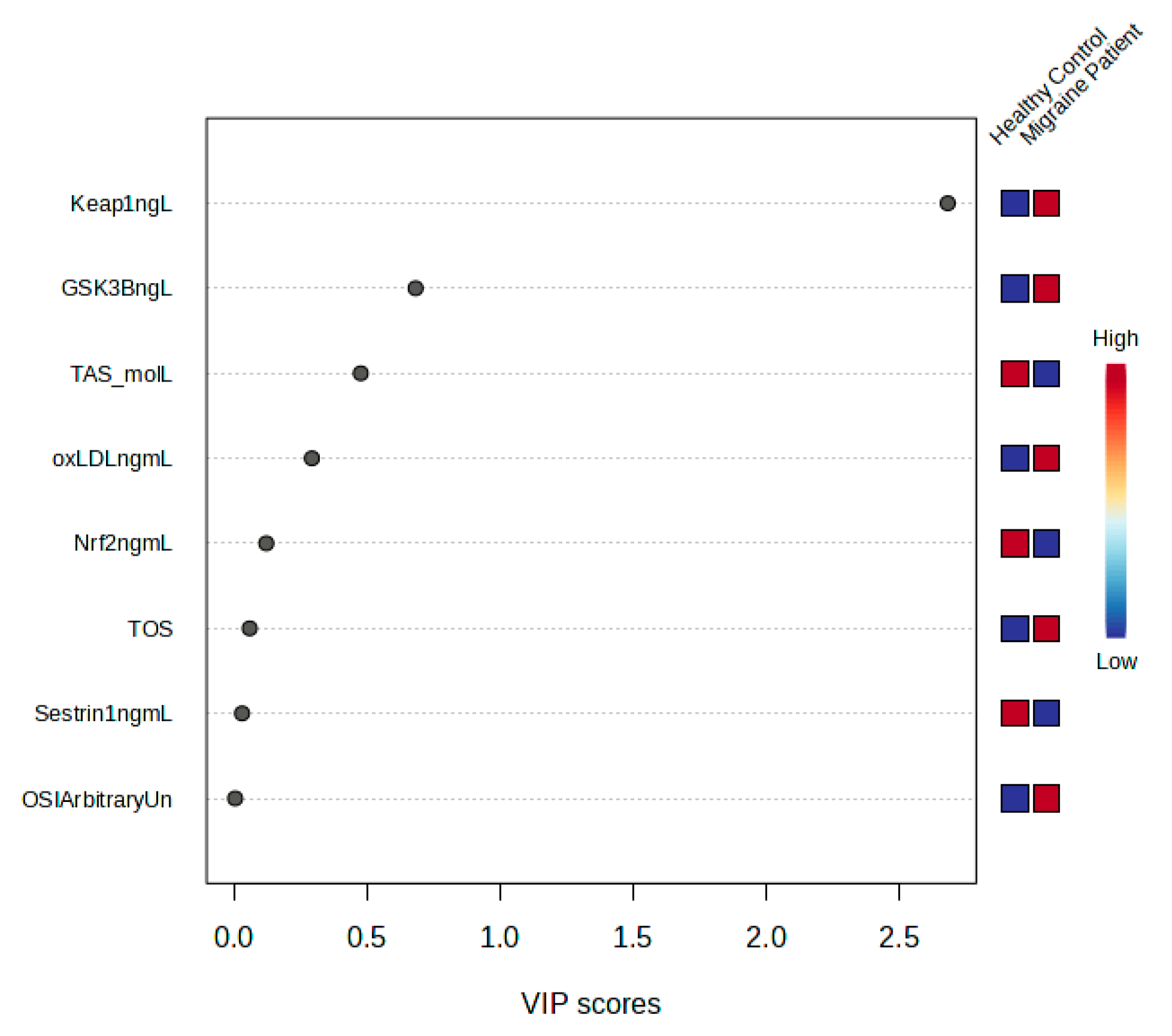
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.-C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Migraine, A. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P.; et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [PubMed]
- Hovaguimian, A.; Roth, J. Management of chronic migraine. BMJ 2022, 379, e067670. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Lipton, R.; Dodick, D.; Silberstein, S.; Saper, J.; Aurora, S.; Goadsby, P.; Charles, A. Migraine headache is present in the aura phase–a prospective study. J. Headache Pain. 2013, 14, P130. [Google Scholar] [CrossRef][Green Version]
- Natoli, J.; Manack, A.; Dean, B.; Butler, Q.; Turkel, C.; Stovner, L.; Lipton, R. Global prevalence of chronic migraine: A systematic review. Cephalalgia 2010, 30, 599–609. [Google Scholar] [CrossRef]
- Schwedt, T.J. Chronic migraine. BMJ 2014, 348, g1416. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Ashina, M.; Buse, D.C.; Ashina, H.; Pozo-Rosich, P.; Peres, M.F.; Lee, M.J.; Terwindt, G.M.; Singh, R.H.; Tassorelli, C.; Do, T.P. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet 2021, 397, 1505–1518. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Lipton, R.B.; Dodick, D.W.; Ailani, J.; McGill, L.; Hirman, J.; Cady, R. Patient-identified most bothersome symptom in preventive migraine treatment with eptinezumab: A novel patient-centered outcome. Headache J. Head Face Pain 2021, 61, 766–776. [Google Scholar] [CrossRef]
- Lampl, C.; Thomas, H.; Stovner, L.J.; Tassorelli, C.; Katsarava, Z.; Laínez, J.M.; Lantéri-Minet, M.; Rastenyte, D.; Ruiz de la Torre, E.; Andrée, C. Interictal burden attributable to episodic headache: Findings from the Eurolight project. J. Headache Pain 2016, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. A phase-by-phase review of migraine pathophysiology. Headache J. Head Face Pain 2018, 58, 4–16. [Google Scholar] [CrossRef]
- Karsan, N.; Goadsby, P.J. Biological insights from the premonitory symptoms of migraine. Nat. Rev. Neurol. 2018, 14, 699–710. [Google Scholar] [CrossRef]
- Charles, A. Migraine: A brain state. Curr. Opin. Neurol. 2013, 26, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Maniyar, F.H.; Sprenger, T.; Monteith, T.; Schankin, C.; Goadsby, P.J. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014, 137, 232–241. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016, 139, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterhout, W.; Van Someren, E.; Schoonman, G.; Louter, M.; Lammers, G.; Ferrari, M.D.; Terwindt, G. Chronotypes and circadian timing in migraine. Cephalalgia 2018, 38, 617–625. [Google Scholar] [PubMed]
- Puledda, F.; Silva, E.M.; Suwanlaong, K.; Goadsby, P.J. Migraine: From pathophysiology to treatment. J. Neurol. 2023, 270, 3654–3666. [Google Scholar] [CrossRef]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R. An Update: Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef]
- Ho, T.W.; Edvinsson, L.; Goadsby, P.J. CGRP and its receptors provide new insights into migraine pathophysiology. Nat. Rev. Neurol. 2010, 6, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the trigeminal system in migraine. Headache J. Head Face Pain. 2019, 59, 659–681. [Google Scholar]
- Zagami, A.S.; Edvinsson, L.; Goadsby, P.J. Pituitary adenylate cyclase activating polypeptide and migraine. Ann. Clin. Transl. Neurol. 2014, 1, 1036–1040. [Google Scholar] [CrossRef]
- Amin, F.M.; Hougaard, A.; Schytz, H.W.; Asghar, M.S.; Lundholm, E.; Parvaiz, A.I.; de Koning, P.J.; Andersen, M.R.; Larsson, H.B.; Fahrenkrug, J. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014, 137, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Messlinger, K.; Fischer, M.J.; Lennerz, J.K. Neuropeptide effects in the trigeminal system: Pathophysiology and clinical relevance in migraine. Keio J. Med. 2011, 60, 82–89. [Google Scholar] [CrossRef]
- Moulton, E.A.; Becerra, L.; Johnson, A.; Burstein, R.; Borsook, D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS ONE 2014, 9, e95508. [Google Scholar] [CrossRef]
- Lai, T.-H.; Fuh, J.-L.; Wang, S.-J. Cranial autonomic symptoms in migraine: Characteristics and comparison with cluster headache. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1116–1119. [Google Scholar] [CrossRef]
- Gazerani, P.; Cairns, B.E. Dysautonomia in the pathogenesis of migraine. Expert Rev. Neurother. 2018, 18, 153–165. [Google Scholar] [CrossRef]
- Burstein, R.; Jakubowski, M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J. Comp. Neurol. 2005, 493, 9–14. [Google Scholar] [CrossRef]
- Lindblad, M.; Hougaard, A.; Amin, F.M.; Ashina, M. Can migraine aura be provoked experimentally? A systematic review of potential methods for the provocation of migraine aura. Cephalalgia 2017, 37, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, A.; Amin, F.; Hauge, A.W.; Ashina, M.; Olesen, J. Provocation of migraine with aura using natural trigger factors. Neurology 2013, 80, 428–431. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Espada-Rubio, S.; Agúndez, J.A. Oxidative Stress and Migraine. Mol. Neurobiol. 2024, 61, 8344–8360. [Google Scholar] [CrossRef]
- Kowalska, M.; Prendecki, M.; Kozubski, W.; Lianeri, M.; Dorszewska, J. Molecular factors in migraine. Oncotarget 2016, 7, 50708. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, E.; Navarro-Muñoz, S.; Ayuso, P.; Rodríguez, C.; Serrador, M.; Alonso-Navarro, H.; Calleja, M.; Navacerrada, F.; Turpín-Fenoll, L.; Recio-Bermejo, M. Lack of association between common LAG3/CD4 variants and risk of migraine. Int. J. Mol. Sci. 2023, 24, 1292. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Liddell, J.R. Are astrocytes the predominant cell type for activation of Nrf2 in aging and neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef]
- Geyik, S.; Altunısık, E.; Neyal, A.M.; Taysi, S. Oxidative stress and DNA damage in patients with migraine. J. Headache Pain 2016, 17, 10. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Zobdeh, F.; Eremenko, I.I.; Akan, M.A.; Tarasov, V.V.; Chubarev, V.N.; Schiöth, H.B.; Mwinyi, J. The epigenetics of migraine. Int. J. Mol. Sci. 2023, 24, 9127. [Google Scholar] [CrossRef]
- Sharma, V.; Mehdi, M.M. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Shojaei, M.; Sahebkar, A.; Khorvash, F.; Fallahpour, S.; Askari, G.; Bagherniya, M. The effects of phytosomal curcumin supplementation on clinical symptoms, and inflammatory and oxidative stress biomarkers in patients with migraine: A protocol for a randomized double-blind placebo-controlled trial. Avicenna J. Phytomed. 2023, 13, 45. [Google Scholar] [PubMed]
- Dalkara, T.; Nozari, A.; Moskowitz, M.A. Migraine aura pathophysiology: The role of blood vessels and microembolisation. Lancet Neurol. 2010, 9, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache: J. Head Face Pain 2016, 56, 12–35. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M. Migraine, lipid profile, and cardiovascular disease. Eur. J. Neurol. 2009, 17, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.J.; Bernecker, C.; Pailer, S.; Lechner, A.; Horejsi, R.; Möller, R.; Fazekas, F.; Truschnig-Wilders, M. Lipid profile in normal weight migraineurs–evidence for cardiovascular risk. Eur. J. Neurol. 2010, 17, 419–425. [Google Scholar] [CrossRef]
- Scher, A.; Terwindt, G.; Picavet, H.; Verschuren, W.; Ferrari, M.; Launer, L. Cardiovascular risk factors and migraine: The GEM population-based study. Neurology 2005, 64, 614–620. [Google Scholar] [CrossRef]
- Azoulay-Alfaguter, I.; Elya, R.; Avrahami, L.; Katz, A.; Eldar-Finkelman, H. Combined regulation of mTORC1 and lysosomal acidification by GSK-3 suppresses autophagy and contributes to cancer cell growth. Oncogene 2015, 34, 4613–4623. [Google Scholar] [CrossRef]
- Gao, C.; Hoelscher, C.; Liu, Y.; Li, L. GSK3: A key target for the development of novel treatments for type 2 diabetes mellitus and Alzheimer disease. Prog. Neurobiol. 2011, 23, 1–11. [Google Scholar] [CrossRef]
- Li, S.-y.; Chen, X.; Chen, Y.-l.; Tan, L.; Zhao, Y.-l.; Wang, J.-t.; Xiang, Q.; Luo, A.-l. Role of GSK-3β in isoflurane-induced neuroinflammation and cognitive dysfunction in aged rats. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2013, 33, 530–535. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I.; Adlard, P.A. GSK-3 in neurodegenerative diseases. Int. J. Alzheimer’s Dis. 2011, 2011, 189246. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Hemmati, F.; Ibrahim, N.M.; Rahmani, B.; Mohamed, Z.; Raymond, A.A.; Dargahi, L.; Ghasemi, R.; Ahmadiani, A. Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson’s disease. Pharmacol. Res. 2015, 97, 16–26. [Google Scholar] [CrossRef]
- Chen, S.-D.; Yang, J.-L.; Lin, T.-K.; Yang, D.-I. Emerging roles of sestrins in neurodegenerative diseases: Counteracting oxidative stress and beyond. J. Clin. Med. 2019, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Y.; Liu, J.; Ye, J.; Yuan, W.; Jiang, H.; Wang, Z.; Jiang, H.; Wan, J. Recent insights into the biological functions of sestrins in health and disease. Cell. Physiol. Biochem. 2018, 43, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Lee, J.H.; Karin, M. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2010, 2, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Cho, C.-S.; Namkoong, S.; Cho, U.-S.; Lee, J.H. Biochemical basis of sestrin physiological activities. Trends Biochem. Sci. 2016, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]


| Migraine Group | Control Group | p a | |||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
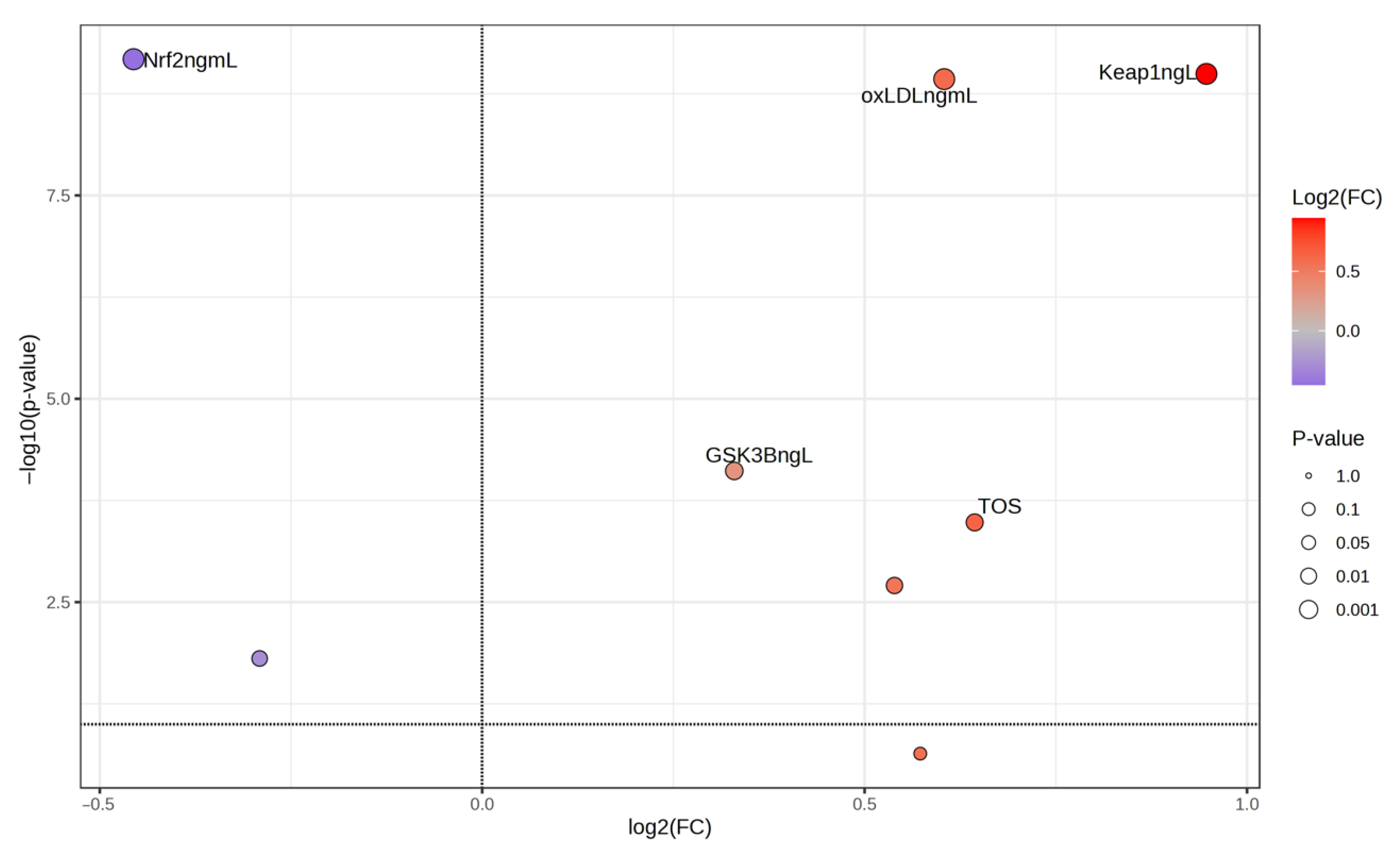
| GSK-3β (ng/L) | 209.83 | 385.53 | 285.87 ± 39.39 | 174.53 | 327.78 | 242.41 ± 37.27 | 0.001 ** |
| Keap1 (ng/L) | 353.79 | 571.10 | 480.65 ± 42.24 | 153.90 | 493.54 | 257.3 ± 81.57 | 0.001 ** |
| Nrf2 (ng/mL) | 22.04 | 34.67 | 28.92 ± 3.09 | 30.22 | 46.10 | 39.22 ± 3.31 | 0.001 ** |
| oxLDL (ng/mL) | 42.65 | 86.90 | 72.74 ± 8.64 | 44.17 | 65.31 | 52.22 ± 4.52 | 0.001 ** |
| Sestrin1 (ng/mL) | 7.37 | 16.91 | 12.54 ± 3.27 | 10.01 | 21.23 | 14.69 ± 2.41 | 0.012 * |
| TOS (μmol/L) | 5.77 | 28.53 | 14.06 ± 5.74 | 5.13 | 14.80 | 10.16 ± 2.4 | 0.001 ** |
| TAS (μmol/L) | 675.10 | 1076.50 | 877.54 ± 87.86 | 719.80 | 1286.00 | 973.4 ± 140.94 | 0.001 ** |
| OSI (Arbitrary Unit) | 0.57 | 3.62 | 1.62 ± 0.69 | 0.51 | 1.79 | 1.07 ± 0.31 | 0.001 ** |
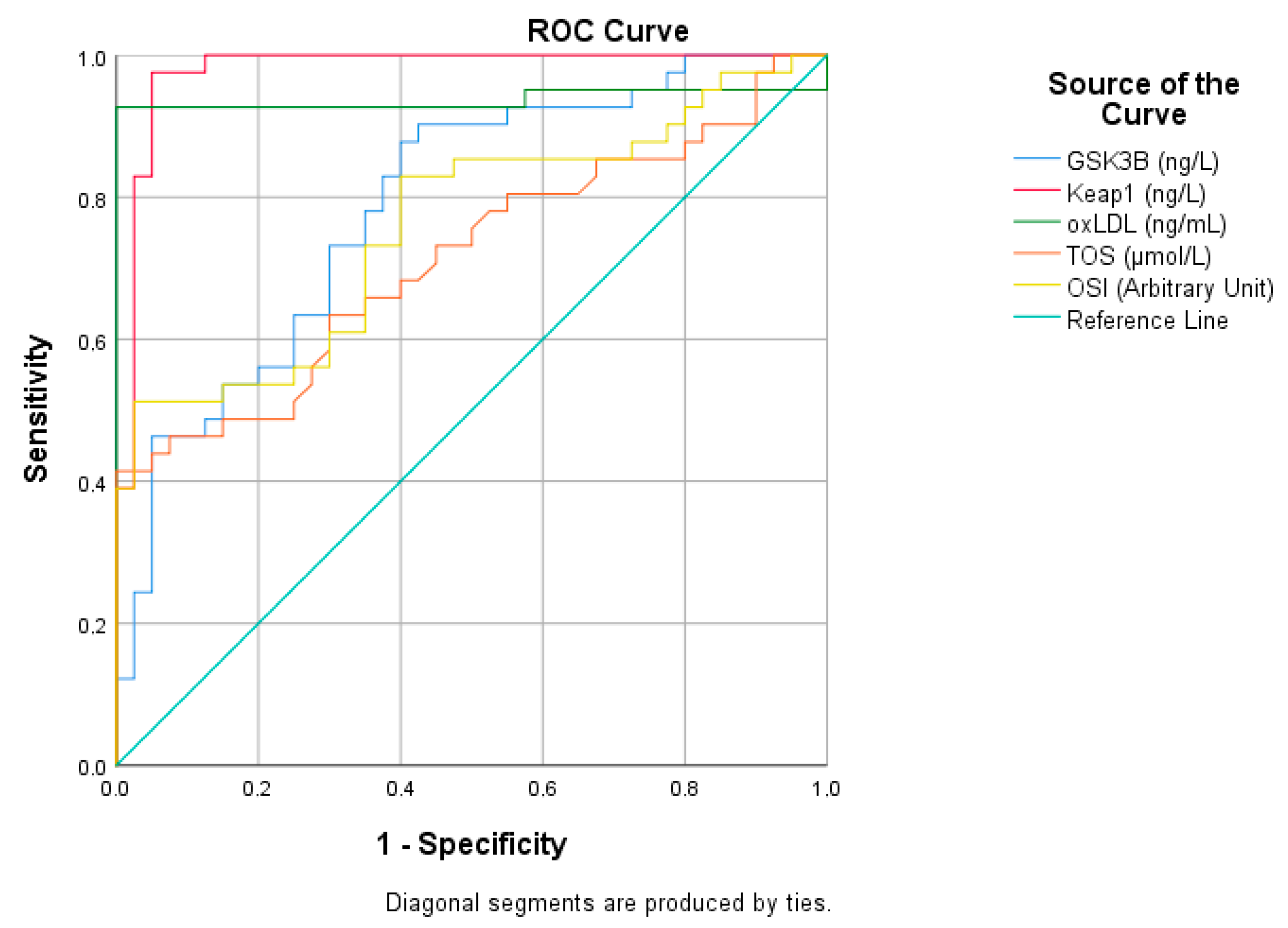
| Risk Factor | AUC (%95) | Cutt Off | p | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| GSK3B (ng/L) | 0.788 (0.690–0.886) | 263.14 | 0.001 | 70 | 70,000 |
| Keap1 (ng/L) | 0.978 (0.947–1) | 393.21 | 0.001 | 98 | 95 |
| oxLDL (ng/mL) | 0.937 (0.867–1) | 65.14 | 0.001 | 93 | 100 |
| TOS (μmol/L) | 0.714 (0.601–0.827) | 10.8 | 0.001 | 66 | 65 |
| OSI (Arbitrary Unit) | 0.759 (0.654–0.865) | 1.12 | 0.001 | 73 | 65 |
| Risk Factor | AUC (%95) | Cutt Off | p | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Nrf2 (ng/mL) | 0.988 (0.968–1) | 33.36 | 0.001 | 95 | 98 |
| Sestrin1 (ng/mL) | 0.662 (0.542–0.781) | 13.87 | 0.012 | 56 | 65 |
| TAS (μmol/L) | 0.706 (0.558–0.825) | 928.65 | 0.001 | 75 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koçtürk, F.; Emekli, F.; Eği, K.; Taysi, S. Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine. Medicina 2025, 61, 1732. https://doi.org/10.3390/medicina61101732
Koçtürk F, Emekli F, Eği K, Taysi S. Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine. Medicina. 2025; 61(10):1732. https://doi.org/10.3390/medicina61101732
Chicago/Turabian StyleKoçtürk, Fatih, Firdevs Emekli, Kadir Eği, and Seyithan Taysi. 2025. "Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine" Medicina 61, no. 10: 1732. https://doi.org/10.3390/medicina61101732
APA StyleKoçtürk, F., Emekli, F., Eği, K., & Taysi, S. (2025). Evaluation of Nrf2/Keap1 Pathway in Patients with Migraine. Medicina, 61(10), 1732. https://doi.org/10.3390/medicina61101732







