Comparison of Total Mesopancreatic Excision and Conventional Pancreaticoduodenectomy in the Surgical Treatment of Pancreatic Head Adenocarcinoma: Early Postoperative Outcomes
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Approval and Patient Selection
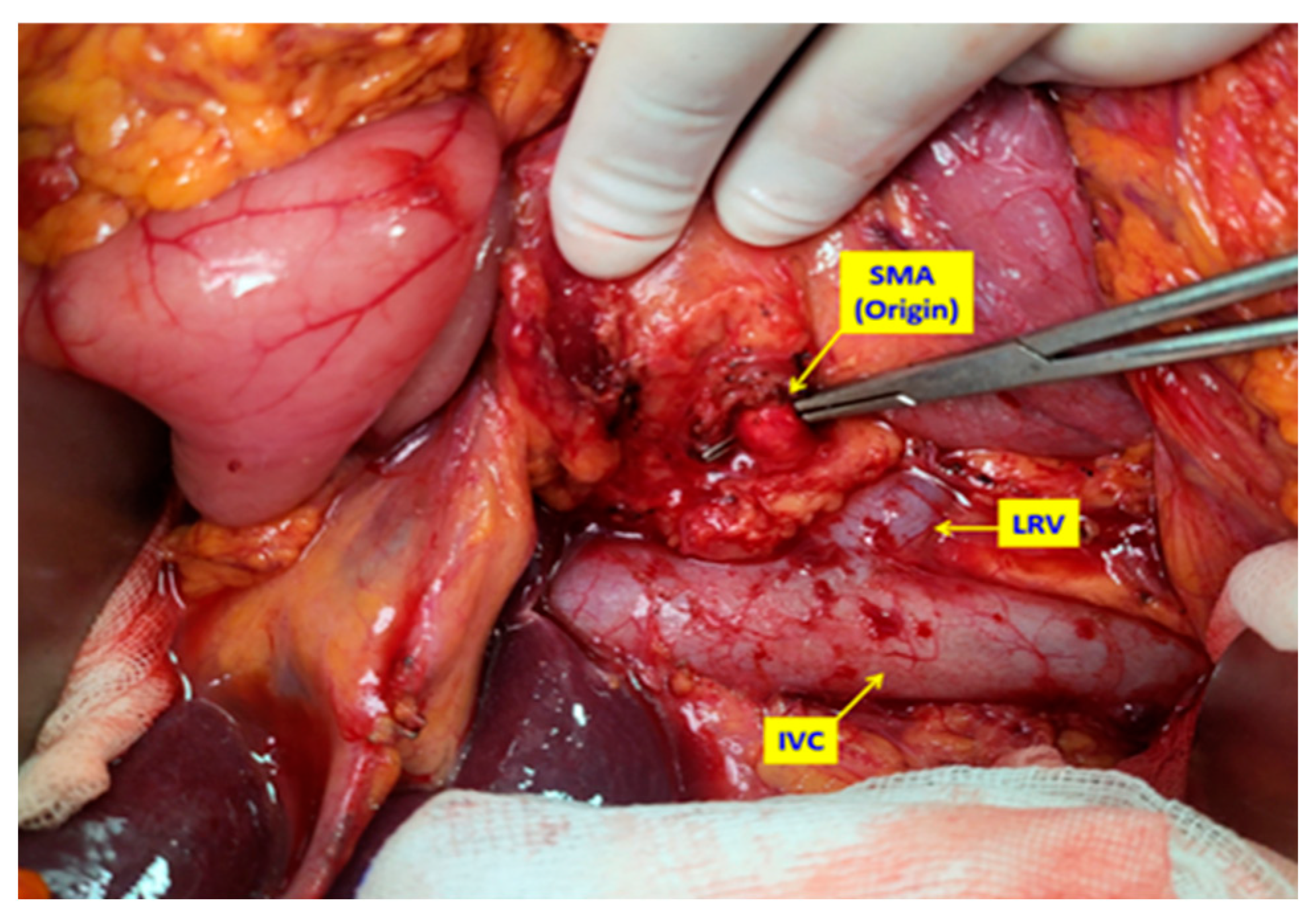
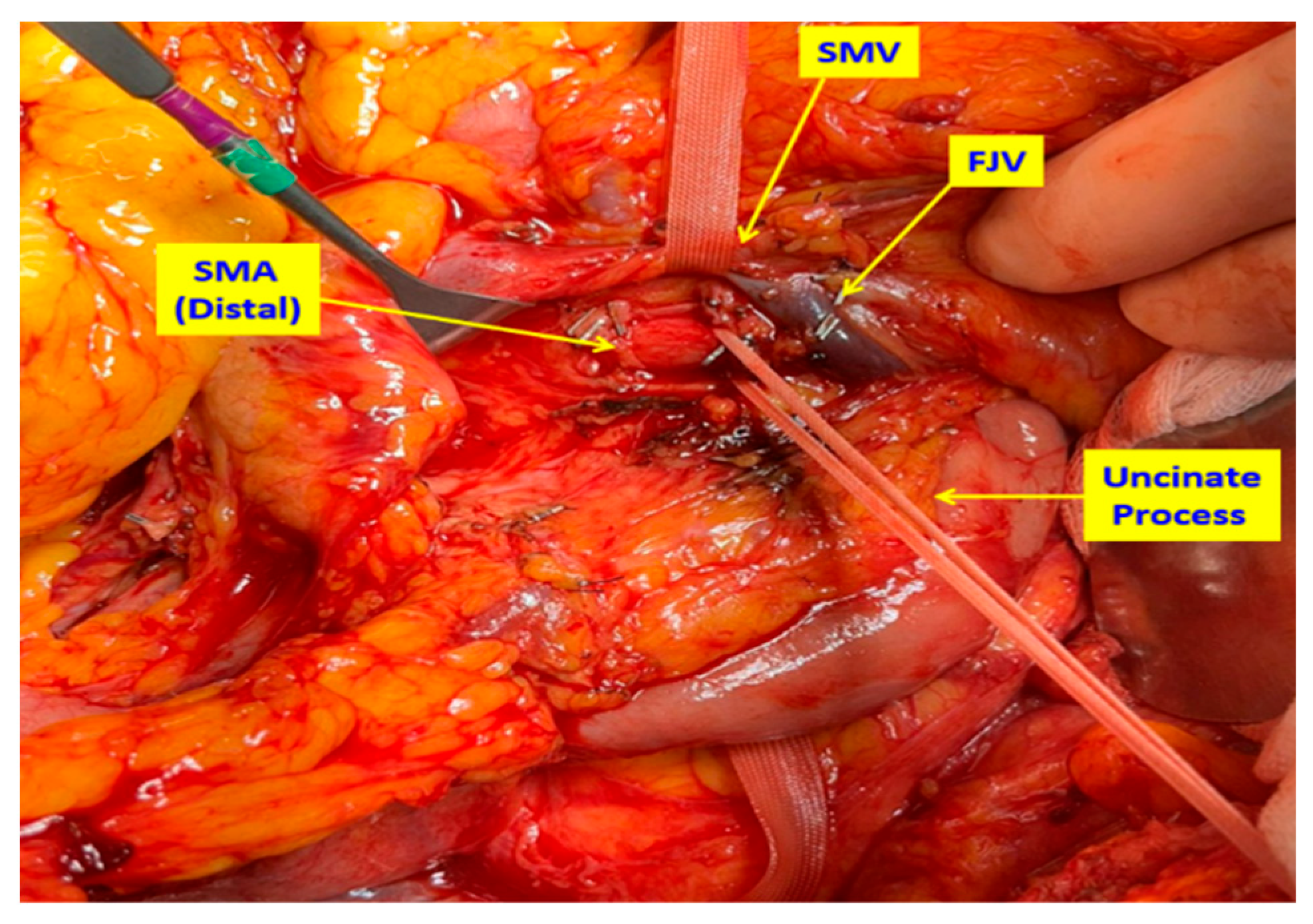
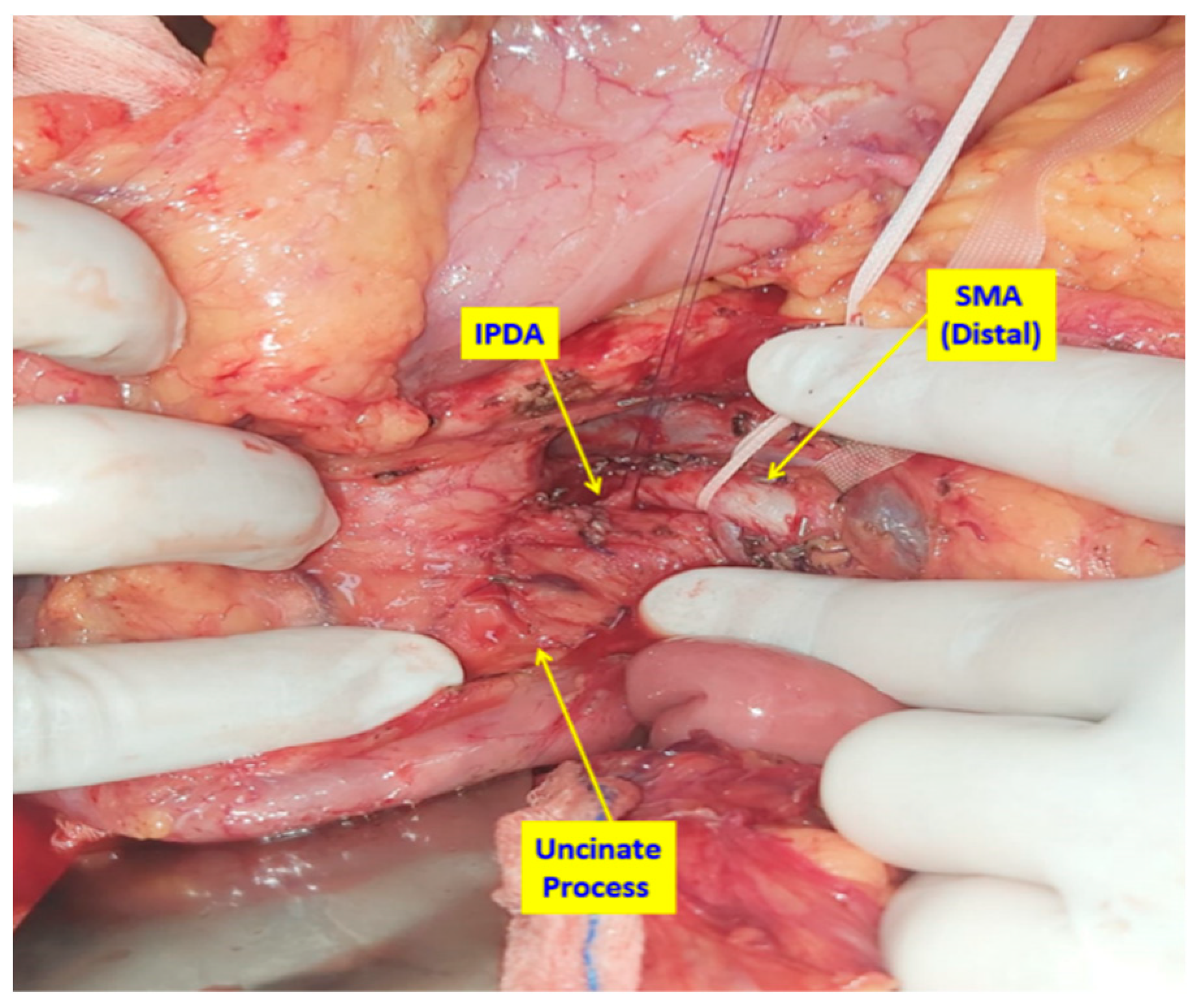
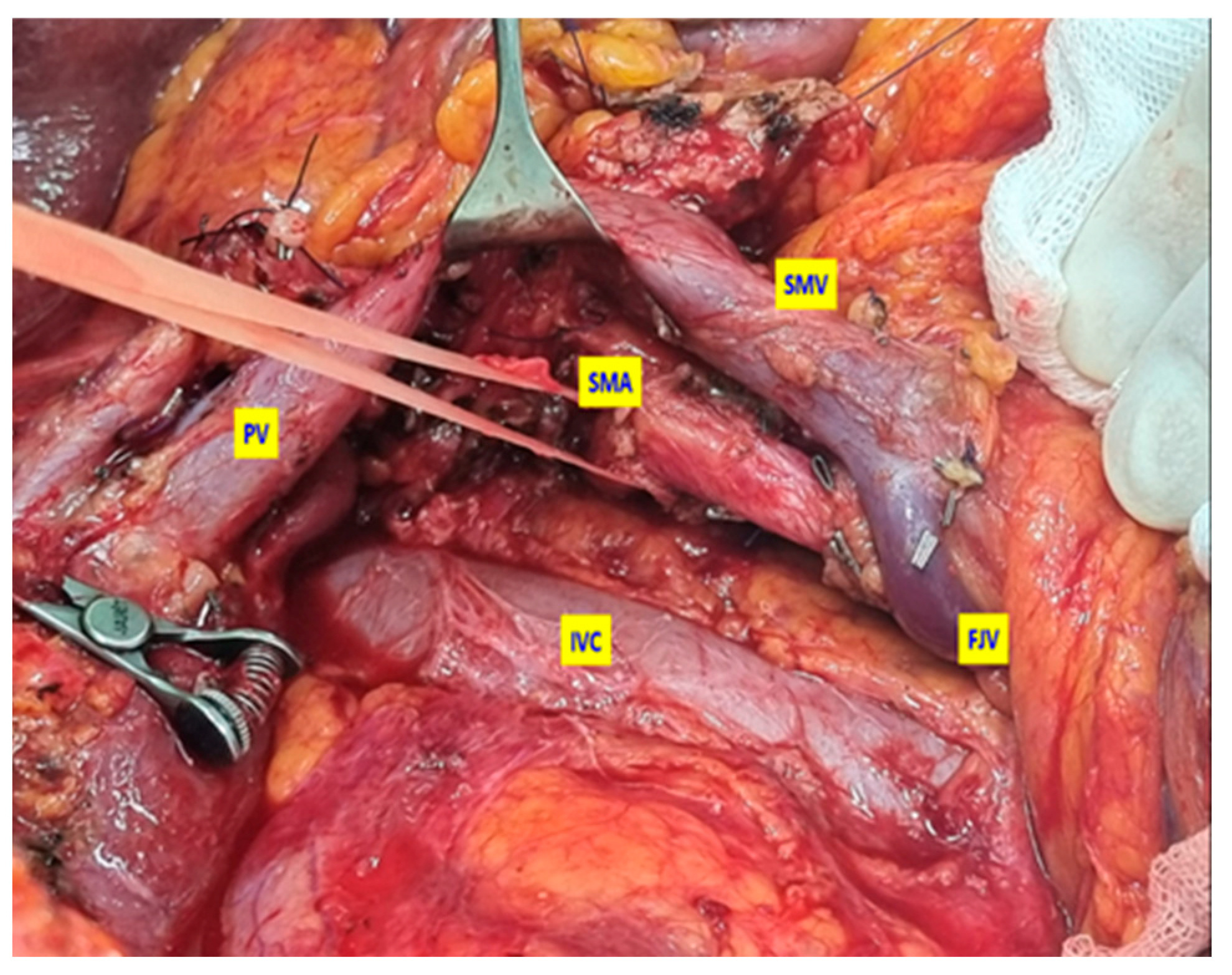
2.2. Surgical Technique
2.3. Statistical Analysis
3. Results
Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Are, C.; Dhir, M.; Ravipati, L. History of pancreaticoduodenectomy: Early misconceptions, initial milestones and the pioneers. HPB 2011, 13, 377–384. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y.; et al. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: A synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S.; DiCarlo, V.; Dionigi, R.; Mosca, F.; Pederzoli, P.; Pasquali, C.; Klöppel, G.; Dhaene, K.; Michelassi, F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. Ann. Surg. 1998, 228, 508–517. [Google Scholar] [CrossRef]
- Augustinus, S.; Schafrat, P.J.M.; Janssen, B.V.; Bonsing, B.A.; Brosens, L.A.A.; Busch, O.R.; Crobach, S.; Doukas, M.; van Eijck, C.H.; van der Geest, L.G.M.; et al. Nationwide impact of centralization, neoadjuvant therapy, minimally invasive surgery, and standardized pathology reporting on R0 resection and overall survival in pancreatoduodenectomy for pancreatic cancer. Ann. Surg. Oncol. 2023, 30, 5051–5060. [Google Scholar] [CrossRef] [PubMed]
- Farnell, M.B.; Pearson, R.K.; Sarr, M.G.; DiMagno, E.P.; Burgart, L.J.; Dahl, T.R.; Foster, N.; Sargent, D.J. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005, 138, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sohn, T.A.; Campbell, K.A.; Sauter, P.K.; Coleman, J.; Abrams, R.A.; Hruban, R.H. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann. Surg. 2002, 236, 355–366. [Google Scholar] [CrossRef]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Delpero, J.R.; Bachellier, P.; Regenet, N.; Le Treut, Y.P.; Paye, F.; Carrere, N.; Sauvanet, A.; Autret, A.; Turrini, O.; Monges-Ranchin, G.; et al. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB 2014, 16, 20–33. [Google Scholar] [CrossRef]
- Kalisvaart, M.; Broadhurst, D.; Marcon, F.; Pande, R.; Schlegel, A.; Sutcliffe, R.; Marudanayagam, R.; Mirza, D.; Chatzizacharias, N.; Abradelo, M.; et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: Systematic review and a single-centre retrospective study. HPB 2020, 22, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: Results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Ghaneh, P.; Kleeff, J.; Halloran, C.M.; Raraty, M.; Jackson, R.; Melling, J.; Jones, O.; Palmer, D.H.; Cox, T.F.; Smith, C.J.; et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann. Surg. 2019, 269, 520–529. [Google Scholar] [CrossRef]
- Gockel, I.; Domeyer, M.; Wolloscheck, T.; Konerding, M.A.; Junginger, T. Resection of the mesopancreas (RMP): A new surgical classification of a known anatomical space. World J. Surg. Oncol. 2007, 5, 44. [Google Scholar] [CrossRef]
- Gaedcke, J.; Gunawan, B.; Grade, M.; Szöke, R.; Liersch, T.; Becker, H.; Ghadimi, B.M. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: Relevance for clinical trials. Langenbeck’s Arch. Surg. 2010, 395, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Adham, M.; Singhirunnusorn, J. Surgical technique and results of total mesopancreas excision (TMpE) in pancreatic tumors. Eur. J. Surg Oncol. 2012, 38, 340–345. [Google Scholar] [CrossRef]
- Bouassida, M.; Mighri, M.M.; Chtourou, M.F.; Sassi, S.; Touinsi, H.; Hajji, H.; Sassi, S. Retroportal lamina or mesopancreas? Lessons learned by anatomical and histological study of thirty-three cadaveric dissections. Int. J. Surg. 2013, 11, 834–836. [Google Scholar] [CrossRef]
- Kawabata, Y.; Tanaka, T.; Nishi, T.; Monma, H.; Yano, S.; Tajima, Y. Appraisal of a total meso-pancreatoduodenum excision with pancreaticoduodenectomy for pancreatic head carcinoma. Eur. J. Surg. Oncol. 2012, 38, 574–579. [Google Scholar] [CrossRef]
- Fernandes, E.S.M.; Strobel, O.; Girão, C.; Moraes-Junior, J.M.A.; Torres, O.J.M. What do surgeons need to know about the mesopancreas. Langenbeck’s Arch. Surg. 2021, 406, 2621–2632. [Google Scholar] [CrossRef]
- da Silva, L.F.L.; Belotto, M.; de Almeida, L.F.C.; Samuel, J.; Pereira, L.H.; Albagli, R.O.; de Araujo, M.S.; Ramia, J.M. Radicality and safety of total mesopancreatic excision in pancreatoduodenectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2024, 22, 217. [Google Scholar] [CrossRef]
- Shyr, B.U.; Shyr, B.S.; Chen, S.C.; Shyr, Y.M.; Wang, S.E. Mesopancreas level 3 dissection in robotic pancreaticoduodenectomy. Surgery 2021, 169, 362–368. [Google Scholar] [CrossRef]
- D’Cruz, J.R.; Misra, S.; Menon, G.; Shamsudeen, S. Pancreaticoduodenectomy (Whipple Procedure). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Absun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPF) definition and grading of postoperative pancreatic fistula: Eleven years after. Surgery 2024, 176, 988–989, Erratum in Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- The jamovi Project, version 2.6; Computer Software; jamovi: Sydney, Australia, 2025; Available online: https://www.jamovi.org (accessed on 19 August 2025).
- Zhang, Z. Model building strategy for logistic regression: Purposeful selection. Ann. Transl. Med. 2016, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Salama, I.A.; Badawy, M.T.; Sweed, D.M.; Elzakaky, N.; Ibrahim, T.M. Total mesopancreas excision in pancreaticoduodenectomy and its prognostic surgical outcomes in cancer head pancreas. Egypt. J. Surg. 2025, 44, 331–341. [Google Scholar] [CrossRef]
- Wellner, U.F.; Krauss, T.; Csanadi, A.; Lapshyn, H.; Bolm, L.; Timme, S.; Kulemann, B.; Hoeppner, J.; Kuesters, S.; Seifert, G.; et al. Mesopancreatic stromal clearance defines curative resection of pancreatic head cancer and can be predicted preoperatively by radiologic parameters: A retrospective study. Medicine 2016, 95, e2529. [Google Scholar] [CrossRef]
- Fernández-del Castillo, C.; Morales-Oyarvide, V.; McGrath, D.; Wargo, J.A.; Ferrone, C.R.; Thayer, S.P.; Lillemoe, K.D.; Warshaw, A.L. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 2012, 152 (Suppl. S1), S56–S63. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Tanaka, M.; Mihaljevic, A.L.; Probst, P.; Heckler, M.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br. J. Surg. 2019, 106, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, X.; Chen, Y.; Ma, Y.; Liu, C.; Tian, L.; Wang, J.; Dong, J.; Cui, D.; Wang, Y.; et al. Total mesopancreas excision for the treatment of pancreatic head cancer. J. Cancer 2017, 8, 3575–3584. [Google Scholar] [CrossRef]
- Chowdappa, R.; Challa, V.R. Mesopancreas in pancreatic cancer: Where do we stand? Review of literature. Indian J. Surg. Oncol. 2015, 6, 69–74. [Google Scholar] [CrossRef][Green Version]
- Peparini, N.; Chirletti, P. Mesopancreas: A boundless structure, namely R1 risk in pancreaticoduodenectomy for pancreatic head carcinoma. Eur. J. Surg. Oncol. 2013, 39, 1303–1308. [Google Scholar] [CrossRef]
- Yi, S.; Nagakawa, Y.; Ren, K.; Dai, Y.D.; Zhang, M.; Chen, J.F.; Wang, Z.D.; Miwa, Y.; Liu, T.; Lu, X.M. The mesopancreas and pancreatic head plexus: Morphological, developmental, and clinical perspectives. Surg. Radiol. Anat. 2020, 42, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. Artery-first’ approaches to pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef]
- Kimura, W. Strategies for the treatment of invasive ductal carcinoma of the pancreas and how to achieve zero mortality for pancreaticoduodenectomy. J. Hepatobiliary Pancreat. Surg. 2008, 15, 270–277. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Yamada, S.; Satoi, S.; Takami, H.; Yamamoto, T.; Yoshioka, I.; Sonohara, F.; Yamaki, S.; Shibuya, K.; Hayashi, M.; Hashimoto, D.; et al. Multicenter randomized phase II trial of prophylactic right-half dissection of superior mesenteric artery nerve plexus in pancreatoduodenectomy for pancreatic head cancer. Ann. Gastroenterol. Surg. 2021, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, N.; Ono, Y.; Sato, T.; Inoue, Y.; Oba, A.; Ito, H.; Mise, Y.; Saiura, A.; Takahashi, Y. Long-term outcome of patients with postoperative refractory diarrhea after tailored nerve plexus dissection around the major visceral arteries during pancreatoduodenectomy for pancreatic cancer. World J. Surg. 2022, 46, 1172–1182. [Google Scholar] [CrossRef]
- Dumitrascu, T.; Popescu, I. Total mesopancreas excision in pancreatic head adenocarcinoma: The same impact as total mesorectal excision in rectal carcinoma? Eur. J. Surg. Oncol. 2012, 38, 725. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, M.; Nagakawa, T.; Ohta, T.; Kitagawa, H.; Miyazaki, I. Surgical strategy for carcinoma of the papilla of Vater based on lymphatic spread and mode of recurrence. Surgery 1997, 121, 611–617. [Google Scholar] [CrossRef]
- Peparini, N.; Chirletti, P. Clearance of the retropancreatic margin in pancreatic carcinomas: Total mesopancreas excision or extended lymphadenectomy? Eur. J. Surg. Oncol. 2012, 38, 1146. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Saiura, A.; Yoshioka, R.; Ono, Y.; Takahashi, M.; Arita, J.; Takahashi, Y.; Koga, R. Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery-first approach. Ann. Surg. 2015, 262, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, Y.; Yi, S.; Takishita, C.; Sahara, Y.; Osakabe, H.; Kiya, Y.; Yamaguchi, H.; Miwa, Y.; Sato, I.; Tsuchida, A. Precise anatomical resection based on structures of nerve and fibrous tissue around the superior mesenteric artery for mesopancreas dissection in pancreaticoduodenectomy for pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2020, 27, 342–351. [Google Scholar] [CrossRef]
| All (n = 41) | TMpE-PD (n = 17) | Co-PD (n = 24) | p Value | |
|---|---|---|---|---|
| Age (years) | 64.6 ± 10.5 | 66.4 ± 10.8 | 63.4 ± 10.3 | 0.382 |
| Sex | ||||
| Female | 16 (39.0) | 5 (29.4) | 11 (45.8) | 0.288 |
| Male | 25 (61.0) | 12 (70.6) | 13 (54.2) | |
| Comorbidity | 31 (75.6) | 13 (76.5) | 18 (75.0) | 0.914 |
| Smoking | 20 (48.8) | 9 (52.9) | 11 (45.8) | 0.654 |
| BMI (kg/m2) | 25.0 (23.0–27.0) | 25.4 (22.9–27.0) | 24.6 (23.0–27.8) | 0.952 |
| Preoperative biliary drainage | 25 (61.0) | 11 (64.7) | 14 (58.3) | 0.680 |
| Neoadjuvant chemotherapy | 1 (2.4) | - | 1 (4.2) | 1.000 |
| Vascular resection (Venous) | 5 (12.2) | 2 (11.8) | 3 (12.5) | 1.000 |
| Operation duration (minutes) | 480 (400–495) | 485 (480–495) | 423 (398–485) | 0.067 |
| R status | ||||
| R0 | 21 (51.2) | 10 (58.8) | 11 (45.8) | 0.412 |
| R1 | 20 (48.8) | 7 (41.2) | 13 (54.2) | |
| Pathological stage | ||||
| Stage I | 3 (7.3) | - | 3 (12.5) | 0.250 |
| Stage II | 18 (43.9) | 7 (41.2) | 11 (45.8) | |
| Stage III | 20 (48.8) | 10 (58.8) | 10 (41.7) | |
| Stage IV | - | - | - | |
| EBL (ml) | 250 (150–300) | 250 (150–350) | 250 (188–300) | 0.139 |
| Number of removed lymph nodes | 29.0 (23.0–38.0) | 30.0 (25.0–39.0) | 26.0 (21.8–36.3) | 0.757 |
| Pancreatic duct diameter (mm) | 4.0 (3.0–6.0) | 4.0 (3.0–5.0) | 3.5 (3.0–6.3) | 0.788 |
| Pancreatic gland texture | ||||
| Firm | 5 (12.2) | 1 (5.9) | 4 (16.7) | 0.245 |
| Moderate | 32 (78.0) | 13 (76.5) | 19 (79.2) | |
| Soft | 4 (9.8) | 3 (17.6) | 1 (4.2) |
| All (n = 41) | TMpE-PD (n = 17) | Co-PD (n = 24) | p Value |
|---|---|---|---|
| All complications 26 (63.4) | 14 (82.4) | 12 (50.0) | 0.034 |
| Surgical complications 24 (58.5) | 12 (70.6) | 12 (50.0) | 0.187 |
| Surgical side infections 15 (36.6) | 8 (47.1) | 7 (29.2) | 0.241 |
| Intra-abdominal abscess 3 (7.3) | 1 (5.9) | 2 (8.3) | 1.000 |
| Biliary leakage x 1 (2.4) | 1 (5.9) | - | 0.415 |
| Chylous leakage 2 (4.9) | 2 (11.8) | - | 0.166 |
| POPF y 5 (12.2) | 2 (11.8) | 3 (12.5) | 1.000 |
| Systemic complications 2 (4.9) | 2 (11.8) | - | 0.166 |
| Atelectasis 2 (4.9) | 2 (11.8) | - | 0.166 |
| Cardiac complications 1 (2.4) | 1 (2.4) | - | 0.415 |
| Complication grade z | |||
| I 12 (29.3) | 8 (47.1) | 4 (16.7) | 0.161 |
| II 7 (17.1) | 4 (23.5) | 3 (12.5) | |
| III-A 4 (9.8) | 1 (5.9) | 3 (12.5) | |
| III-B 1 (2.4) | - | 1 (4.2) | |
| IV-A - | - | - | |
| IV-B - | - | - | |
| V 2 (4.9) | 1 (5.9) | 1 (4.2) | |
| ≥grade-III complications 7 (17.1) | 2 (11.8) | 5 (20.8) | 0.447 |
| Reoperation 1 (2.4) | 1 (5.9) | - | 0.415 |
| Length of hospital stay (days) 18 (12–21) | 18 (12–21) | 19.5 (11.8–23.0) | 0.730 |
| 30-day readmissions 1 (2.4) | - | 1 (4.2) | 1.000 |
| 90-day mortality 4 (9.8) | 1 (5.9) | 3 (12.5) | 0.629 |
| No Complications (n = 15) | Complications (n = 26) | OR (95% CI, p Value) | |
|---|---|---|---|
| Age (years) | 64.7 ± 8.87 | 64.6 ± 11.4 | 1.00 (0.94–1.06, p = 0.988) |
| Sex | |||
| Female | 8 (53.3) | 8 (30.8) | - |
| Male | 7 (46.7) | 18 (69.2) | 2.57 (0.69–9.55, p = 0.158) |
| Comorbidity | |||
| No | 2 (13.3) | 8 (30.8) | - |
| Yes | 13 (86.7) | 18 (69.2) | 0.35 (0.06–1.91, p = 0.223) |
| Smoking | |||
| No | 9 (60.0) | 12 (46.2) | - |
| Yes | 6 (40.0) | 14 (53.8) | 1.75 (0.48–6.35, p = 0.395) |
| BMI (kg/m2) | 24.0 (22.9–26.6) | 25.2 (23.9–27.9) | 1.04 (0.88–1.22, p = 0.662) |
| Preoperative biliary drainage | |||
| No | 5 (33.3) | 11 (42.3) | - |
| Yes | 10 (66.7) | 15 (57.7) | 0.68 (0.18–2.57, p = 0.571) |
| Vascular resection | - | ||
| No | 13 (86.7) | 23 (88.5) | |
| Yes | 2 (13.3) | 3 (11.5) | 0.85 (0.13–5.75, p = 0.866) |
| Operation duration (minutes) | 480 (423–488) | 468 (400–495) | 1.00 (0.99–1.01, p = 0.582) |
| EBL (ml) | 250 (175–300) | 250 (150–338) | 1.00 (0.99–1.01, p = 0.465) |
| Pathological stage | |||
| Stage I–II | 10 (66.7) | 11 (42.3) | - |
| Stage III | 5 (33.3) | 15 (57.7) | 2.73 (0.72–10.27, p = 0.138) |
| Pancreatic duct diameter (mm) | 5.0 (3.5–7.0) | 3.5 (3.0–5.0) | 0.75 (0.56–1.00, p = 0.053) |
| Pancreatic gland texture | |||
| Firm | 3 (20.0) | 2 (7.7) | - |
| Moderate/Soft | 12 (80.0) | 24 (92.3) | 3.00 (0.44–20.44, p = 0.262) |
| Number of removed lymph nodes | 26.0 (22.5–36.0) | 29.5 (23.0–37.8) | 1.00 (0.95–1.05, p = 0.959) |
| No Complications (n = 15) | Complications (n = 26) | Adjusted OR * (95% CI, p Value) | |
|---|---|---|---|
| Surgical technique | |||
| Co-PD | 12 (80.0) | 12 (46.2) | - |
| TMpE-PD | 3 (20.0) | 14 (53.8) | 4.84 (0.90–25.95, p = 0.065) |
| R status | |||
| R0 | 8 (53.3) | 13 (50.0) | - |
| R1 | 7 (46.7) | 13 (50.0) | 1.14 (0.27–4.86, p = 0.862) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egeli, T.; Unek, T.; Ozbilgin, M.; Agalar, C.; Agalar, A.A.; Unek, I.T.; Bektas, C.; Kazancı, G.K.; Sakaoglu, B.; Karadeniz, E.; et al. Comparison of Total Mesopancreatic Excision and Conventional Pancreaticoduodenectomy in the Surgical Treatment of Pancreatic Head Adenocarcinoma: Early Postoperative Outcomes. Medicina 2025, 61, 1725. https://doi.org/10.3390/medicina61101725
Egeli T, Unek T, Ozbilgin M, Agalar C, Agalar AA, Unek IT, Bektas C, Kazancı GK, Sakaoglu B, Karadeniz E, et al. Comparison of Total Mesopancreatic Excision and Conventional Pancreaticoduodenectomy in the Surgical Treatment of Pancreatic Head Adenocarcinoma: Early Postoperative Outcomes. Medicina. 2025; 61(10):1725. https://doi.org/10.3390/medicina61101725
Chicago/Turabian StyleEgeli, Tufan, Tarkan Unek, Mucahit Ozbilgin, Cihan Agalar, Anıl Aysal Agalar, Ilkay Tugba Unek, Caner Bektas, Gokce Kıran Kazancı, Berkay Sakaoglu, Emre Karadeniz, and et al. 2025. "Comparison of Total Mesopancreatic Excision and Conventional Pancreaticoduodenectomy in the Surgical Treatment of Pancreatic Head Adenocarcinoma: Early Postoperative Outcomes" Medicina 61, no. 10: 1725. https://doi.org/10.3390/medicina61101725
APA StyleEgeli, T., Unek, T., Ozbilgin, M., Agalar, C., Agalar, A. A., Unek, I. T., Bektas, C., Kazancı, G. K., Sakaoglu, B., Karadeniz, E., & Sagol, O. (2025). Comparison of Total Mesopancreatic Excision and Conventional Pancreaticoduodenectomy in the Surgical Treatment of Pancreatic Head Adenocarcinoma: Early Postoperative Outcomes. Medicina, 61(10), 1725. https://doi.org/10.3390/medicina61101725






