Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. FMD Measurement
2.3. Measurement of Serum Biomarkers
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial Function Testing as a Biomarker of Vascular Disease. Circulation 2003, 108, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, M.; Veleba, J.; Sojakova, D.; Marhefkova, N.; Fejfarova, V.; Jude, E.B. Endothelial Dysfunction in Diabetes Mellitus: New Insights. Int. J. Mol. Sci. 2023, 24, 10705. [Google Scholar] [CrossRef] [PubMed]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial Function Assessment by Flow-Mediated Dilation Method: A Valuable Tool in the Evaluation of the Cardiovascular System. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.; Shah, P.; Phalgune, D.S. Correlation of endothelial dysfunction measured by flow-mediated vasodilatation to severity of coronary artery disease. Indian Heart J. 2018, 70, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, A.; Ciracì, L.; Andrè, L.; Trio, O.; Manganaro, R.; Saporito, F.; Oreto, G.; Andò, G. Endothelial Dysfunction in Patients with Coronary Artery Disease: Insights from a Flow-Mediated Dilation Study. Clin. Appl. Thromb. Hemost. 2014, 20, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Endothelial Dysfunction and Atherosclerosis. Eur. Heart J. 1997, 18, E19–E29. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Niki, H.; Nagai, H.; Nishikawa, M.; Nakagawa, H. Serotonin Potentiates High-Glucose–Induced Endothelial Injury: The Role of Serotonin an d5-HT2AReceptors in Promoting Thrombosis in Diabetes. J. Pharmacol. Sci. 2012, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial dysfunction in diabetes. Biomedicines 2020, 8, 182. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Vavere, A.L.; Sinsakul, M.; Ongstad, E.L.; Yang, Y.; Varma, V.; Jones, C.; Goodman, J.; Dubois, V.F.S.; Quartino, A.L.; Karathanasis, S.K.; et al. Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1 Inhibition in Type 2 diabetes: Phase1 results. J. Am. Heart Assoc. 2023, 12, e027540. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.; VanMil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Kajikawa, M.; Kishimoto, S.; Hashimoto, H.; Takaeko, Y.; Yamaji, T.; Harada, T.; Han, Y.; Aibara, Y.; Yusoff, F.M.; et al. Diagnostic criteria of flow-mediated vasodilation for normal endothelial function and nitroglycerin-induced vasodilation for normal vascular smooth muscle function of the brachial artery. J. Am. Heart Assoc. 2020, 9, e013915. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Bapir, M.; Skene, S.S.; Sies, H.; Kelm, M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc. Res. 2023, 119, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K. Role of platelets in atherothrombosis. Am. J. Cardiol. 2009, 103, 4A–10A. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Lerman, A. Endothelial dysfunction and coronary artery disease: Assessment, prognosis, and treatment. Coron. Artery Dis. 2014, 25, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Gutierrez, E.; Flammer, A.J.; Lerman, L.O.; Elizaga1, J.; Lerman, A.; Fernandez-Avile, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef]
- DeVriese, A.S.; Verbeuren, T.J.; Van De Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, K.; Hansa, G.; Bansal, M.; Tandon, S.; Kasliwal, R.R. Endothelium-dependent brachial artery flow mediated vasodilatation in patients with diabetes mellitus with and without coronary artery disease. J. Assoc. Physicians India. 2003, 51, 355–358. [Google Scholar]
- Kirma, C.; Akcakoyun, M.; Esen, A.M.; Barutcu, I.; Karakaya, O.; Saglam, M.; Kargin, R.; Turkmen, M.; Boztosun, B.; Izgi, A.; et al. Relationship between endothelial function and coronary risk factors in patients with stable coronary artery disease. Circ. J. 2007, 71, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soffer, G.; Holleran, S.; DiTullio, M.R.; Homma, S.; Boden-Albala, B.; Ramakrishnan, R.; Elkind, M.S.; Sacco, R.L.; Ginsberg, H.N. Endothelial function in individuals with coronary artery disease with and without type 2 diabetes mellitus. Metabolism 2010, 59, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Simova, I.I.; Denchev, S.V.; Dimitrov, S.I.; Ivanova, R. Endothelial function in patients with and without diabetes mellitus with different degrees of coronary artery stenosis. J. Clin. Ultrasound 2009, 37, 35–39. [Google Scholar] [CrossRef]
- Vikenes, K.; Farstad, M.; Nordrehaug, J.E. Serotonin is associated with coronary artery disease and cardiac events. Circulation 1999, 100, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Barradas, M.A.; Gill, D.S.; Fonseca, V.A.; Mikhailidis, D.P.; Dandona, P. Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur. J. Clin. Investig. 1988, 18, 399–404. [Google Scholar] [CrossRef]
- Malyszko, J.; Urano, T.; Knofler, R.; Taminato, A.; Yoshimi, T.; Takada, Y.; Takada, A. Daily variations of platelet aggregation in relation to blood and plasma serotonin in diabetes. Thromb. Res. 1994, 75, 569–576. [Google Scholar] [CrossRef]
- Bir, S.C.; Fujita, M.; Marui, A.; Hirose, K.; Arai, Y.; Sakaguchi, H.; Huang, Y.; Esaki, J.; Ikeda, T.; Tabata, Y.; et al. New therapeutic approach for impaired arteriogenesis in diabetic mouse hindlimb ischemia. Circ. J. 2008, 72, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Park, S.; Kim, H. Serotoninas a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Oh, C.M.; Ohara-Imaizumi, M.; Park, S.; Namkung, J.; Yadav, V.K.; Tamarina, N.A.; Roe, M.W.; Philipson, L.H.; Karsenty, G.; et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology 2015, 156, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Ezzeldin, E.; Souror, W.A.; El-Nahhas, T.; Soudi, A.N.; Shahat, A.A. Biochemical and neurotransmitters changes associated with tramadol in streptozotocin-induced diabetes in rats. Bio Med. Res. Int. 2014, 2014, 238780. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, H.; Xu, Z.; Duan, J.K. Serotonin and Its Receptor as a New Antioxidant Therapeutic Target for Diabetic Kidney Disease. J. Diabetes Res. 2017, 2017, 7680576. [Google Scholar] [CrossRef]
- Sugiura, T.; Dohi, Y.; Hirowatari, Y.; Yamashita, S.; Ohte, N.; Kimura, G.; Fujii, S. Cigarette smoking induces vascular damage and persistent elevation of plasma serotonin unresponsive to 8 weeks of smoking cessation. Int. J. Cardiol. 2013, 166, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Dohi, Y.; Yamashita, S.; Hirowatari, Y.; Fujii, S.; Ohte, N. Serotoninin peripheral blood reflects oxidative stress and plays a crucial role in atherosclerosis: Novel insights toward holistic anti-atherothrombotic strategy. Atherosclerosis 2016, 246, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.; Hinderliter, A.L.; Watkins, L.L.; Waugh, R.A.; Blumenthal, J.A. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J. Am. Coll. Cardiol. 2005, 46, 656–659. [Google Scholar] [CrossRef]
- Enatescu, V.R.; Cozma, D.; Tint, D.; Enatescu, I.; Simu, M.; Giurgi-Oncu, C.; Lazar, M.A.; Mornos, C. The Relationship Between Type D Personality and the Complexity of Coronary Artery Disease. Neuropsychiatr. Dis. Treat. 2021, 18, 809–820. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.S.; Kim, M.G.; Song, Y.K.; Kim, Y.; Jang, H.; Kim, J.H.; Han, N.; Ji, E.; Kim, I.W.; et al. The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events: A meta-analysis of randomized-controlled studies in depression. Int. Clin. Psychopharmacol. 2019, 34, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Delialis, D.; Mavraganis, G.; Dimoula, A.; Patras, R.; Dimopoulou, A.M.; Sianis, A.; Ajdini, E.; Maneta, E.; Kokras, N.; Stamatelopoulos, K.; et al. A systematic review and meta-analysis on the effect of selective serotonin reuptake inhibitors on endothelial function. J. Affect. Disord. 2022, 316, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Almuwaqqat, Z.; Jokhadar, M.; Norby, F.L.; Lutsey, P.L.; O’Neal, W.T.; Seyerle, A.; Soliman, E.Z.; Chen, L.Y.; Bremner, J.D.; Vaccarino, V.; et al. Association of Antidepressant Medication Type with the Incidence of Cardiovascular Disease in the ARIC Study. J. Am. Heart Assoc. 2019, 8, e012503. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Chun, E.J.; Hur, J.H.; Min, S.H.; Lee, J.-E.; Oh, T.J.; Kim, K.M.; Jang, H.C.; Han, S.J.; Kang, D.K.; et al. Effect of sarpogrelate, a selective 5-HT2A receptor antagonist, on characteristics of coronary artery disease in patients with type 2 diabetes. Atherosclerosis 2017, 257, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; DelRizzo, D.F.; Zahradka, P.; Bhangu, S.K.; Werner, J.P.; Takeda, N.; Dhalla, N.S. Sarpogrelate inhibits Serotonin-induced proliferation of porcine coronary artery smooth muscle cells: Implications for long-term graft patency. Ann. Thorac. Surg. 2001, 71, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Tanaka, N.; Kuwabara, M.; Yamashita, A.; Matsuo, Y.; Kanai, T.; Onitsuka, T.; Asada, Y.; Hisa, H. Relative contributions of 5-hydroxytryptamine (5-HT) receptor subtypes in 5-HT-induced vasoconstriction of the distended human saphenous vein as a coronary artery bypass graft. Biol. Pharm. Bull. 2011, 34, 82–86. [Google Scholar] [CrossRef]
- Tanak-Totoribe, N.; Hidaka, M.; Gamoh, S.; Yokota, A.; Nakamura, E.; Kuwabara, M.; Tsunezumi, J.; Yamamoto, R. Effects of M-1, a major metabolite of sarpogrelate, on 5-HT-induced constriction of isolated human internal thoracic artery. Biol. Pharm. Bull. 2020, 43, 1979–1982. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.R.; Ting-Ting, L.; Chang, H.T.; Xuan, G.; Bian, H.; Wei-Min, L. Elevated Levels of Plasma Superoxide Dismutases 1 and 2 in Patients with Coronary Artery Disease. BioMed Res. Int. 2016, 2016, 3708905. [Google Scholar] [CrossRef]
- Yan, M.; Mehta, J.L.; Zhang, W.; Hu, C. LOX-1, Oxidative Stress and Inflammation: A Novel Mechanism for Diabetic Cardiovascular Complications. Cardiovasc. Drugs Ther. 2011, 25, 451–459. [Google Scholar] [CrossRef] [PubMed]


| Patients’ Characteristics | T2DM (n = 33) | Non-T2DM (n = 51) | p |
|---|---|---|---|
| 1. Age (years), mean ± SD (min–max) | 65.24 ± 6.96 (46–78) | 65 ± 8.82 (47–88) | 0.69 |
| 2. Male, n (%) | 26 (78.78) | 41 (80.39) | 0.858 |
| 3. BMI (kg/m2), mean ± SD, (min–max), | 28.48 ± 4.4 (20–36.3) | 28.28 ± 4.17 (18.98–38.5) | 0.759 |
| 4. Smoking status, n (%) | 9 (27.27) | 8 (15.68) | 0.197 |
| 5. Dyslipidemia, n (%) | 31 (93.93) | 49 (96.07) | 0.653 |
| 6. Hypertension, n (%) | 33 (100) | 46 (90.19) | 0.064 |
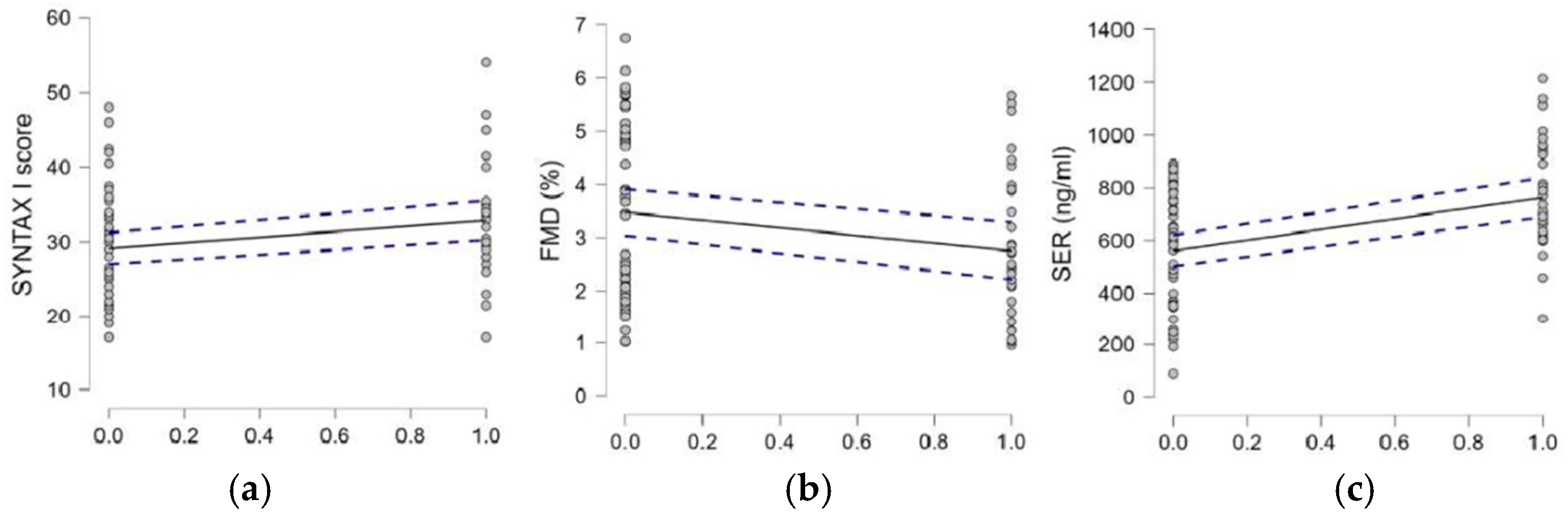
| 7. SYNTAXI score, median, interquartile range (min–max) | 30.5, 7.12 (17–54) | 29, 10.50 (17–48) | 0.050 |
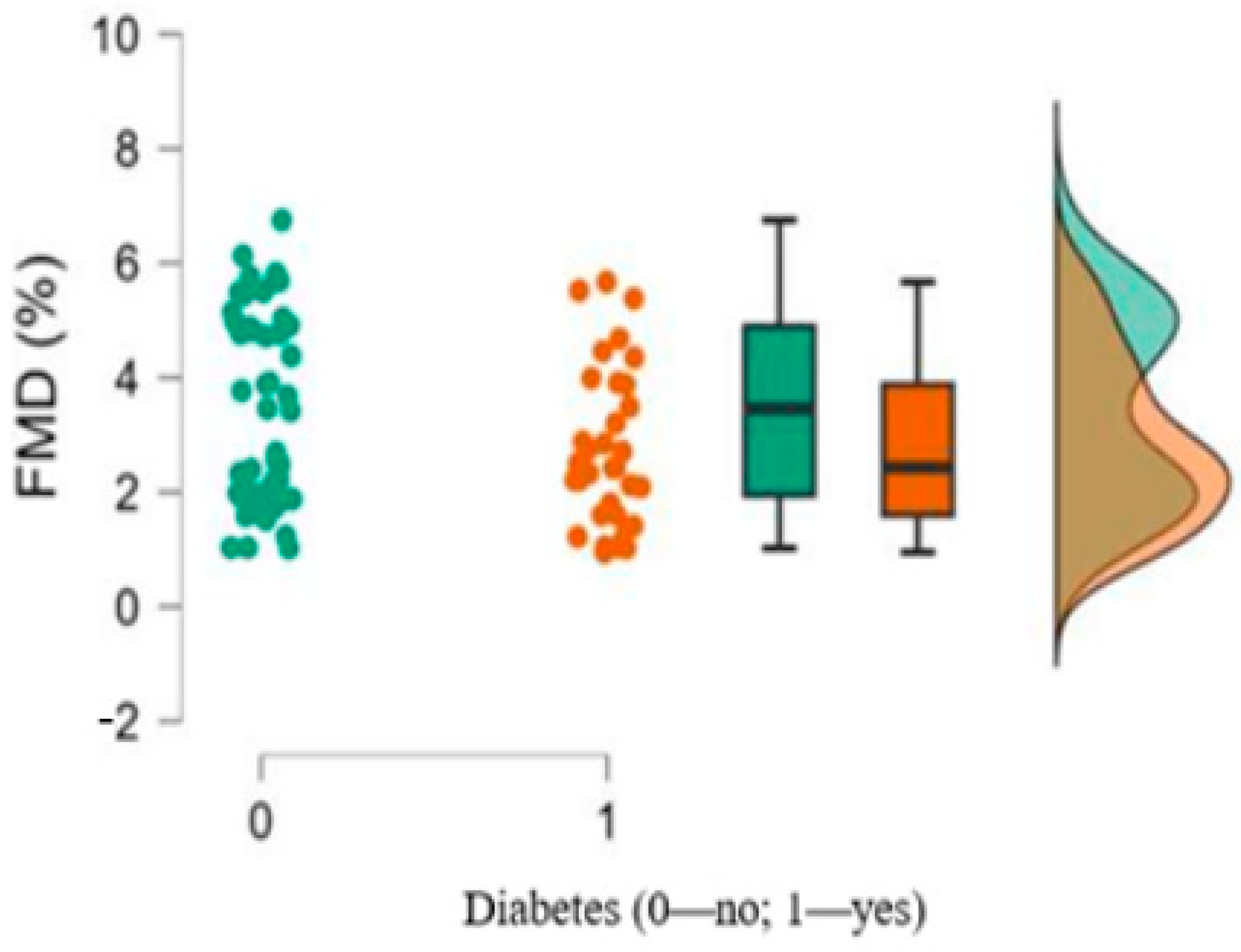
| 8. FMD%, median, interquartile range (min–max) | 2.43, 2.28 (0.95–5.67) | 3.46, 2.95 (1.02–6.75) | 0.079 |
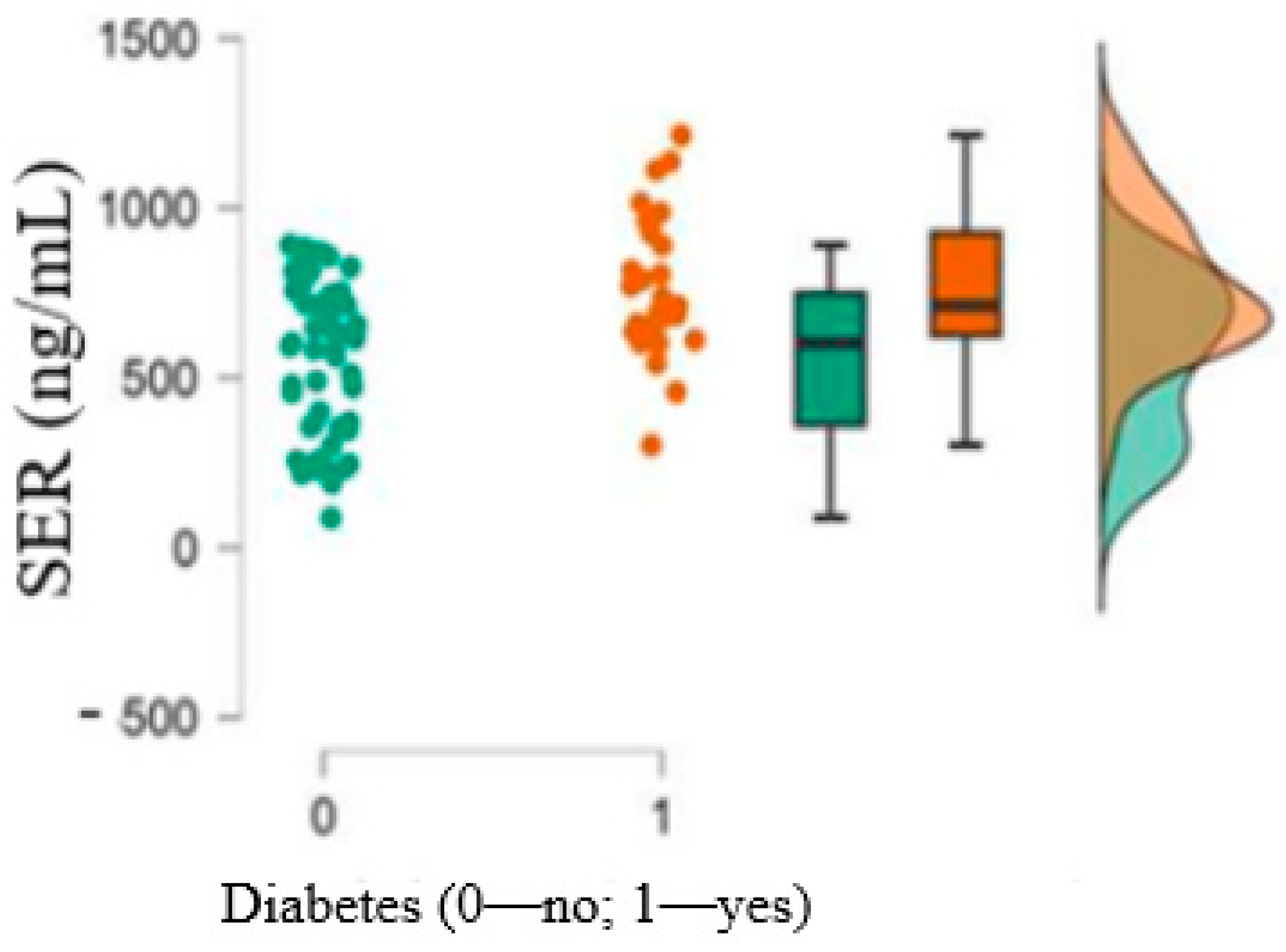
| 9. 5-HT (ng/mL),mean ± SD (min–max) | 764.78 ± 201.44 (301.75–1214.57) | 561.06 ± 224.3 (87.97–891.56) | <0.001 |
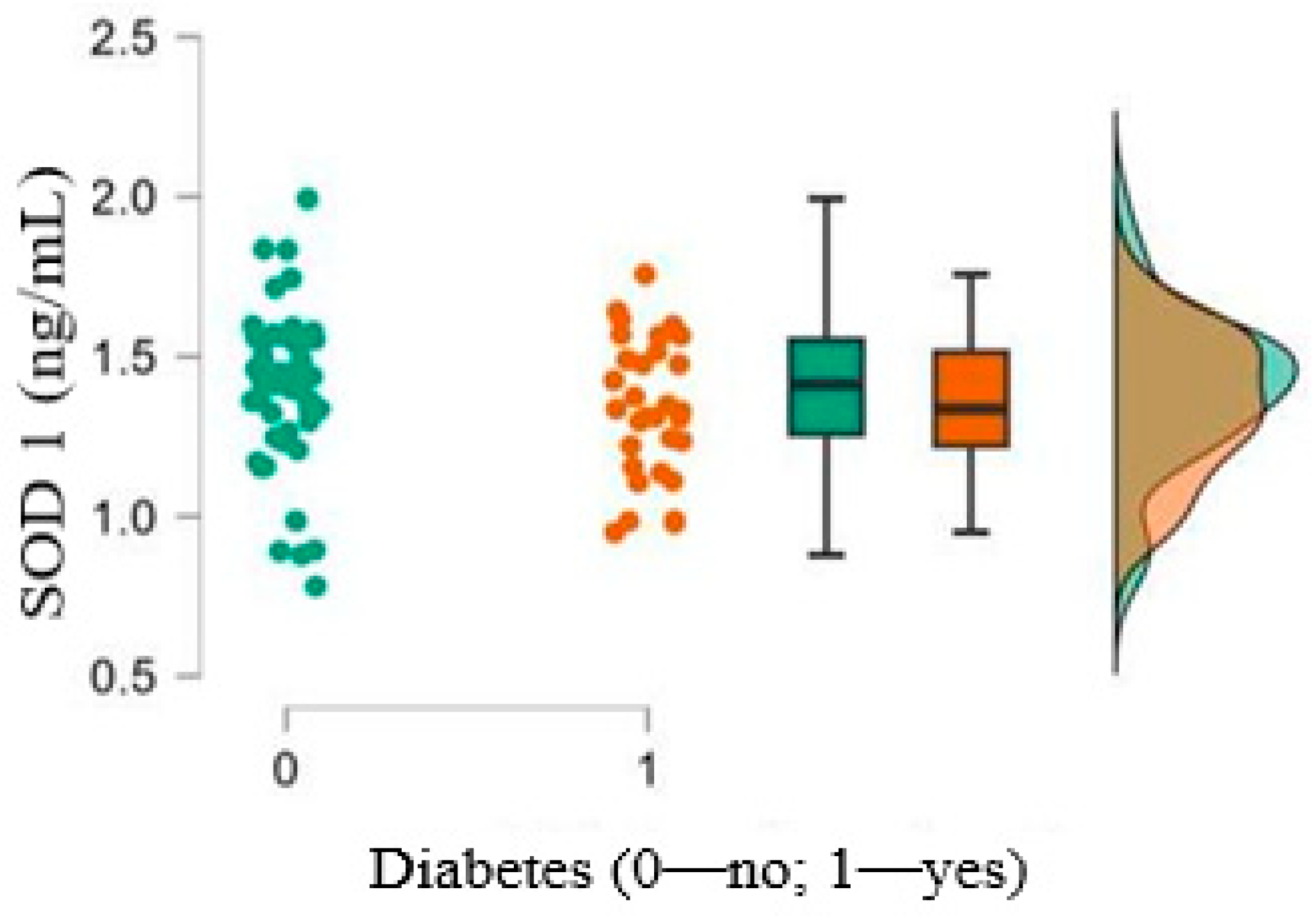
| 10. SOD1 (ng/mL),mean ± SD (min–max) | 1.34 ± 0.21 (0.95–1.75) | 1.39 ± 0.24 (0.78–1.99) | 0.362 |
| 11. LOX1 (pg/mL), median, interquartilerange (min–max) | 1157.169, 191.90 (789.00–1420.54) | 1207.10, 187.846 (874.12–1512.56) | 0.536 |
| Pearson’s Correlations | |||||||
|---|---|---|---|---|---|---|---|
| Variable | SYNTAX Score | FMD (%) | 5-HT (ng/mL) | SOD 1 (ng/mL) | LOX 1 (pg/mL) | Diabetes (0—No; 1—Yes) | |
| 1. SYNTAXI score | Pearson’s | - | |||||
| p-value | - | ||||||
| 2. FMD (%) | Pearson’s | −0.786 *** | |||||
| p-value | <0.001 | ||||||
| 3. 5-HT | Pearson’s | 0.159 | −0.141 | - | |||
| (ng/mL) | p-value | 0.149 | 0.200 | - | |||
| 4. SOD | Pearson’s | −0.067 | 0.099 | 0.038 | - | ||
| 1 (ng/mL) | p-value | 0.547 | 0.372 | 0.731 | - | ||
| 5. LOX | Pearson’s | −0.104 | 0.105 | −0.035 | 0.185 | - | |
| 1 (pg/mL) | p-value | 0.348 | 0.341 | 0.751 | 0.092 | - | |
| 6. Diabetes | Pearson’s | 0.238 * | −0.223 * | 0.423 *** | −0.106 | −0.048 | - |
| (0—no; 1—yes) | p-value | 0.029 | 0.042 | <0.001 | 0.338 | 0.666 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boieriu, A.M.; Luca, C.D.; Neculoiu, C.D.; Bisoc, A.; Țînț, D. Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role? Medicina 2025, 61, 135. https://doi.org/10.3390/medicina61010135
Boieriu AM, Luca CD, Neculoiu CD, Bisoc A, Țînț D. Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role? Medicina. 2025; 61(1):135. https://doi.org/10.3390/medicina61010135
Chicago/Turabian StyleBoieriu, Alexandra Maria, Cezar Dumitrel Luca, Carmen Daniela Neculoiu, Alina Bisoc, and Diana Țînț. 2025. "Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role?" Medicina 61, no. 1: 135. https://doi.org/10.3390/medicina61010135
APA StyleBoieriu, A. M., Luca, C. D., Neculoiu, C. D., Bisoc, A., & Țînț, D. (2025). Endothelial Dysfunction and Oxidative Stress in Patients with Severe Coronary Artery Disease: Does Diabetes Play a Contributing Role? Medicina, 61(1), 135. https://doi.org/10.3390/medicina61010135





