Abstract
Background and Objectives: Up to 27% of anterior cruciate ligament (ACL) reconstruction cases result in a residual positive pivot shift sign, indicating anteroposterior and rotational instability. This instability can compromise returning to sports and increase the risk of further injuries. The biomechanical role of the anterolateral ligament (ALL) in controlling internal knee rotation is well known. However, there are no clinical trials comparing isolated ACL repairs to those combined with ALL reconstruction. Our objective is to compare the results of these techniques, with a primary focus on assessing knee stability and graft survival, to provide evidence for optimizing surgical approaches, particularly for athletes and physically active individuals. Materials and Methods: An observational study using paired score matching as a method of pseudo-randomization was conducted. Subjects were divided into an ACL group and an ACL+ALL group. Demographic and clinical variables were collected, as well as those related to complications and survival. Results: A total of 236 patients were included, which was reduced to 74 ACL and 37 ACL+ALL after pseudo-randomization, resulting in preoperatively comparable groups. During follow-up, differences in favor of ALL reinforcement were found on the pivot shift test (p = 0.007). No differences were found with regard to the Lachman test (p = 0.201), the International Knee Documentation Committee (IKDC) knee score (p = 0.169), the IKDC subjective score (p = 0.095), intensity of pain (p = 0.928), or complications (p = 0.529). Nor were differences found in the limb symmetry index; the single hop test (p = 0.710); the triple hop test (p = 0.653); the crossover hop test (p = 0.682); the 6 meter timed hop test (p = 0.360); the normalized Y-balance test (YBT) (p = 0.459 anterior; p = 0.898 posterolateral; and p = 0.211 posteromedial directions); or the limb symmetry index of the composite YBT (p = 0.488). There were no differences either with respect to return to sports practice (p = 0.723) or survival (p = 0.798). Conclusions: Patients treated by means of the ACL+ALL technique obtained higher rotational stability, as measured by the pivot shift test, than those subjected to an isolated ACL repair. No differences were found with respect to Lachman test, complications, IKDC, pain, or survival.
1. Introduction
The overarching aim of anterior cruciate ligament (ACL) reconstruction surgery is to restore knee stability and allow patients to resume their routine activities, reducing the risk of cartilage degeneration [1]. ACL reconstruction surgery protects the medial meniscus [2,3,4], minimizing the risk of arthritic knee degeneration [5,6,7,8,9,10,11,12]. However, for surgical ACL reconstruction to be successful, the surgeon must carefully manage the knee’s anteroposterior and, particularly, rotational laxity [13], as up to 27% of intra-articular ACL reconstruction cases were reported to result in a residual positive pivot shift sign [13,14,15,16,17,18].
The pivot shift phenomenon is typically indicative of a combination of anteroposterior and rotational instability [13], which could prevent participation in pivoting sports and result in failure of the reconstruction or in injuries to cartilage or the menisci [14]. Although a positive pivot shift sign was traditionally considered to be tantamount to an ACL injury, the truth is that this sign is typically observed when both the ACL and the knee’s peripheral structures are damaged [13,16,19,20,21]. In fact, some isolated injuries to these peripheral structures often present with a pivot shift sign in the absence of an ACL injury, and not all ACL lesions result in a pivot shift sign [13,20,21].
The torsional laxity that results from some intra-articular reconstructions together with the well-known biomechanical role played by the anterolateral ligament (ALL) in controlling internal knee rotation [22,23,24,25,26,27,28,29,30,31,32,33,34,35] have understandably led to a renewed interest in the use of lateral extra-articular tenodeses to improve the results of ACL repair procedures [36,37,38]. As they are located in the periphery of the knee, ALL-augmented reconstructions provide a more efficient lever arm for controlling internal tibial rotation compared to isolated reconstructions (Figure 1) [13]. Indeed, the Anterolateral Ligament Expert Group determined that the main function of the ALL is to restrict internal tibial rotation, which may affect pivot shift in cases of ACL insufficiency [35].
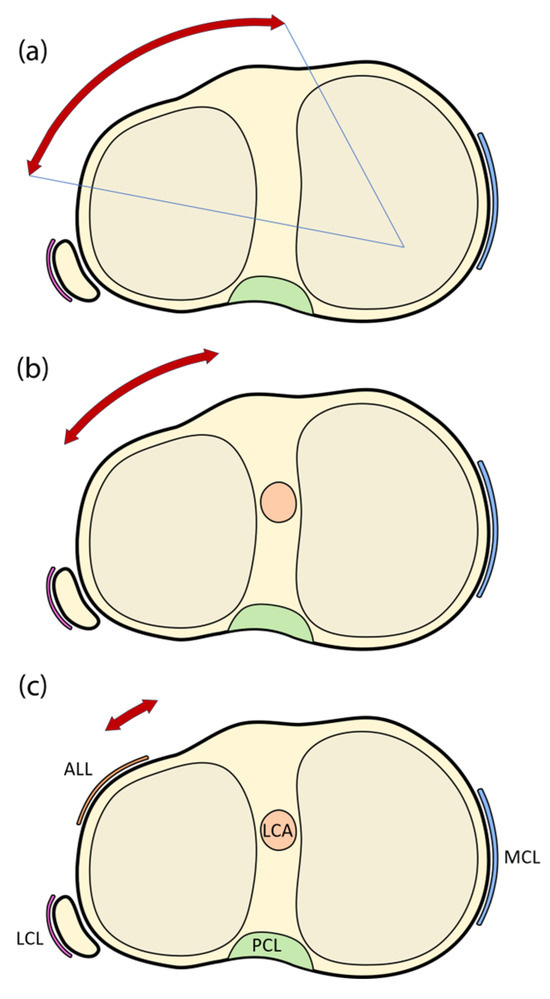
Figure 1.
Schematic representation showing instances of knee joint instability, with the red arrows indicating the residual rotational instability. (a) without anterior cruciate or anterolateral ligament; (b) with anterior cruciate ligament but without anterolateral ligament; and (c) with all articular structures present. ALL: anterolateral ligament; LCA: anterior cruciate ligament; PCL: posterior cruciate ligament; MCL: medial collateral ligament; LCL: lateral collateral ligament.
Although intra- and extra-articular reconstruction techniques were, in the past, used in isolation, combined reconstructions of the ACL and the ALL act synergistically, making it possible to control anteroposterior and rotational laxity [14,39,40,41,42,43,44,45,46,47], protecting the graft during the biological healing phase, and offering an additional restraint against pivot shift instability. Indeed, an in vitro study showed that extra-articular reconstructions are able to reduce the forces borne from an intra-articular graft by 43% [13].
However, despite the biomechanical advantages of ALL reinforcement, its clinical implications remain controversial. Some studies suggest that ALL reinforcement may accelerate degeneration of the lateral compartment [48,49], while others attribute such outcomes to outdated surgical techniques, excessive tensioning, or inadequate postoperative care. Furthermore, many studies lack long-term follow-ups or present conflicting results regarding stability and survival rates [50,51]. These gaps in the literature limit the ability to draw definitive conclusions about the clinical benefits and risks of ALL reinforcement.
Given the novelty of this technique, there are no clinical trials comparing the outcomes of isolated ACL repairs with those of an ACL repair combined with an extra-articular ALL reconstruction. The purpose of the present study is to compare both techniques, as well as to analyze the survival and complications rates associated with each of them.
2. Materials and Methods
This study was approved by the Ethics Committee for Drug Research of the Principality of Asturias on 16 March 2020 (code: 2020.111). All subjects gave their informed consent prior to inclusion.
The study analyzed all patients subjected to an ACL repair (isolated or combined with an ALL reconstruction) from 1 January 2000 to 31 December 2021, who agreed to participate in the study. Since the objective of our analysis was to assess a new treatment strategy, a single-arm before-and-after study design was chosen [52,53]. Prior to 2018, all cases were treated with isolated ACL repair, while ALL augmentation was introduced from that year forward. Subjects were divided into one of the following groups:
- −
- ACL group: Isolated intra-articular arthroscopic reconstruction of the ACL.
- −
- ACL+ALL group: The intra-articular reconstruction was augmented by an extra-articular reinforcement that stemmed from the lateral aspect of the femur, slightly proximal and posterior to the epicondyle, and attached distally to the tibia, 15 mm below the joint line and half way between the fibular head and Gerdy’s tubercle [14,54,55,56,57].
The variables analyzed included the demographic data (age, sex, height, weight, etc.); the mechanism of injury; comorbidities and previous surgeries; Lachman test [58]; the pivot shift test [59]; Tegner score [60]; range of motion; the type of surgery (isolated ACL repair or ACL repair reinforced with a repair of the ALL); the type of repair; tunnel and graft thickness; associated maneuvers; complications; pain, as measured by the Visual analog scale (VAS) [61]; failed repairs; hop test (simple, triple, crossover and 6 meter times tests) [62]; the Y-balance test (YBT) [63,64,65,66]; the International Knee Documentation Committee (IKDC) subjective score [67]; and the IKDC knee score. All the data, except for functional scores, was collected both preoperatively and in the course of follow-up.
2.1. Anatomy and Biomechanics of ALL
Although not the primary focus of this study, a brief overview of the anatomy and biomechanics of the anterolateral ligament (ALL) is provided. Recent research suggests the ALL is a distinct ligament in the anterolateral portion of the knee, attaching near the lateral epicondyle of the femur. Its distal insertion point is located between the fibular head and Gerdy’s tubercle [24,25]. Biomechanical studies show that the length of the ALL increases with knee flexion and tibial internal rotation, suggesting it plays a key role in resisting internal rotation. The ALL acts as a secondary stabilizer, particularly in ACL-deficient knees, by restricting tibial internal rotation and influencing the pivot shift test [24,35].
2.2. Statistical Analysis
Given that the goal of our study is to evaluate a new treatment strategy and that all patients were initially treated with isolated ACL reconstruction, with the addition of ALL reinforcement introduced later, randomization was not feasible [52]. In this context, propensity score matching (PSM) using nearest neighbor matching with a 2:1 ratio was conducted. This approach consolidates multiple covariates into a single scalar function, enabling the comparison of subjects with similar characteristics and improving the validity of treatment effect estimates [68,69]. Once paired, a descriptive analysis of the data was performed using measures of central tendency and dispersion. Either parametric or non-parametric tests of mean differences were used to make comparisons between the groups, depending on the normality of the samples. Qualitative variables were analyzed using Pearson’s chi-squared test or the Fisher Exact Test, depending on the magnitude of the expected values. Survival was analyzed by means of the Kaplan–Meier method, using failure for any cause as an endpoint. All cases, and not only the paired ones, were included in the survival analysis to avoid bias. Differences in survival were evaluated using a log rank test. Statistical significance was set, in all cases, at a p-value of 0.05. The data were analyzed using R software (R Development Core Team), version 4.1.3 [70]. Propensity score matching was applied using the MatchIt package [71].
3. Results
The study included 236 patients subjected to ACL reconstruction surgery, of whom 199 (84.3%) were included in the isolated ACL repair group and 37 (15.7%) in the ALL augmentation group (ACL+ALL). After PSM, 37 patients were retained in the ACL+ALL group (33.3%) and 74 (66.6%) in the ACL group. Figure 2 shows a significant reduction in the absolute standardized mean difference, increasing comparability between the groups.
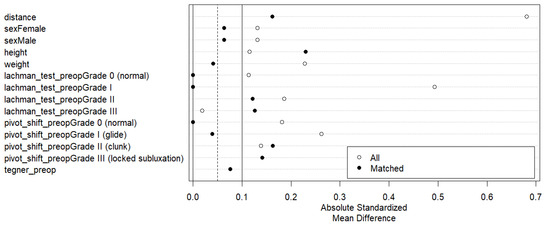
Figure 2.
Love plot showing reduction in absolute standardized mean difference among groups after application of paired score matching method.
3.1. Anthropometry and Diagnosis
The anthropometric and diagnostic data obtained (Table 1) showed that both groups were similar in terms of sex distribution (78% of males in the ACL group vs. 76% in the ACL+ALL group, p = 0.748); laterality (45% right legs in the ACL group vs. 51% in the ACL+ALL group, p = 0.542); age (30.9 years in the ACL group vs. 30.2 in the ACL+ALL group, p = 0.781); height (171.2 cm in the ACL group vs. 173.4 in the ACL+ALL group, p = 0.927); and weight (73.2 kg in the ACL group vs. 72.3 in the ACL+ALL group, p = 0.714). Mean length of follow-up was, however, considerably longer in patients in the isolated reconstruction group (90.1 months vs. 29.9 months in the ACL+ALL group, p < 0.001).

Table 1.
Anthropometric and diagnostic data.
3.2. Preoperative Evaluation
The preoperative clinical evaluation (Table 2) did not reveal statistically significant differences between the groups in terms of flexion (108.6 degrees in the ACL group vs. 113.5 in the ACL+ALL group, p = 0.169) or extension (−3.6 degrees in the ACL group vs. −3.8 in the ACL+ALL group, p = 0.747) of the injured limb. In addition, anteroposterior knee stability, as measured by the Lachman test, was also similar in both groups (p = 0.350) (Figure 3). Nor was statistical significance achieved (p = 0.756) with respect to rotational stability, as measured by the pivot shift test (Figure 4).

Table 2.
Preoperative clinical evaluation.
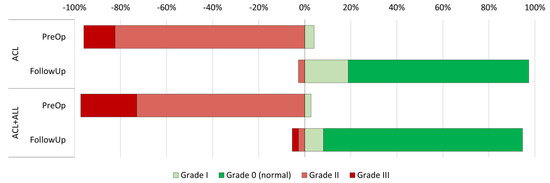
Figure 3.
Representation of progression of Lachman test results in both groups between preoperative and postoperative period.
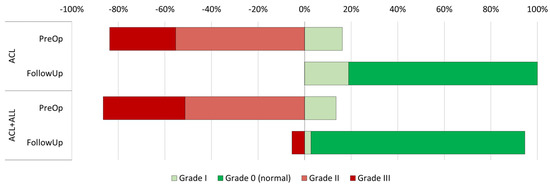
Figure 4.
Representation of progression of pivot shift test results in both groups between preoperative and postoperative period.
3.3. Surgical Information
All the information about the surgical procedure is contained in Table 3. All repairs in this study were performed using the semitendinosus and the gracilis. No statistically significant differences were found between the groups with respect to the percentage of meniscus repairs (61% in the ACL group vs. 65% in the ACL+ALL group, p = 0.678) or chondral repairs (4% in the ACL group vs. 11% in the ACL+ALL group, p = 0.219) performed. Similarly, although the percentage of intraoperative complications was higher in the group without ALL reinforcement, the difference did not reach statistical significance (4% in the ACL group vs. 0% in the ACL+ALL group, p = 0.299).

Table 3.
Surgical information.
3.4. Evaluation During Follow-Up Period
Table 4 and Table 5 describe the clinical assessment carried out during the follow-up period. No statistically significant or clinically relevant differences were found between the groups in terms of knee extension (p = 0.191). However, a difference was observed in flexion (133.2° in ACL vs. 129.6° in ACL+ALL; p = 0.037), although this difference is small and lacks clinical relevance, as indicated by the effect size (Cohen’s d = 0.425).

Table 4.
Clinical evaluation at follow-up (I).

Table 5.
Clinical evaluation at follow-up (II).
The Lachman test did not find differences during the follow-up period (Figure 2) (p = 0.201). Contrarily, the rotational stability achieved by patients with an anterolateral reinforcement, as measured by the pivot shift test, was superior to that achieved by the group where the standard technique was employed (92% grade in the ACL+ALL group vs. 81% in the ACL group, p = 0.007) (Figure 3).
Results on the IKDC knee score were similar among groups (p = 0.169) (Figure 4). Also, the differences observed in the IKDC subjective score were not statistically significant (82.4% in the ACL group vs. 83.0% in the ACL+ALL group; p = 0.095). No differences were observed with respect to pain, as measured by VAS (0.70 in the ACL group vs. 0.68 in the ACL+ALL group, p = 0.928), or the percentage of patients experiencing complications in the course of the follow-up (9% in the ACL group vs. 14% in the ACL+ALL group, p = 0.529).
No statistically significant differences were observed in any of the hop tests evaluated using the limb symmetry index (LSI). Indeed, the difference between the groups was 95.3% for the ACL group vs. 94.2% for the ACL+ALL group (p = 0.710) in the single hop test; 96.4% for the ACL group vs. 98.0% for the ACL+ALL group (p = 0.653) in the triple hop test; 94.6% for the ACL group vs. 95.8% for the ACL+ALL group (p = 0.682) in the crossover hop test; and 102.7% for the ACL group vs. 105.5% for the ACL+ALL group (p = 0.360) in the 6 meter timed hop test.
No differences were found between the groups with respect to the various YBT measures, as normalized to the subjects’ leg length, when comparing the differences between the healthy and the injured leg in the three directions of space (p = 0.459 for the anterior direction; p = 0.898 for the posterolateral direction; and p = 0.211 for the posteromedial direction), or to the LSI of the composite YBT (p = 0.488).
3.5. Return to Sports Practice
The proportion of patients who were able to return to the same or a higher level of sports performance was similar in both groups (83.8% in the ACL group vs. 81.1% in the ACL+ALL group, p = 0.723) (Table 6).

Table 6.
Evolution of Tegner score (return to physical activity) by groups.
No differences were found when comparing the subgroups traditionally associated with a lower rate of return to sports practice such as women (78.3% for women vs. 84.1% for men, p = 0.539) or subjects with a meniscus lesion (81.0% for subjects without a meniscus injury vs. 84.1% for subjects with a meniscus injury, p = 0.674) (Table 7).

Table 7.
Evolution of Tegner score (Return to physical activity). By other factors.
3.6. Survival
Survival, as estimated by the Kaplan–Meier method, was 95.3% in the ACL group and 97.2% in the ACL+ALL group, with no statistically significant differences being found between the groups on the log rank test (p = 0.798) (Figure 5).
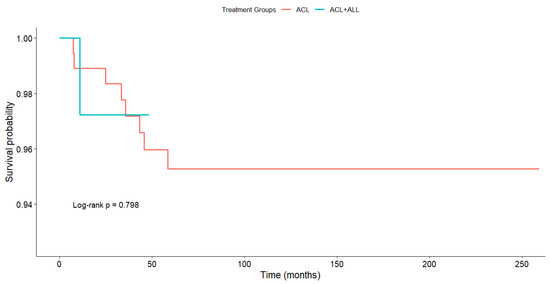
Figure 5.
Estimated survival according to Kaplan–Meier method.
4. Discussion
The most significant finding of the present study is that ACL repairs that are augmented by restoration of the ALL result in greater knee stability, as measured by the pivot shift test. In the following section, we shall provide a detailed analysis of our results.
4.1. Pivot Shift Test and Lachman Test
The pivot shift test [59] is recognized as an important tool to measure clinical outcomes following an ACL repair [10,72,73]. Several studies have confirmed that the presence of a positive pivot shift sign is indicative of instability and poorer objective and subjective results, as well as of an impossibility to return to preinjury levels of sports performance [74,75]. Under this perspective, given that the extra-articular augmentation of ACL repairs prevents tibial plateau subluxations and protects the intra-articular graft [76,77,78] and that various studies have demonstrated that intra- and extra-articular repairs act synergistically in controlling the pivot shift phenomenon [14,39,40,41,42,43,44], it is only logical to expect the ACL+ALL group to be more capable of overcoming this kind of instability. It must be said though that some authors have argued that ALL augmentations can only limit pivot shift instability at knee positions around 90 degrees of flexion [79].
The present series provides confirmation of the hypothesis above, as we found a higher percentage of patients without pivot shift instability (grade 0) in the ACL+ALL group (92% in the ACL group vs. 81% in the ACL+ALL group, p = 0.007). Our findings are in line with those of Song et al. [47], who found a mean positive pivot shift prevalence of 27.2% in their ACL group (26% in our series) and 13.3% in their ACL+ALL group (8% in our series), indicating a clear superiority of ALL reinforcement. The results reported in the literature are somewhat inconsistent. The study with the longest follow-up to date [80] was also unable to find statistically significant differences between the groups with respect to the presence of a residual pivot shift sign (p = 0.230), although its authors—like us—consider that patients in the ALL reinforcement group are able to control their residual pivot shift instability more effectively. Similarly, Hewison et al. [81] performed a systematic review where they analyzed eight articles of which three did not find any pivot shift differences between the groups analyzed, four found differences in favor of ALL reinforcement, and one found differences in favor of the isolated ACL reconstruction. An analysis of 70 professional athletes [82] obtained better results in terms of pivot shift reduction (5.7% positive cases), although the reported reoperations and failure rates were considerably higher (15.7% and 5.7%, respectively).
As far as the Lachman test is concerned, we did not find differences among the groups (p = 0.201). This is in line with the fact that ALL reinforcement was not found to exert a very marked effect on anteroposterior knee stability.
4.2. Return to Sports Practice
The return-to-sports rates reported in the literature on ACL repairs are highly heterogeneous. A systematic review of 7556 cases found that only 65% of patients were able to go back to their preinjury activity levels [83], whereas another one, which only included patients operated using extra-articular augmentation techniques combined with an intra-articular repair [84], reported rates between 64% and 100% but failed to provide a mean value. Other authors found considerably lower return-to-sports rates [85] (44.2% for patients with an isolated ACL repair and 52% for those with combined ACL+ALL repairs). Overall, a majority of authors appear to be more favorable to ALL reinforcement, even if practically none of the studies achieved statistical significance [86,87]. This evidence is consistent with our data.
Some of the factors influencing the likelihood for a patient to go back to preinjury activity levels are sex, the presence or absence of meniscal damage, and the type of graft used [10,36]. Our study found no differences in the rate of return to preinjury levels of activity between sexes (p = 0.539), or in the presence or absence of concomitant meniscal damage (p = 0.674). The role of the type of graft used could not be evaluated because the same type of repair was performed on all the patients in this study.
4.3. IKDC and Pain
Most studies in the literature have found no association between patient-reported outcomes and the use, or otherwise, of ALL reinforcement [36,47,80,85,86,88]. Our study was no different in that regard as neither the IKDC subjective score nor VAS, used to measure the intensity of pain, found any statistically significant or clinically relevant differences between the groups (p = 0.095 and p = 0.928, respectively). Helito et al. [89] did find statistically significantly different clinical results, as measured by the IKDC subjective score, when applying the ACL+ALL technique to patients where over 12 months had elapsed between the injury and the repair (87.1 for the ACL group vs. 92.7 for the ACL+ALL group, p = 0.001). This may be due to the marked anterolateral knee laxity observed in these patients with chronic ACL insufficiency. In this regard, our proportion of chronic patients was insufficient to verify this observation. Sonnery-Cottet et al. [86] found statistically significant differences in favor of an isolated ACL repair on the KOOS pain subscale. Nonetheless, these authors do not consider the difference to be clinically relevant.
4.4. YBT and Hop Tests
Interlimb asymmetries in YBT were used to estimate the risk of sustaining an injury in the lower limbs [64,90,91] and to evaluate the outcome of surgical procedures in the lower extremities, including ACL repair [92,93,94]. When administering YBT, we used reach distances normalized to limb length, which allowed us to combine males and females in the same analysis, as it was shown that differences between the sexes disappear when results are normalized to limb length [95].
Our analysis did not find statistically significant asymmetries between the groups in any of the three dimensions of space analyzed or in the composite scores. Moreover, we did not find any of the YBT parameters evaluated in our study to fall outside the mean lateral asymmetry value for the general population, which was reported to range between 3% and 8% [64]. Similarly, we found no differences in the limb symmetry index between any of the hop tests analyzed, which is in line with previous findings [88].
4.5. Survival
Analyzing survival is no easy task given the plethora of factors affecting the survival of ACL reconstruction [10,96,97] and the high variability of the reported failure rates, which range from 1.4% to 28% in high-risk patients [36,98,99]. In addition, few published articles look into the influence of ALL reinforcement on the rupture of ACL repairs [36]. In our case, survival, as estimated by the Kaplan–Meier method, did not exhibit any statistically significant differences between the two reconstruction techniques (p = 0.798). In a study of 502 patients where they compared three techniques including ALL reinforcement, Sonnery-Cottet et al. [36] did find a correlation between the rupture rate and the technique used, with a rupture rate of 4.13% in the hamstring tendon (HT)+ALL group, 10.77% in the quadrupled hamstring tendon graft (4HT) group, and 16.77% in the bone–patellar tendon–bone graft (B-PT-B) group.
4.6. The Strengths and Limitations of the Study
The present study is not exempt from limitations. The most obvious one has to do with its retrospective and non-randomized nature. However, before-and-after designs, which are usually interventional rather than observational, are justified in situations where it would be deemed unethical to withhold essential treatment from patients in the control group, as is the case here [52,53]. Additionally, these designs may offer a high level of generalizability [100]. Also, PSM is a valuable way to control for bias and achieve pseudo-randomization in retrospective observation studies [68,69]. Other limitations include a failure to analyze the progression of osteoarthritis, which would have been desirable as techniques such as ALL+ACL reconstruction were blamed for increasing the risk of developing the disease. Nevertheless, in these circumstances, where there are no randomized clinical trials comparing these two treatments–and, given the ethical limitations, we believe there will not be any—this comparison using PSM provides a valuable opportunity to understand the effects of anterolateral reinforcement in ACL repair.
5. Conclusions
Patients treated by means of the ACL+ALL technique obtained higher rotational stability, as measured by the pivot shift test, than those subjected to an isolated ACL repair. No differences were found with respect to Lachman test, complications, IKDC, pain, or survival. Therefore, and given that the anterolateral reinforcement is a surgical gesture with low morbidity, it would be advisable to perform it as a complement in all surgeries where residual instability is suspected.
Author Contributions
A.M. conceptualized the study along with N.R., I.P., C.C. and A.M. also designed the study. A.M., N.R., I.P., C.T., L.L., F.M. and S.M. were involved in patient treatment, and all authors contributed to data collection. A.M. participated in data analysis and led the manuscript drafting. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Drug Research of the Principality of Asturias on 16 March 2020 (code: 2020.111).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Datasets are available on request from the authors.
Acknowledgments
The authors would like to thank María Baena Sánchez, Manrique García Riera, Roberto González Alonso, and Elisa Vallina Fernández for their disinterested assistance in the collection of the clinical data. They would also like to thank Tania Iglesias Cabo from the Statistical Consultancy Unit of the Scientific and Technical Department of the University of Oviedo for performing the statistical analysis of the data. Finally, a special thank you goes to María Rabanal Rubio and Pablo Oscar Roza Miguel from the MBA Chair for Medical and Biomechanical Research for their collaboration with the design of the paper, the preparation of the databases, figures, illustrations, and tables, and the formatting of the final manuscript. This paper would not have been possible without the collaboration of the aforementioned individuals.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACL | Anterior cruciate ligament |
| ALL | Anterolateral ligament |
| ACL+ALL | Anterior cruciate ligament repair combined with anterolateral ligament reconstruction |
| PCL | Posterior cruciate ligament |
| MCL | Medial collateral ligament |
| LCL | Lateral collateral ligament |
| YBT | Y-balance test |
| IKDC | International Knee Documentation Committee |
| PSM | Propensity score matching |
| VAS | Visual analog scale for pain |
References
- Bousquet, B.A.; O’Brien, L.; Singleton, S.; Beggs, M. Post-operative criterion based rehabilitation of acl repairs: A clinical commentary. Int. J. Sports Phys. Ther. 2018, 13, 293–305. [Google Scholar] [CrossRef]
- Kennedy, J.; Jackson, M.P.; O’Kelly, P.; Moran, R. Timing of Reconstruction of the Anterior Cruciate Ligament in Athletes and the Incidence of Secondary Pathology within the Knee. J. Bone Jt. Surg. Br. 2010, 92-B, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.C.; Ahn, J.H.; Lee, S.H.; Yoon, Y.C. Increasing Incidence of Medial Meniscal Tears in Nonoperatively Treated Anterior Cruciate Ligament Insufficiency Patients Documented by Serial Magnetic Resonance Imaging Studies. Am. J. Sports Med. 2009, 37, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Saithna, A.; Blakeney, W.G.; Ouanezar, H.; Borade, A.; Daggett, M.; Thaunat, M.; Fayard, J.-M.; Delaloye, J.-R. Anterolateral Ligament Reconstruction Protects the Repaired Medial Meniscus: A Comparative Study of 383 Anterior Cruciate Ligament Reconstructions from the SANTI Study Group with a Minimum Follow-up of 2 Years. Am. J. Sports Med. 2018, 46, 1819–1826. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Gray, T. Results of Anterior Cruciate Ligament Reconstruction Based on Meniscus and Articular Cartilage Status at the Time of Surgery. Am. J. Sports Med. 2000, 28, 446–452. [Google Scholar] [CrossRef]
- Sommerlath, K.; Lysholm, J.; Gillquist, J. The Long-Term Course after Treatment of Acute Anterior Cruciate Ligament Ruptures. Am. J. Sports Med. 1991, 19, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, R.; Mansour, A.; Carey, J.; Spindler, K. Meniscus Status at Anterior Cruciate Ligament Reconstruction Associated with Radiographic Signs of Osteoarthritis at 5- to 10-Year Follow-Up—A Systematic Review. J. Knee Surg. 2009, 22, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Keays, S.L.; Newcombe, P.A.; Bullock-Saxton, J.E.; Bullock, M.I.; Keays, A.C. Factors Involved in the Development of Osteoarthritis after Anterior Cruciate Ligament Surgery. Am. J. Sports Med. 2010, 38, 455–463. [Google Scholar] [CrossRef]
- Wu, W.H.; Hackett, T.; Richmond, J.C. Effects of Meniscal and Articular Surface Status on Knee Stability, Function, and Symptoms after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2002, 30, 845–850. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Adachi, N.; Koga, H.; Kondo, E.; Kuroda, R.; Mae, T.; Uchio, Y. Japanese Orthopaedic Association (JOA) Clinical Practice Guidelines on the Management of Anterior Cruciate Ligament Injury—Secondary Publication. J. Orthop. Sci. 2020, 25, 6–45. [Google Scholar] [CrossRef]
- Longo, U.G.; Marino, M.; Arias, C.; Ruzzini, L.; Papalia, R.; Denaro, V. A Narrative Review of Anterior Cruciate Ligament Reconstruction in Skeletally Immature Patients. Minerva Orthop. 2024, 75, 163–170. [Google Scholar] [CrossRef]
- Passaretti, A.; Colò, G.; Bulgheroni, A.; Vulcano, E.; Surace, M.F. Gonarthrosis and ACL Lesion: An Intraoperative Analysis and Correlations in Patients Who Underwent Total Knee Arthroplasty. Minerva Orthop. 2024, 75, 331–336. [Google Scholar] [CrossRef]
- Dodds, A.L.; Gupte, C.M.; Neyret, P.; Williams, A.M.; Amis, A.A. Extra-Articular Techniques in Anterior Cruciate Ligament Reconstruction. J. Bone Jt. Surg. Br. 2011, 93, 1440–1448. [Google Scholar] [CrossRef]
- Zein, A.; Ali, M.; Ali, H.; Saleh Elsaid, A.N.; Mahmoud, A.Z.; Osman, M.K.; Mohamed Soliman, A.M. Combined Anatomic Reconstruction of the Anterior Cruciate and Anterolateral Ligaments Using Hamstring Graft Through a Single Femoral Tunnel and With a Single Femoral Fixation. Arthrosc. Tech. 2017, 6, e567–e577. [Google Scholar] [CrossRef] [PubMed]
- Chambat, P.; Guier, C.; Sonnery-Cottet, B.; Fayard, J.-M.; Thaunat, M. The Evolution of ACL Reconstruction over the Last Fifty Years. Int. Orthop. 2013, 37, 181–186. [Google Scholar] [CrossRef]
- Losee, R.E.; Johnson, T.R.; Southwick, W.O. Anterior Subluxation of the Lateral Tibial Plateau. A Diagnostic Test and Operative Repair. J. Bone Jt. Surg. Am. 1978, 60, 1015–1030. [Google Scholar] [CrossRef]
- Howe, J.G.; Johnson, R.J.; Kaplan, M.J.; Fleming, B.; Jarvinen, M. Anterior Cruciate Ligament Reconstruction Using Quadriceps Patellar Tendon Graft. Am. J. Sports Med. 1991, 19, 447–457. [Google Scholar] [CrossRef]
- Crawford, S.N.; Waterman, M.B.R.; Lubowitz, J.H. Long-Term Failure of Anterior Cruciate Ligament Reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1566–1571. [Google Scholar] [CrossRef]
- Bull, A.M.J.; Amis, A.A. The Pivot-Shift Phenomenon: A Clinical and Biomechanical Perspective. Knee 1998, 5, 141–158. [Google Scholar] [CrossRef]
- Norwood, L.A.; Andrews, J.R.; Meisterling, R.C.; Glancy, G.L. Acute Anterolateral Rotatory Instability of the Knee. J. Bone Jt. Surg. Am. 1979, 61, 704–709. [Google Scholar] [CrossRef]
- Fetto, J.F.; Marshall, J.L. Injury to the Anterior Cruciate Ligament Producing the Pivot-Shift Sign. J. Bone Jt. Surg. Am. 1979, 61, 710–714. [Google Scholar] [CrossRef]
- Dodds, A.L.; Halewood, C.; Gupte, C.M.; Williams, A.; Amis, A.A. The Anterolateral Ligament: Anatomy, Length Changes and Association with the Segond Fracture. Bone Jt. J. 2014, 96-B, 325–331. [Google Scholar] [CrossRef]
- Kittl, C.; Halewood, C.; Stephen, J.M.; Gupte, C.M.; Weiler, A.; Williams, A.; Amis, A.A. Length Change Patterns in the Lateral Extra-Articular Structures of the Knee and Related Reconstructions. Am. J. Sports Med. 2015, 43, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Patel, N.A.; Lin, C.C.; Lee, T.Q. The Anterolateral Ligament of the Knee Joint: A Review of the Anatomy, Biomechanics, and Anterolateral Ligament Surgery. Knee Surg. Relat. Res. 2019, 31, 12. [Google Scholar] [CrossRef]
- Ariel de Lima, D.; Helito, C.P.; Lacerda de Lima, L.; de Castro Silva, D.; Costa Cavalcante, M.L.; Dias Leite, J.A. Anatomy of the Anterolateral Ligament of the Knee: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 670–681. [Google Scholar] [CrossRef]
- Pomajzl, R.; Maerz, T.; Shams, C.; Guettler, J.; Bicos, J. A Review of the Anterolateral Ligament of the Knee: Current Knowledge Regarding Its Incidence, Anatomy, Biomechanics, and Surgical Dissection. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 583–591. [Google Scholar] [CrossRef]
- Daggett, M.; Stephenson, C.; Dobson, J.; Whitaker, A.; Redler, A.; Monaco, E.; Wright, B.; Saithna, A.; Sonnery-Cottet, B. Anatomic and Histological Study of the Anterolateral Aspect of the Knee: A SANTI Group Investigation. Orthop. J. Sport. Med. 2018, 6, 232596711879997. [Google Scholar] [CrossRef]
- Parsons, E.M.; Gee, A.O.; Spiekerman, C.; Cavanagh, P.R. The Biomechanical Function of the Anterolateral Ligament of the Knee. Am. J. Sports Med. 2015, 43, 669–674. [Google Scholar] [CrossRef]
- Helito, C.P.; Demange, M.K.; Bonadio, M.B.; Tírico, L.E.P.; Gobbi, R.G.; Pécora, J.R.; Camanho, G.L. Anatomy and Histology of the Knee Anterolateral Ligament. Orthop. J. Sport. Med. 2013, 1, 232596711351354. [Google Scholar] [CrossRef]
- Vincent, J.-P.; Magnussen, R.A.; Gezmez, F.; Uguen, A.; Jacobi, M.; Weppe, F.; Al-Saati, M.F.; Lustig, S.; Demey, G.; Servien, E.; et al. The Anterolateral Ligament of the Human Knee: An Anatomic and Histologic Study. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 147–152. [Google Scholar] [CrossRef]
- Zein, A. Step-by-Step Arthroscopic Assessment of the Anterolateral Ligament of the Knee Using Anatomic Landmarks. Arthrosc. Tech. 2015, 4, e825–e831. [Google Scholar] [CrossRef] [PubMed]
- Helito, C.P.; Demange, M.K.; Bonadio, M.B.; Tirico, L.E.P.; Gobbi, R.G.; Pecora, J.R.; Camanho, G.L. Radiographic Landmarks for Locating the Femoral Origin and Tibial Insertion of the Knee Anterolateral Ligament. Am. J. Sports Med. 2014, 42, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Claes, S.; Vereecke, E.; Maes, M.; Victor, J.; Verdonk, P.; Bellemans, J. Anatomy of the Anterolateral Ligament of the Knee. J. Anat. 2013, 223, 321–328. [Google Scholar] [CrossRef]
- Claes, S.; Bartholomeeusen, S.; Bellemans, J. High Prevalence of Anterolateral Ligament Abnormalities in Magnetic Resonance Images of Anterior Cruciate Ligament-Injured Knees. Acta Orthop. Belg. 2014, 80, 45–49. [Google Scholar] [PubMed]
- Sonnery-Cottet, B.; Daggett, M.; Fayard, J.-M.; Ferretti, A.; Helito, C.P.; Lind, M.; Monaco, E.; de Pádua, V.B.C.; Thaunat, M.; Wilson, A.; et al. Anterolateral Ligament Expert Group Consensus Paper on the Management of Internal Rotation and Instability of the Anterior Cruciate Ligament—Deficient Knee. J. Orthop. Traumatol. 2017, 18, 91–106. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Cavalier, M.; Kajetanek, C.; Temponi, E.F.; Daggett, M.; Helito, C.P.; Thaunat, M. Anterolateral Ligament Reconstruction Is Associated with Significantly Reduced ACL Graft Rupture Rates at a Minimum Follow-up of 2 Years: A Prospective Comparative Study of 502 Patients from the SANTI Study Group. Am. J. Sports Med. 2017, 45, 1547–1557. [Google Scholar] [CrossRef]
- Tramer, J.S.; Fidai, M.S.; Kadri, O.; Jildeh, T.R.; Hooda, Z.; Makhni, E.C.; Lock, T. Anterolateral Ligament Reconstruction Practice Patterns Across the United States. Orthop. J. Sport. Med. 2018, 6, 232596711881106. [Google Scholar] [CrossRef] [PubMed]
- Reider, B. ACL or ACL+. Am. J. Sports Med. 2020, 48, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Thaunat, M.; Freychet, B.; Pupim, B.H.B.; Murphy, C.G.; Claes, S. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique with a Minimum 2-Year Follow-Up. Am. J. Sports Med. 2015, 43, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Clancy, W.G.; Nelson, D.A.; Reider, B.; Narechania, R.G. Anterior Cruciate Ligament Reconstruction Using One-Third of the Patellar Ligament, Augmented by Extra-Articular Tendon Transfers. J. Bone Jt. Surg. Am. 1982, 64, 352–359. [Google Scholar] [CrossRef]
- Dejour, H.; Dejour, D.; Aït Si Selmi, T. Laxité Chronique Du Genou Traité Par Une Greffe de Tendon Rotulien Libre et Une Plastie Extra Articulaire Antérolatérale. 10 Ans de Recul. 148 Cas. Rev. Chir. Orthop. Réparatrice Appar. Locomot. 1999, 85, 777–789. [Google Scholar]
- Marcacci, M.; Zaffagnini, S.; Iacono, F.; Neri, M.P.; Loreti, I.; Petitto, A. Arthroscopic Intra- and Extra-Articular Anterior Cruciate Ligament Reconstruction with Gracilis and Semitendinosus Tendons. Knee Surg. Sport. Traumatol. Arthrosc. 1998, 6, 68–75. [Google Scholar] [CrossRef]
- Vadalà, A.P.; Iorio, R.; De Carli, A.; Bonifazi, A.; Iorio, C.; Gatti, A.; Rossi, C.; Ferretti, A. An Extra-Articular Procedure Improves the Clinical Outcome in Anterior Cruciate Ligament Reconstruction with Hamstrings in Female Athletes. Int. Orthop. 2013, 37, 187–192. [Google Scholar] [CrossRef]
- Monaco, E.; Maestri, B.; Conteduca, F.; Mazza, D.; Iorio, C.; Ferretti, A. Extra-Articular ACL Reconstruction and Pivot Shift. Am. J. Sports Med. 2014, 42, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Duthon, V.B.; Magnussen, R.A.; Servien, E.; Neyret, P. ACL Reconstruction and Extra-Articular Tenodesis. Clin. Sports Med. 2013, 32, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Colombet, P.D. Navigated Intra-Articular ACL Reconstruction with Additional Extra-Articular Tenodesis Using the Same Hamstring Graft. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 384–389. [Google Scholar] [CrossRef]
- Song, G.; Hong, L.; Zhang, H.; Zhang, J.; Li, Y.; Feng, H. Clinical Outcomes of Combined Lateral Extra-Articular Tenodesis and Intra-Articular Anterior Cruciate Ligament Reconstruction in Addressing High-Grade Pivot-Shift Phenomenon. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 898–905. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Warren, R.F.; Pavlov, H.; Panariello, R.; Wickiewicz, T.L. Reconstruction of the Chronically Insufficient Anterior Cruciate Ligament with the Central Third of the Patellar Ligament. J. Bone Jt. Surg. Am. 1991, 73, 278–286. [Google Scholar] [CrossRef]
- Roth, J.H.; Kennedy, J.C.; Lockstadt, H.; McCallum, C.L.; Cunning, L.A. Intra-Articular Reconstruction of the Anterior Cruciate Ligament with and without Extra-Articular Supplementation by Transfer of the Biceps Femoris Tendon. J. Bone Jt. Surg. Am. 1987, 69, 275–278. [Google Scholar] [CrossRef]
- Pernin, J.; Verdonk, P.; Si Selmi, T.A.; Massin, P.; Neyret, P. Long-Term Follow-Up of 24.5 Years After Intra-Articular Anterior Cruciate Ligament Reconstruction with Lateral Extra-Articular Augmentation. Am. J. Sports Med. 2010, 38, 1094–1102. [Google Scholar] [CrossRef]
- Ferretti, A.; Monaco, E.; Ponzo, A.; Basiglini, L.; Iorio, R.; Caperna, L.; Conteduca, F. Combined Intra-Articular and Extra-Articular Reconstruction in Anterior Cruciate Ligament–Deficient Knee: 25 Years Later. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 2039–2047. [Google Scholar] [CrossRef]
- Thiese, M.S. Observational and Interventional Study Design Types; an Overview. Biochem. Medica 2014, 24, 199–210. [Google Scholar] [CrossRef]
- López Capdevilla, L.; Santamaría Fumas, A.; Sales Pérez, J.M.; Domínguez Sevilla, A.; del Corral Cuervo, J.; Varela-Quintana, C.; Rabanal Rubio, M.; Roza Miguel, P. Amputation versus Circular External Fixation in the Treatment of Diabetic Foot with Osteomyelitis: A Cost and Quality-of-Life Analysis. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241271795. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Maestro Fernández, A.; Pipa Muñiz, I.; Rodríguez García, N.; Maestro, A. Two-Stage Anterior Cruciate Ligament Reconstruction Revision Surgery for Severe Bone Defects with Anterolateral Ligament Reconstruction Technique. Arthrosc. Tech. 2020, 9, e327–e337. [Google Scholar] [CrossRef]
- Boutsiadis, A.; Brossard, P.; Panisset, J.-C.; Graveleau, N.; Barth, J. Minimally Invasive Combined Anterior and Anterolateral Stabilization of the Knee Using Hamstring Tendons and Adjustable-Loop Suspensory Fixation Device: Surgical Technique. Arthrosc. Tech. 2017, 6, e419–e425. [Google Scholar] [CrossRef] [PubMed]
- Saithna, A.; Thaunat, M.; Delaloye, J.R.; Ouanezar, H.; Fayard, J.M.; Sonnery-Cottet, B. Combined ACL and Anterolateral Ligament Reconstruction. JBJS Essent. Surg. Tech. 2018, 8, e2. [Google Scholar] [CrossRef]
- Helito, C.P.; Bonadio, M.B.; Gobbi, R.G.; da Mota e Albuquerque, R.F.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Combined Intra- and Extra-Articular Reconstruction of the Anterior Cruciate Ligament: The Reconstruction of the Knee Anterolateral Ligament. Arthrosc. Tech. 2015, 4, e239–e244. [Google Scholar] [CrossRef]
- Gurtler, R.A.; Stine, R.; Torg, J.S. Lachman Test Evaluated. Quantification of a Clinical Observation. Clin. Orthop. Relat. Res. 1987, 216, 141–150. [Google Scholar] [CrossRef]
- Galway, R.D.; Beaupre, A.; McIntosh, D.L. Pivot Shift: A Clinical Sign of Symptomatic Anterior Cruciate Insufficiency. Bone Jt. Surg. Br. 1972, 54B, 763–764. [Google Scholar]
- Tegner, Y.; Lysholm, J. Rating Systems in the Evaluation of Knee Ligament Injuries. Clin. Orthop. Relat. Res. 1985, 198, 43–49. [Google Scholar] [CrossRef]
- Hayes, M.; Patterson, D. Experimental Development of the Graphic Rating Method. Psychol. Bull. 1921, 18, 98–99. [Google Scholar]
- Gustavsson, A.; Neeter, C.; Thomeé, P.; Grävare Silbernagel, K.; Augustsson, J.; Thomeé, R.; Karlsson, J. A Test Battery for Evaluating Hop Performance in Patients with an ACL Injury and Patients Who Have Undergone ACL Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Braham, R.A.; Hale, S.A.; Olmsted-Kramer, L.C. Simplifying the Star Excursion Balance Test: Analyses of Subjects with and Without Chronic Ankle Instability. J. Orthop. Sports Phys. Ther. 2006, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stiffler, M.R.; Sanfilippo, J.L.; Brooks, M.A.; Heiderscheit, B.C. Star Excursion Balance Test Performance Varies by Sport in Healthy Division I Collegiate Athletes. J. Orthop. Sports Phys. Ther. 2015, 45, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J. Sensorimotor Deficits with Ankle Sprains and Chronic Ankle Instability. Clin. Sports Med. 2008, 27, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.A.; Hertel, J.; Plisky, P. Using the Star Excursion Balance Test to Assess Dynamic Postural-Control Deficits and Outcomes in Lower Extremity Injury: A Literature and Systematic Review. J. Athl. Train. 2012, 47, 339–357. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L.; Harner, C.D.; Kurosaka, M.; Neyret, P.; Richmond, J.C.; Shelborne, K.D. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sports Med. 2001, 29, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-Y.; Luo, J.-C.; Su, Y.; Zhang, Y.-J.; Tu, G.-W.; Luo, Z. Propensity Score Matching with R: Conventional Methods and New Features. Ann. Transl. Med. 2021, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Maldonado, D.R.; Kowalski, B.L.; Miecznikowski, K.B.; Kyin, C.; Gornbein, J.A.; Domb, B.G. Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 632–642. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://ropensci.org/blog/2021/11/16/h (accessed on 1 April 2019).
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Meuffels, D.E.; Poldervaart, M.T.; Diercks, R.L.; Fievez, A.W.F.M.; Patt, T.W.; Van Der Hart, C.P.; Hammacher, E.R.; Van Der Meer, F.; Goedhart, E.A.; Lenssen, A.F.; et al. Guideline on Anterior Cruciate Ligament Injury. Acta Orthop. 2012, 83, 379–386. [Google Scholar] [CrossRef]
- Carey, J.L.; Shea, K.G. AAOS Clinical Practice Guideline. J. Am. Acad. Orthop. Surg. 2015, 23, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.S.; Steadman, J.R.; Briggs, K.K.; Sterett, W.I.; Hawkins, R.J. Relationships between Objective Assessment of Ligament Stability and Subjective Assessment of Symptoms and Function after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2004, 32, 629–634. [Google Scholar] [CrossRef]
- Kubo, S.; Muratsu, H.; Yoshiya, S.; Mizuno, K.; Kurosaka, M. Reliability and Usefulness of a New In Vivo Measurement System of the Pivot Shift. Clin. Orthop. Relat. Res. 2007, 454, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Acquitter, Y.; Hulet, C.; Locker, B.; Delbarre, J.-C.; Jambou, S.; Vielpeau, C. Patellar Tendon-Bone Autograft Reconstruction of the Anterior Cruciate Ligament for Advanced-Stage Chronic Anterior Laxity: Is an Extra-Articular Plasty Necessary? A Prospective Randomized Study of 100 Patients with Five Year Follow-Up. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2003, 89, 413–422. [Google Scholar]
- Draganich, L.F.; Reider, B.; Ling, M.; Samuelson, M. An in Vitro Study of an Intraarticular and Extraarticular Reconstruction in the Anterior Cruciate Ligament Deficient Knee. Am. J. Sports Med. 1990, 18, 262–266. [Google Scholar] [CrossRef]
- Engebretsen, L.; Lew, W.D.; Lewis, J.L.; Hunter, R.E. The Effect of an Iliotibial Tenodesis on Intraarticular Graft Forces and Knee Joint Motion. Am. J. Sports Med. 1990, 18, 169–176. [Google Scholar] [CrossRef]
- Colombet, P. Knee Laxity Control in Revision Anterior Cruciate Ligament Reconstruction Versus Anterior Cruciate Ligament Reconstruction and Lateral Tenodesis. Am. J. Sports Med. 2011, 39, 1248–1254. [Google Scholar] [CrossRef]
- Giraud, B.; Besse, J.-L.; Cladière, F.; Ecochard, R.; Moyen, B.; Lerat, J.-L. Influence d’une Ligamentoplastie Extra-Articulaire Latérale Sur Les Résultats de La Reconstruction Du Ligament Croisé Antérieur Avec Le Ligament Patellaire Avec 7 Ans de Recul. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2006, 92, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Hewison, C.E.; Tran, M.N.; Kaniki, N.; Remtulla, A.; Bryant, D.; Getgood, A.M. Lateral Extra-Articular Tenodesis Reduces Rotational Laxity When Combined with Anterior Cruciate Ligament Reconstruction: A Systematic Review of the Literature. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 2022–2034. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, N.; Praz, C.; Ouanezar, H.; Saithna, A.; Fournier, Y.; Hager, J.-P.; Thaunat, M.; Sonnery-Cottet, B. Combined Anterior Cruciate and Anterolateral Ligament Reconstruction in the Professional Athlete: Clinical Outcomes from the Scientific Anterior Cruciate Ligament Network International Study Group in a Series of 70 Patients with a Minimum Follow-Up of 2 Y. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 885–892. [Google Scholar] [CrossRef]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-Five per Cent Return to Competitive Sport Following Anterior Cruciate Ligament Reconstruction Surgery: An Updated Systematic Review and Meta-Analysis Including Aspects of Physical Functioning and Contextual Factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T.; Manjunath, A.K.; Strauss, E.J.; Jazrawi, L.M.; Alaia, M.J. Return to Play After Anterior Cruciate Ligament Reconstruction with Extra-Articular Augmentation: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 381–387. [Google Scholar] [CrossRef]
- Laboudie, P.; Douiri, A.; Bouguennec, N.; Biset, A.; Graveleau, N. Combined ACL and ALL Reconstruction Reduces the Rate of Reoperation for Graft Failure or Secondary Meniscal Lesions in Young Athletes. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Haidar, I.; Rayes, J.; Fradin, T.; Ngbilo, C.; Vieira, T.D.; Freychet, B.; Ouanezar, H.; Saithna, A. Long-Term Graft Rupture Rates After Combined ACL and Anterolateral Ligament Reconstruction Versus Isolated ACL Reconstruction: A Matched-Pair Analysis from the SANTI Study Group. Am. J. Sports Med. 2021, 49, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Coquard, M.; Carrozzo, A.; Saithna, A.; Vigne, G.; Le Guen, M.; Fournier, Y.; Hager, J.-P.; Vieira, T.D.; Sonnery-Cottet, B. Anterolateral Ligament Reconstruction Does Not Delay Functional Recovery, Rehabilitation, and Return to Sport After Anterior Cruciate Ligament Reconstruction: A Matched-Pair Analysis from the SANTI (Scientific ACL Network International) Study Group. Arthrosc. Sports Med. Rehabil. 2022, 4, e9–e16. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.; Hewison, C.; Bryant, D.; Litchfield, R.; Heard, M.; Buchko, G.; Hiemstra, L.A.; Willits, K.R.; Firth, A.; MacDonald, P. No Difference in Functional Outcomes When Lateral Extra-Articular Tenodesis Is Added to Anterior Cruciate Ligament Reconstruction in Young Active Patients: The Stability Study. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1690–1701. [Google Scholar] [CrossRef]
- Helito, C.P.; Camargo, D.B.; Sobrado, M.F.; Bonadio, M.B.; Giglio, P.N.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Combined Reconstruction of the Anterolateral Ligament in Chronic ACL Injuries Leads to Better Clinical Outcomes than Isolated ACL Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3652–3659. [Google Scholar] [CrossRef]
- Plisky, P.J.; Rauh, M.J.; Kaminski, T.W.; Underwood, F.B. Star Excursion Balance Test as a Predictor of Lower Extremity Injury in High School Basketball Players. J. Orthop. Sports Phys. Ther. 2006, 36, 911–919. [Google Scholar] [CrossRef]
- Butler, R.J.; Lehr, M.E.; Fink, M.L.; Kiesel, K.B.; Plisky, P.J. Dynamic Balance Performance and Noncontact Lower Extremity Injury in College Football Players. Sports Health A Multidiscip. Approach 2013, 5, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.M.; Greenberg, E.T.; Ganley, T.J.; Lawrence, J.T.R. Strength and Functional Performance Recovery After Anterior Cruciate Ligament Reconstruction in Preadolescent Athletes. Sports Health A Multidiscip. Approach 2014, 6, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Lockie, R.G.; Callaghan, S.J.; Berry, S.P.; Cooke, E.R.A.; Jordan, C.A.; Luczo, T.M.; Jeffriess, M.D. Relationship Between Unilateral Jumping Ability and Asymmetry on Multidirectional Speed in Team-Sport Athletes. J. Strength Cond. Res. 2014, 28, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Sannicandro, I.; Cofano, G.; Rosa, R.A.; Piccinno, A. Balance Training Exercises Decrease Lower-Limb Strength Asymmetry in Young Tennis Players. J. Sports Sci. Med. 2014, 13, 397–402. [Google Scholar] [PubMed]
- Gribble, P.A.; Hertel, J. Considerations for Normalizing Measures of the Star Excursion Balance Test. Meas. Phys. Educ. Exerc. Sci. 2003, 7, 89–100. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Reinke, E.K.; Huston, L.J.; Hewett, T.E.; Spindler, K.P.; Andrish, J.T.; Jones, M.H.; Parker, R.D.; McCarty, E.C.; Marx, R.G.; et al. Effect of High-Grade Preoperative Knee Laxity on Anterior Cruciate Ligament Reconstruction Outcomes. Am. J. Sports Med. 2016, 44, 3077–3082. [Google Scholar] [CrossRef]
- Hopper, G.P.; Pioger, C.; Philippe, C.; El Helou, A.; Campos, J.P.; Gousopoulos, L.; Carrozzo, A.; Vieira, T.D.; Sonnery-Cottet, B. Risk Factors for Anterior Cruciate Ligament Graft Failure in Professional Athletes: An Analysis of 342 Patients with a Mean Follow-up of 100 Months from the SANTI Study Group. Am. J. Sports Med. 2022, 50, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Feller, J.A. Exploring the High Reinjury Rate in Younger Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2016, 44, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Kamath, G.V.; Murphy, T.; Creighton, R.A.; Viradia, N.; Taft, T.N.; Spang, J.T. Anterior Cruciate Ligament Injury, Return to Play, and Reinjury in the Elite Collegiate Athlete. Am. J. Sports Med. 2014, 42, 1638–1643. [Google Scholar] [CrossRef]
- Carey, T.; Sanders, G.; Viswanathan, M. Framework for Considering Study Designs for Future Research Needs [Internet]. Agency for Healthcare Research and Quality (US). Available online: https://www.ncbi.nlm.nih.gov/books/NBK95280/ (accessed on 20 September 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).