Current Status of Anti-Reflux Surgery as a Treatment for GERD
Abstract
1. Introduction
2. Materials and Methods
3. Efficacy of ARS in Refractory GERD
4. Comparison between ARS and PPI
5. Cost-Effectiveness of ARS
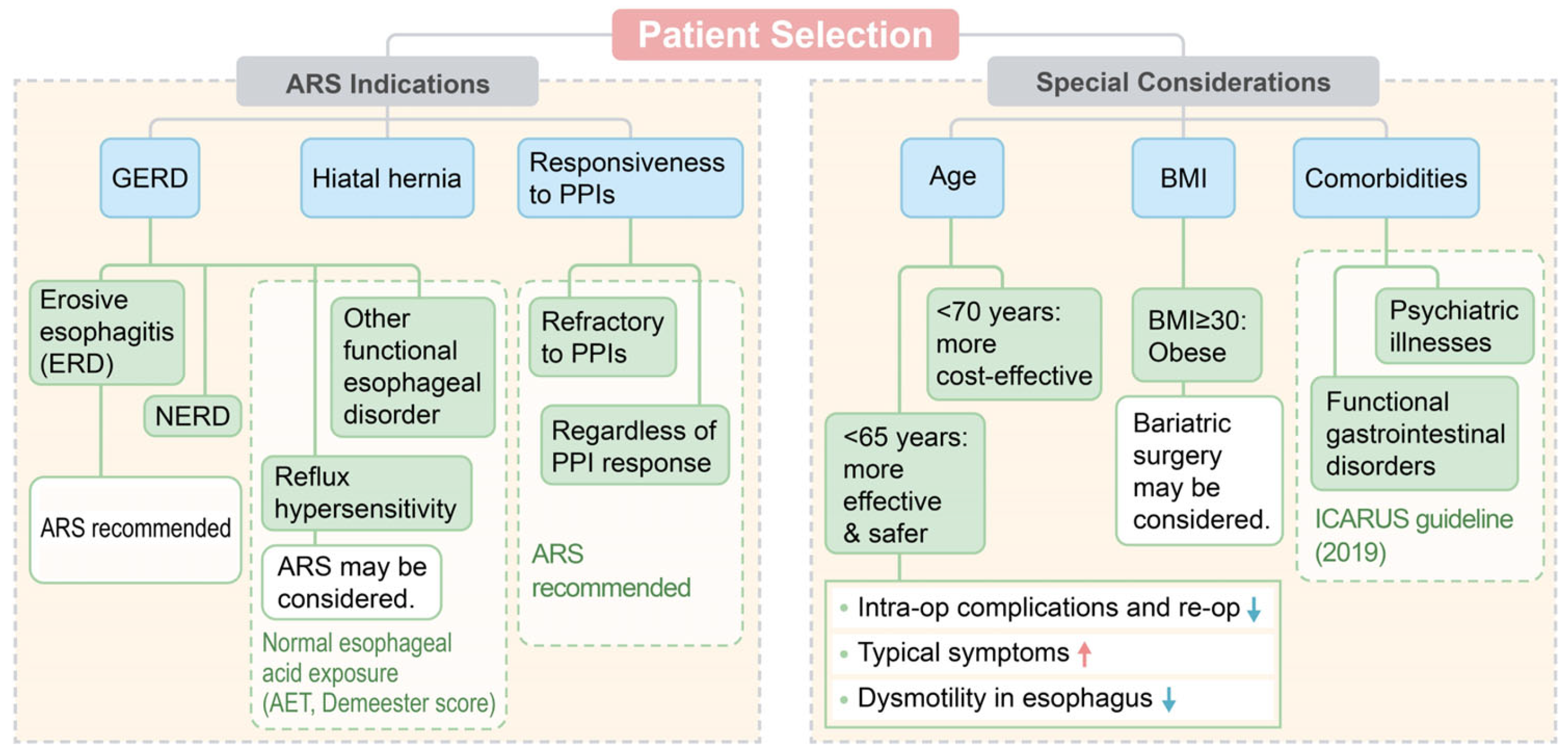
6. Patient Selection of ARS
6.1. Association between Symptoms and Reflux Events
6.2. Age
6.3. Body Mass Index
6.4. Comorbidities
7. Complications and Failure of ARS
8. Nissen Fundoplication and Partial Fundoplication
9. Public and Health Workers’ Awareness of ARS
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.U.; Conway, B.R.; Ghori, M.U. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): Systematic review with meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef]
- Jung, H.K.; Tae, C.H.; Song, K.H.; Kang, S.J.; Park, J.K.; Gong, E.J.; Shin, J.E.; Lim, H.C.; Lee, S.K.; Jung, D.H.; et al. 2020 Seoul consensus on the diagnosis and management of gastroesophageal reflux disease. J. Neurogastroenterol. Motil. 2021, 27, 453–481. [Google Scholar] [CrossRef]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef]
- Kahrilas, P.J.; Boeckxstaens, G.; Smout, A.J. Management of the patient with incomplete response to PPI therapy. Best. Pract. Res. Clin. Gastroenterol. 2013, 27, 401–414. [Google Scholar] [CrossRef]
- El-Serag, H.; Becher, A.; Jones, R. Systematic review: Persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment. Pharmacol. Ther. 2010, 32, 720–737. [Google Scholar] [CrossRef]
- Becher, A.; El-Serag, H. Systematic review: The association between symptomatic response to proton pump inhibitors and health-related quality of life in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2011, 34, 618–627. [Google Scholar] [CrossRef]
- Yadlapati, R.; Vaezi, M.F.; Vela, M.F.; Spechler, S.J.; Shaheen, N.J.; Richter, J.; Lacy, B.E.; Katzka, D.; Katz, P.O.; Kahrilas, P.J.; et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: Recommendations from an expert panel. Am. J. Gastroenterol. 2018, 113, 980–986. [Google Scholar] [CrossRef]
- Sifrim, D.; Zerbib, F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012, 61, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- Eom, C.S.; Jeon, C.Y.; Lim, J.W.; Cho, E.G.; Park, S.M.; Lee, K.S. Use of acid-suppressive drugs and risk of pneumonia: A systematic review and meta-analysis. CMAJ 2011, 183, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Lix, L.M.; Metge, C.J.; Prior, H.J.; Leung, S.; Leslie, W.D. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008, 179, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Gupta, A.; Golzy, M.; Patel, N.; Carter, R.L.; Jalal, K.; Lohr, J.W. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016, 17, 112. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Bin Abdulhak, A.A.; Riaz, M.; Alasmari, F.A.; Garbati, M.A.; AlGhamdi, M.; Khan, A.R.; Al Tannir, M.; Erwin, P.J.; Ibrahim, T.; et al. Association between proton pump inhibitor therapy and clostridium difficile infection: A contemporary systematic review and meta-analysis. PLoS ONE 2012, 7, e50836. [Google Scholar] [CrossRef]
- Tristão, L.S.; Tustumi, F.; Tavares, G.; Bernardo, W.M. Fundoplication versus oral proton pump inhibitors for gastroesophageal reflux disease: A systematic review and meta-analysis of randomized clinical trials. Esophagus 2021, 18, 173–180. [Google Scholar] [CrossRef]
- Kahrilas, P.J.; Lin, S.; Chen, J.; Manka, M. The effect of hiatus hernia on gastro-oesophageal junction pressure. Gut 1999, 44, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kohn, G.P.; Price, R.R.; DeMeester, S.R.; Zehetner, J.; Muensterer, O.J.; Awad, Z.; Mittal, S.K.; Richardson, W.S.; Stefanidis, D.; Fanelli, R.D. Guidelines for the management of hiatal hernia. Surg. Endosc. 2013, 27, 4409–4428. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Dirks, R.C.; McKinley, S.K.; Ansari, M.T.; Kohn, G.P.; Thosani, N.; Qumseya, B.; Billmeier, S.; Daly, S.; Crawford, C.; et al. SAGES guidelines for the surgical treatment of gastroesophageal reflux (GERD). Surg. Endosc. 2021, 35, 4903–4917. [Google Scholar] [CrossRef]
- Cookson, R.; Flood, C.; Koo, B.; Mahon, D.; Rhodes, M. Short-term cost effectiveness and long-term cost analysis comparing laparoscopic Nissen fundoplication with proton-pump inhibitor maintenance for gastro-oesophageal reflux disease. Br. J. Surg. 2005, 92, 700–706. [Google Scholar] [CrossRef]
- Epstein, D.; Bojke, L.; Sculpher, M.J. Laparoscopic fundoplication compared with medical management for gastro-oesophageal reflux disease: Cost effectiveness study. BMJ 2009, 339, b2576. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Maradey-Romero, C.; Ganocy, S.; Frazier, R.; Fass, R. Utilisation of surgical fundoplication for patients with gastro-oesophageal reflux disease in the USA has declined rapidly between 2009 and 2013. Aliment. Pharmacol. Ther. 2016, 43, 1124–1131. [Google Scholar] [CrossRef]
- Palser, T.R.; Ceney, A.; Navarro, A.; Swift, S.; Bowrey, D.J.; Beckingham, I.J. Variation in laparoscopic anti-reflux surgery across England: A 5-year review. Surg. Endosc. 2018, 32, 3208–3214. [Google Scholar] [CrossRef]
- Park, S.; Park, J.M.; Kim, J.J.; Lee, I.S.; Han, S.U.; Seo, K.W.; Kwon, J.W. Multicenter prospective study of laparoscopic Nissen fundoplication for gastroesophageal reflux disease in Korea. J. Neurogastroenterol. Motil. 2019, 25, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.C.; Holloway, R.H.; Toouli, J.; Dent, J. Mechanisms underlying the antireflux action of fundoplication. Gut 1993, 34, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sandler, B.; Bhargava, V.; Mittal, R.K. Antireflux action of Nissen fundoplication and stretch-sensitive mechanism of lower esophageal sphincter relaxation. Gastroenterology 2011, 140, 442–449. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Zhang, N.; Xiong, L.; Chen, M.; Xiao, Y. Gastroesophageal flap valve reflected EGJ morphology and correlated to acid reflux. BMC Gastroenterol. 2017, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.; Allen, C. Surgical outcome in gastro-esophageal reflux disease patients with inadequate response to proton pump inhibitors. Surg. Endosc. 2003, 17, 1029–1035. [Google Scholar] [CrossRef]
- Frazzoni, M.; Conigliaro, R.; Melotti, G. Reflux parameters as modified by laparoscopic fundoplication in 40 patients with heartburn/regurgitation persisting despite PPI therapy: A study using impedance-pH monitoring. Dig. Dis. Sci. 2011, 56, 1099–1106. [Google Scholar] [CrossRef]
- Spechler, S.J.; Hunter, J.G.; Jones, K.M.; Lee, R.; Smith, B.R.; Mashimo, H.; Sanchez, V.M.; Dunbar, K.B.; Pham, T.H.; Murthy, U.K.; et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N. Engl. J. Med. 2019, 381, 1513–1523. [Google Scholar] [CrossRef]
- Broeders, J.A.; Draaisma, W.A.; Bredenoord, A.J.; de Vries, D.R.; Rijnhart-de Jong, H.G.; Smout, A.J.; Gooszen, H.G. Oesophageal acid hypersensitivity is not a contraindication to Nissen fundoplication. Br. J. Surg. 2009, 96, 1023–1030. [Google Scholar] [CrossRef]
- Mainie, I.; Tutuian, R.; Agrawal, A.; Adams, D.; Castell, D.O. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br. J. Surg. 2006, 93, 1483–1487. [Google Scholar] [CrossRef]
- Hamdy, E.; El Nakeeb, A.; Hamed, H.; El Hemaly, M.; ElHak, N.G. Outcome of laparoscopic Nissen fundoplication for gastroesophageal reflux disease in non-responders to proton pump inhibitors. J. Gastrointest. Surg. 2014, 18, 1557–1562. [Google Scholar] [CrossRef]
- Lundell, L.; Bell, M.; Ruth, M. Systematic review: Laparoscopic fundoplication for gastroesophageal reflux disease in partial responders to proton pump inhibitors. World J. Gastroenterol. 2014, 20, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Morgenthal, C.B.; Lin, E.; Shane, M.D.; Hunter, J.G.; Smith, C.D. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg. Endosc. 2007, 21, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, P.M.; Stratford, J.; Jones, L.; Sohanpal, J.; Booth, M.I.; Dehn, T.C. A poor response to proton pump inhibition is not a contraindication for laparoscopic antireflux surgery for gastro esophageal reflux disease. Surg. Endosc. 2005, 19, 1272–1277. [Google Scholar] [CrossRef]
- Seo, H.S.; Choi, M.; Son, S.Y.; Kim, M.G.; Han, D.S.; Lee, H.H. Evidence-based practice guideline for surgical treatment of gastroesophageal reflux disease 2018. J. Gastric Cancer 2018, 18, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Gurusamy, K.S. Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst. Rev. 2015, 2015, Cd003243. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.W.; Park, J.M.; Park, S.; Hwang, J.; Seo, K.W. The characteristics of antireflux surgery compared to proton pump inhibitor treatment in Korea: A nationwide study using claim data from 2007 to 2016. Ann. Surg. Treat. Res. 2020, 98, 254–261. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.W.; Park, J.M.; Park, S.; Seo, K.W. Treatment pattern and economic burden of refractory gastroesophageal reflux disease patients in Korea. J. Neurogastroenterol. Motil. 2020, 26, 281–288. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Park, J.M.; Ryu, S.; Hwang, J.; Kwon, J.W.; Seo, K.W. Anti-reflux surgery versus proton pump inhibitors for severe gastroesophageal reflux disease: A cost-effectiveness study in Korea. J. Neurogastroenterol. Motil. 2020, 26, 215–223. [Google Scholar] [CrossRef]
- Pauwels, A.; Boecxstaens, V.; Andrews, C.N.; Attwood, S.E.; Berrisford, R.; Bisschops, R.; Boeckxstaens, G.E.; Bor, S.; Bredenoord, A.J.; Cicala, M.; et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut 2019, 68, 1928–1941. [Google Scholar] [CrossRef]
- Lee, I.; Park, S. Diagnosis and treatment of reflux hypersensitivity with gastroesophageal reflux symptoms from a surgical perspective. Foregut Surg. 2022, 2, 8–16. [Google Scholar] [CrossRef]
- Fernando, H.C.; Schauer, P.R.; Buenaventura, P.O.; Christie, N.A.; Close, J.M.; Luketich, J.D. Outcomes of minimally invasive antireflux operations in the elderly: A comparative review. JSLS J. Soc. Laparoendosc. Surg. 2003, 7, 311–315. [Google Scholar]
- Irino, T.; Takeuchi, H.; Ozawa, S.; Saikawa, Y.; Oyama, T.; Hiraiwa, K.; Yoshikawa, T.; Kitajima, M.; Kitagawa, Y. Age and body mass index: Significant predictive factors for successful laparoscopic antireflux surgery. Surg. Today 2010, 40, 1137–1143. [Google Scholar] [CrossRef]
- Brehant, O.; Pessaux, P.; Arnaud, J.P.; Delattre, J.F.; Meyer, C.; Baulieux, J.; Mosnier, H. Long-term outcome of laparoscopic antireflux surgery in the elderly. J. Gastrointest. Surg. 2006, 10, 439–444. [Google Scholar] [CrossRef]
- Cowgill, S.M.; Arnaoutakis, D.; Villadolid, D.; Al-Saadi, S.; Arnaoutakis, D.; Molloy, D.L.; Thomas, A.; Rakita, S.; Rosemurgy, A., 2nd. Results after laparoscopic fundoplication: Does age matter? Am. Surg. 2006, 72, 778–783. [Google Scholar] [CrossRef]
- Addo, A.; Sanford, Z.; Broda, A.; Zahiri, H.R.; Park, A. Age-related outcomes in laparoscopic hiatal hernia repair: Is there a "too old" for antireflux surgery? Surg. Endosc. 2021, 35, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Rossetti, G.; Moccia, F.; Marra, T.; Guadagno, P.; Docimo, L.; Cimmino, M.; Napolitano, V.; Docimo, G.; Napoletano, D.; et al. Is the advanced age a contraindication to GERD laparoscopic surgery? Results of a long term follow-up. BMC Surg. 2013, 13 (Suppl. 2), S13. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.L.; Pennathur, A.; Mutnick, J.L.; Little, A.G. Obesity correlates with gastroesophageal reflux. Dig. Dis. Sci. 1999, 44, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Wajed, S.A.; Streets, C.G.; Bremner, C.G.; DeMeester, T.R. Elevated body mass disrupts the barrier to gastroesophageal reflux. Arch. Surg. 2001, 136, 1014–1018, discussion 1018–1019. [Google Scholar] [CrossRef]
- Fraser, J.; Watson, D.I.; O’Boyle, C.J.; Jamieson, G.G. Obesity and its effect on outcome of laparoscopic Nissen fundoplication. Dis. Esophagus 2001, 14, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Luketina, R.R.; Koch, O.O.; Köhler, G.; Antoniou, S.A.; Emmanuel, K.; Pointner, R. Obesity does not affect the outcome of laparoscopic antireflux surgery. Surg. Endosc. 2015, 29, 1327–1333. [Google Scholar] [CrossRef]
- Tandon, A.; Rao, R.; Hotouras, A.; Nunes, Q.M.; Hartley, M.; Gunasekera, R.; Howes, N. Safety and effectiveness of antireflux surgery in obese patients. Ann. R. Coll. Surg. Engl. 2017, 99, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, T.; Latif, A.; Chan, D.S.; Jones, H.; Farag, M.; Lewis, W.G.; Havard, T.; Escofet, X. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int. J. Surg. 2018, 51, 76–82. [Google Scholar] [CrossRef]
- Bashir, Y.; Chonchubhair, H.N.; Duggan, S.N.; Memba, R.; Ain, Q.U.; Murphy, A.; McMahon, J.; Ridgway, P.F.; Conlon, K.C. Systematic review and meta-analysis on the effect of obesity on recurrence after laparoscopic anti-reflux surgery. Surgeon 2019, 17, 107–118. [Google Scholar] [CrossRef]
- Varela, J.E.; Hinojosa, M.W.; Nguyen, N.T. Laparoscopic fundoplication compared with laparoscopic gastric bypass in morbidly obese patients with gastroesophageal reflux disease. Surg. Obes. Relat. Dis. 2009, 5, 139–143. [Google Scholar] [CrossRef]
- Patterson, E.J.; Davis, D.G.; Khajanchee, Y.; Swanström, L.L. Comparison of objective outcomes following laparoscopic Nissen fundoplication versus laparoscopic gastric bypass in the morbidly obese with heartburn. Surg. Endosc. 2003, 17, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Melissas, J.; Braghetto, I.; Molina, J.C.; Silecchia, G.; Iossa, A.; Iannelli, A.; Foletto, M. Gastroesophageal reflux disease and sleeve gastrectomy. Obes. Surg. 2015, 25, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Pavone, G.; Tartaglia, N.; Porfido, A.; Panzera, P.; Pacilli, M.; Ambrosi, A. The new onset of GERD after sleeve gastrectomy: A systematic review. Ann. Med. Surg. 2022, 77, 103584. [Google Scholar] [CrossRef]
- Laffin, M.; Chau, J.; Gill, R.S.; Birch, D.W.; Karmali, S. Sleeve gastrectomy and gastroesophageal reflux disease. J. Obes. 2013, 2013, 741097. [Google Scholar] [CrossRef]
- Braghetto, I.; Lanzarini, E.; Korn, O.; Valladares, H.; Molina, J.C.; Henriquez, A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes. Surg. 2010, 20, 357–362. [Google Scholar] [CrossRef]
- Himpens, J.; Dapri, G.; Cadière, G.B. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: Results after 1 and 3 years. Obes. Surg. 2006, 16, 1450–1456. [Google Scholar] [CrossRef]
- Yehoshua, R.T.; Eidelman, L.A.; Stein, M.; Fichman, S.; Mazor, A.; Chen, J.; Bernstine, H.; Singer, P.; Dickman, R.; Beglaibter, N.; et al. Laparoscopic sleeve gastrectomy--volume and pressure assessment. Obes. Surg. 2008, 18, 1083–1088. [Google Scholar] [CrossRef]
- Salman, M.A.; Mikhail, H.M.S.; Abdelsalam, A.; Abdallah, A.; Elshafey, H.E.; Abouelregal, T.E.; Omar, M.G.; Elkassar, H.; Ahmed, R.A.; Atallah, M.; et al. Acceleration of gastric emptying and improvement of GERD outcome after laparoscopic sleeve gastrectomy in non-diabetic obese patients. Obes. Surg. 2020, 30, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, N.; Vanuytsel, T.; Augustijns, P.; Tack, J.; Mertens, A.; Lannoo, M.; Van der Schueren, B.; Matthys, C. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol. Hepatol. 2021, 6, 225–237. [Google Scholar] [CrossRef]
- Carandina, S.; Zulian, V.; Nedelcu, A.; Danan, M.; Vilallonga, R.; Nocca, D.; Nedelcu, M. Is it safe to combine a fundoplication to sleeve gastrectomy? Review of literature. Medicina 2021, 57, 392. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef]
- Broeders, J.A.; Roks, D.J.; Ahmed Ali, U.; Watson, D.I.; Baigrie, R.J.; Cao, Z.; Hartmann, J.; Maddern, G.J. Laparoscopic anterior 180-degree versus nissen fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis of randomized clinical trials. Ann. Surg. 2013, 257, 850–859. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, Y.; Lee, J.H.; Park, J.M.; Kim, J.J.; Song, K.Y.; Ryu, S.W.; Seo, K.W.; Kim, H.I.; Kim, D.J.; et al. Trends in laparoscopic anti-reflux surgery: A Korea nationwide study. Surg. Endosc. 2021, 35, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.G.; Smith, C.D.; Branum, G.D.; Waring, J.P.; Trus, T.L.; Cornwell, M.; Galloway, K. Laparoscopic fundoplication failures: Patterns of failure and response to fundoplication revision. Ann. Surg. 1999, 230, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lafullarde, T.; Watson, D.I.; Jamieson, G.G.; Myers, J.C.; Game, P.A.; Devitt, P.G. Laparoscopic Nissen fundoplication: Five-year results and beyond. Arch. Surg. 2001, 136, 180–184. [Google Scholar] [CrossRef]
- Carlson, M.A.; Frantzides, C.T. Complications and results of primary minimally invasive antireflux procedures: A review of 10,735 reported cases. J. Am. Coll. Surg. 2001, 193, 428–439. [Google Scholar] [CrossRef]
- Furnée, E.J.; Draaisma, W.A.; Broeders, I.A.; Gooszen, H.G. Surgical reintervention after failed antireflux surgery: A systematic review of the literature. J. Gastrointest. Surg. 2009, 13, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; McClusky, D.A.; Rajad, M.A.; Lederman, A.B.; Hunter, J.G. When fundoplication fails: Redo? Ann. Surg. 2005, 241, 861–869, discussion 869–871. [Google Scholar] [CrossRef] [PubMed]
- van Beek, D.B.; Auyang, E.D.; Soper, N.J. A comprehensive review of laparoscopic redo fundoplication. Surg. Endosc. 2011, 25, 706–712. [Google Scholar] [CrossRef]
- Dallemagne, B.; Arenas Sanchez, M.; Francart, D.; Perretta, S.; Weerts, J.; Markiewicz, S.; Jehaes, C. Long-term results after laparoscopic reoperation for failed antireflux procedures. Br. J. Surg. 2011, 98, 1581–1587. [Google Scholar] [CrossRef]
- Byrne, J.P.; Smithers, B.M.; Nathanson, L.K.; Martin, I.; Ong, H.S.; Gotley, D.C. Symptomatic and functional outcome after laparoscopic reoperation for failed antireflux surgery. Br. J. Surg. 2005, 92, 996–1001. [Google Scholar] [CrossRef]
- Furnée, E.J.; Draaisma, W.A.; Broeders, I.A.; Smout, A.J.; Gooszen, H.G. Surgical reintervention after antireflux surgery for gastroesophageal reflux disease: A prospective cohort study in 130 patients. Arch. Surg. 2008, 143, 267–274, discussion 274. [Google Scholar] [CrossRef]
- Awais, O.; Luketich, J.D.; Schuchert, M.J.; Morse, C.R.; Wilson, J.; Gooding, W.E.; Landreneau, R.J.; Pennathur, A. Reoperative antireflux surgery for failed fundoplication: An analysis of outcomes in 275 patients. Ann. Thorac. Surg. 2011, 92, 1083–1089, discussion 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bamehriz, F.; Boghossian, T.; Pottruff, C.G.; Anvari, M. Outcome of laparoscopic redo fundoplication. Surg. Endosc. 2004, 18, 440–443. [Google Scholar] [CrossRef]
- Grover, B.T.; Kothari, S.N. Reoperative antireflux surgery. Surg. Clin. North. Am. 2015, 95, 629–640. [Google Scholar] [CrossRef]
- Schlottmann, F.; Laxague, F.; Angeramo, C.A.; Sadava, E.E.; Herbella, F.A.M.; Patti, M.G. Outcomes of laparoscopic redo fundoplication in patients with failed antireflux surgery: A systematic review and meta-analysis. Ann. Surg. 2021, 274, 78–85. [Google Scholar] [CrossRef]
- Chiappetta, S.; de Falco, N.; Lainas, P.; Kassir, R.; Valizadeh, R.; Kermansaravi, M. Safety and efficacy of Roux-en-Y gastric bypass as revisional bariatric surgery after failed anti-reflux surgery: A systematic review. Surg. Obes. Relat. Dis. 2023, 19, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Soot, S.J.; Eshraghi, N.; Farahmand, M.; Sheppard, B.C.; Deveney, C.W. Transition from open to laparoscopic fundoplication: The learning curve. Arch. Surg. 1999, 134, 278–281, discussion 282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, H.; Turner, L.J.; Blair, N.P. Examining the learning curve of laparoscopic fundoplications at an urban community hospital. Am. J. Surg. 2005, 189, 522–526, discussion 526. [Google Scholar] [CrossRef] [PubMed]
- Endzinas, Z.; Maleckas, A.; Mickevicius, A.; Kiudelis, M. Follow-up results and learning curve in laparoscopic gastrofundoplications. Zentralbl. Chir. 2002, 127, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.N.; Smith, L.T.; Watson, D.I.; Devitt, P.G.; Thompson, S.K.; Jamieson, G.G. Outcomes for trainees vs experienced surgeons undertaking laparoscopic antireflux surgery is equipoise achieved? J. Gastrointest. Surg. 2013, 17, 1173–1180. [Google Scholar] [CrossRef]
- Du, X.; Wu, J.M.; Hu, Z.W.; Wang, F.; Wang, Z.G.; Zhang, C.; Yan, C.; Chen, M.P. Laparoscopic Nissen (total) versus anterior 180° fundoplication for gastro-esophageal reflux disease: A meta-analysis and systematic review. Medicine 2017, 96, e8085. [Google Scholar] [CrossRef]
- Tian, Z.C.; Wang, B.; Shan, C.X.; Zhang, W.; Jiang, D.Z.; Qiu, M. A meta-analysis of randomized controlled trials to compare long-term outcomes of Nissen and Toupet fundoplication for gastroesophageal reflux disease. PLoS ONE 2015, 10, e0127627. [Google Scholar] [CrossRef]
- Broeders, J.A.; Roks, D.J.; Ahmed Ali, U.; Draaisma, W.A.; Smout, A.J.; Hazebroek, E.J. Laparoscopic anterior versus posterior fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis of randomized clinical trials. Ann. Surg. 2011, 254, 39–47. [Google Scholar] [CrossRef]
- Patti, M.G. An Evidence-based approach to the treatment of gastroesophageal reflux disease. JAMA Surg. 2016, 151, 73–78. [Google Scholar] [CrossRef]
- Makris, K.I.; Cassera, M.A.; Kastenmeier, A.S.; Dunst, C.M.; Swanström, L.L. Postoperative dysphagia is not predictive of long-term failure after laparoscopic antireflux surgery. Surg. Endosc. 2012, 26, 451–457. [Google Scholar] [CrossRef]
- Matusitz, J.; Spear, J. Effective doctor-patient communication: An updated examination. Soc. Work. Public. Health 2014, 29, 252–266. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, I.; Oh, Y.; Kim, J.W.; Kwon, Y.; Alromi, A.; Eledreesi, M.; Khalid, A.; Aljarbou, W.; Park, S. Current Status of Anti-Reflux Surgery as a Treatment for GERD. Medicina 2024, 60, 518. https://doi.org/10.3390/medicina60030518
Lee J, Lee I, Oh Y, Kim JW, Kwon Y, Alromi A, Eledreesi M, Khalid A, Aljarbou W, Park S. Current Status of Anti-Reflux Surgery as a Treatment for GERD. Medicina. 2024; 60(3):518. https://doi.org/10.3390/medicina60030518
Chicago/Turabian StyleLee, Jooyeon, Inhyeok Lee, Youjin Oh, Jeong Woo Kim, Yeongkeun Kwon, Ahmad Alromi, Mohannad Eledreesi, Alkadam Khalid, Wafa Aljarbou, and Sungsoo Park. 2024. "Current Status of Anti-Reflux Surgery as a Treatment for GERD" Medicina 60, no. 3: 518. https://doi.org/10.3390/medicina60030518
APA StyleLee, J., Lee, I., Oh, Y., Kim, J. W., Kwon, Y., Alromi, A., Eledreesi, M., Khalid, A., Aljarbou, W., & Park, S. (2024). Current Status of Anti-Reflux Surgery as a Treatment for GERD. Medicina, 60(3), 518. https://doi.org/10.3390/medicina60030518








