Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Measured Outcomes
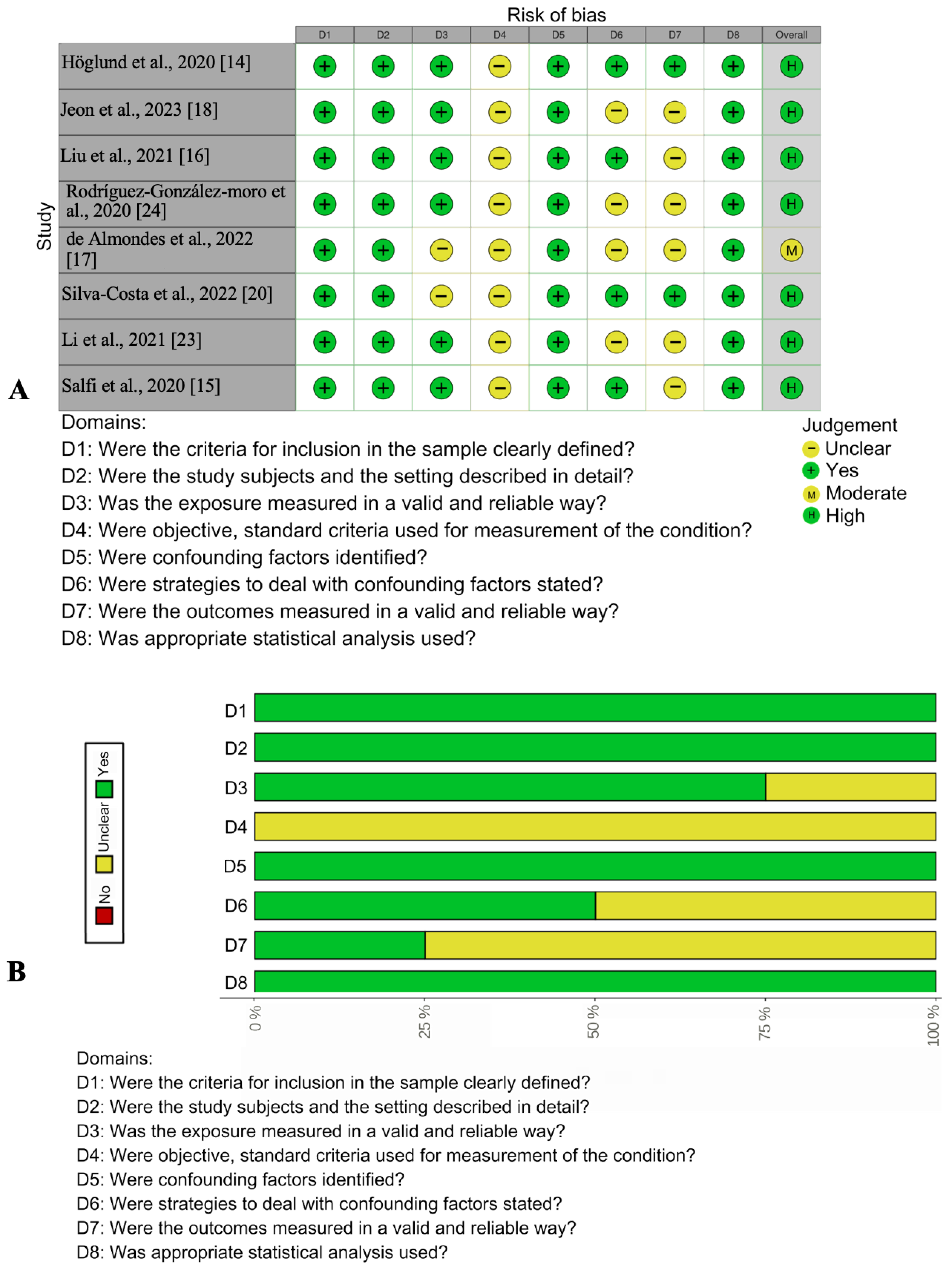
2.4. Assessment of Risk of Bias
2.5. Statistical Analysis
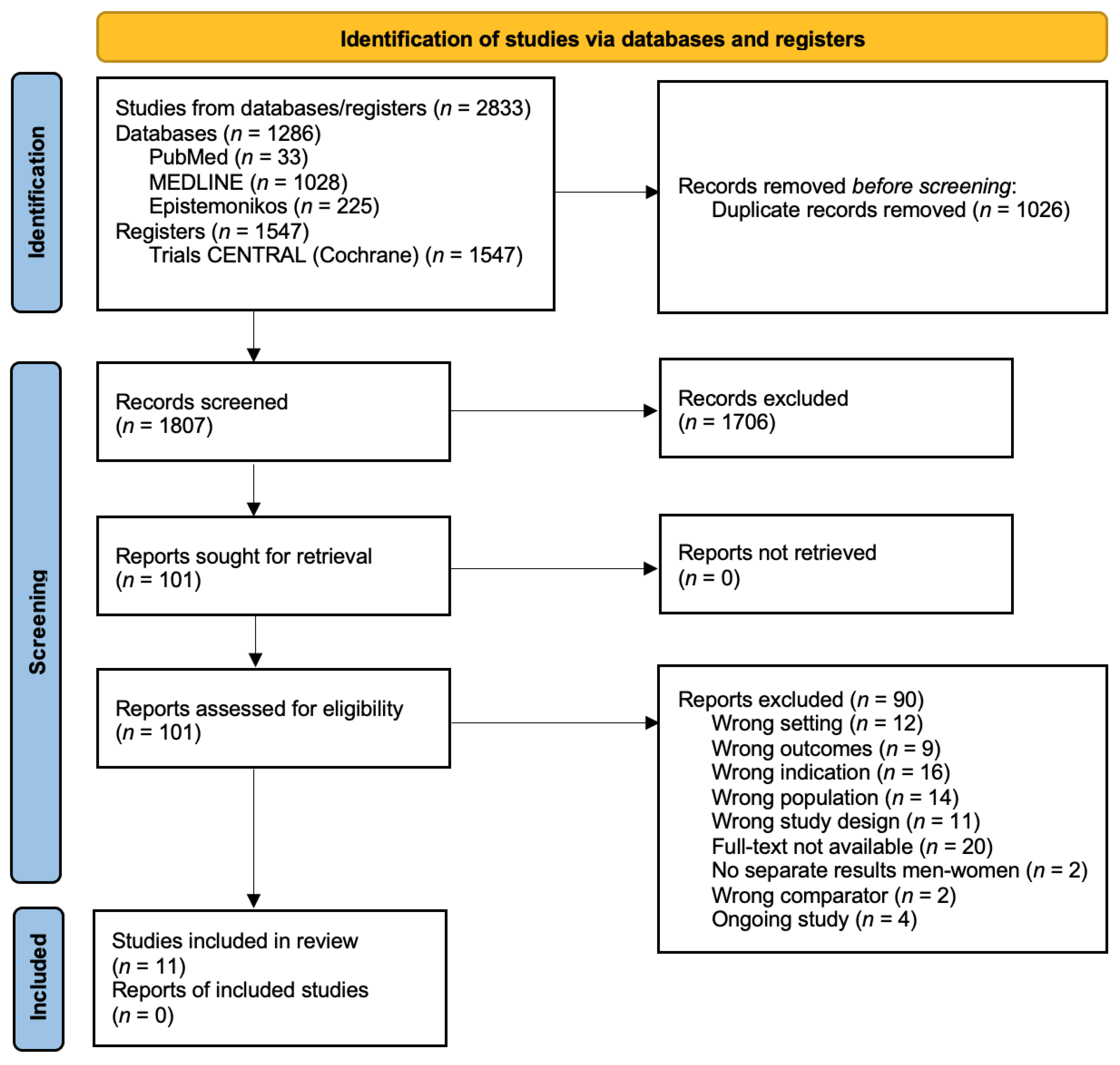
3. Results
3.1. Study Selection and Characteristics
3.2. Quality Appraisal
3.3. Insomnia
3.3.1. Prevalence
3.3.2. Insomnia during the COVID-19 Lockdown
3.3.3. Risk Factors of Insomnia
3.3.4. Long-Term Effects of Insomnia
3.4. Circadian Rhythm Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buysse, D.J. Insomnia. JAMA 2013, 309, 706–716. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; DeLeire, T.; Lauderdale, D.S. Trends in the prevalence of short sleepers in the USA: 1975-2006. Sleep 2010, 33, 37–45. [Google Scholar] [CrossRef]
- Perlis, M.L.; Posner, D.; Riemann, D.; Bastien, C.H.; Teel, J.; Thase, M. Insomnia. Lancet 2022, 400, 1047–1060. [Google Scholar] [CrossRef]
- Kim, J.H.; Elkhadem, A.R.; Duffy, J.F. Circadian Rhythm Sleep-Wake Disorders in Older Adults. Sleep Med. Clin. 2022, 17, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Sarber, K.M.; Lam, D.J.; Ishman, S.L. Sleep Apnea and Sleep Disorders. In Cummings Otolaryngology: Head and Neck Surgery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–235. [Google Scholar]
- Telias, I.; Wilcox, M.E. Sleep and Circadian Rhythm in Critical Illness. Crit. Care 2019, 23, 82. [Google Scholar] [CrossRef]
- Hong, J.K.; Lee, H.J.; Chung, S.; Yoon, I.Y. Differences in sleep measures and waking electroencephalography of patients with insomnia according to age and sex. J. Clin. Sleep Med. 2021, 17, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Shechter, A.; Cain, S.W. Sex Effects and Differences in Circadian Rhythms and Sleep. In Principles and Practice of Sleep Medicine—2 Volume Set, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1734–1741. [Google Scholar]
- Pillai, V.; Roth, T.; Drake, C.L. The Nature of Stable Insomnia Phenotypes. Sleep 2015, 38, 127–138. [Google Scholar] [CrossRef]
- Hale, L.; Do, D.P.; Basurto-Davila, R.; Heron, M.; Finch, B.K.; Dubowitz, T.; Lurie, N.; Bird, C.E. Does mental health history explain gender disparities in insomnia symptoms among young adults? In Sleep Medicine; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1118–1123. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- JBI Critical Appraisal Tools | JBI. Available online: https://jbi.global/critical-appraisal-tools (accessed on 14 November 2023).
- CASP Checklists-Critical Appraisal Skills Programme. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 14 November 2023).
- Höglund, P.; Hakelind, C.; Nordin, S. Severity and prevalence of various types of mental ill-health in a general adult population: Age and sex differences. BMC Psychiatry 2020, 20, 209. [Google Scholar] [CrossRef]
- Salfi, F.; Lauriola, M.; Amicucci, G.; Corigliano, D.; Viselli, L.; Tempesta, D.; Ferrara, M. Gender-related time course of sleep disturbances and psychological symptoms during the COVID-19 lockdown: A longitudinal study on the Italian population. Neurobiol. Stress 2020, 13, 100259. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, D.; Huang, N.; Fu, M.; Ahmed, J.F.; Zhang, Y.; Wang, X.; Wang, Y.; Shahid, M.; Guo, J. The Combined Impact of Gender and Age on Post-traumatic Stress Symptoms, Depression, and Insomnia During COVID-19 Outbreak in China. Front. Public Health 2020, 8, 620023. [Google Scholar] [CrossRef] [PubMed]
- de Almondes, K.M.; Castro, E.d.A.S.; Paiva, T. Morbidities Worsening Index to Sleep in the Older Adults During COVID-19: Potential Moderators. Front. Psychol. 2022, 13, 913644. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Kim, K.T.; Lee, S.Y.; Cho, Y.W. Insomnia during coronavirus disease 2019 pandemic in Korea: A National sleep survey. Sleep Biol. Rhythm. 2023, 1, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Matas, A.; Amaral, L.; Patto, A.V. Is post-ischemic stroke insomnia related to a negative functional and cognitive outcome? Sleep Med. 2022, 94, 1–7. [Google Scholar] [CrossRef]
- Silva-Costa, A.; Rotenberg, L.; Nobre, A.A.; Chor, D.; Aquino, E.M.; Melo, E.C.; Barreto, S.M.; Schmidt, M.I.; Griep, R.H. Sex differences in the association between self-reported sleep duration, insomnia symptoms and cardiometabolic risk factors: Cross-sectional findings from Brazilian longitudinal study of adult health. Arch. Public Health 2020, 78, 48. [Google Scholar] [CrossRef]
- Pudlo, R.; Jaworska, I.; Szczegielniak, A.; Niedziela, J.; Kułaczkowska, Z.; Nowowiejska-Wiewióra, A.; Jaroszewicz, J.; Gąsior, M. Prevalence of Insomnia in the Early Post-COVID-19 Recovery Period. Int. J. Environ. Res. Public Health 2022, 19, 14224. [Google Scholar] [CrossRef]
- Huang, B.H.; del Pozo Cruz, B.; Teixeira-Pinto, A.; Cistulli, P.A.; Stamatakis, E. Influence of poor sleep on cardiovascular disease-free life expectancy: A multi-resource-based population cohort study. BMC Med. 2023, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Somers, V.K.; Lopez-Jimenez, F.; Di, J.; Covassin, N. Demographic characteristics associated with circadian rest-activity rhythm patterns: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Rodríguez-González-moro, M.T.; Rodríguez-González-moro, J.M.; Rivera-Caravaca, J.M.; Vera-Catalán, T.; Simonelli-Muñoz, A.J.; Gallego-Gómez, J.I. Work Shift and Circadian Rhythm as Risk Factors for Poor Sleep Quality in Public Workers from Murcia (Spain). Int. J. Environ. Res. Public Health 2020, 17, 5881. [Google Scholar] [CrossRef]
- Zeng, L.-N.; Zong, Q.-Q.; Yang, Y.; Zhang, L.; Xiang, Y.-F.; Ng, C.H.; Chen, L.-G.; Xiang, Y.-T. Gender Difference in the Prevalence of Insomnia: A Meta-Analysis of Observational Studies. Front. Psychiatry 2020, 1, 577429. [Google Scholar] [CrossRef]
- Bitran, D.; Purdy, R.H.; Kellogg, C.K. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol. Biochem. Behav. 1993, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Fishbein, W. Sex differences in paradoxical sleep: Influences of estrus cycle and ovariectomy. Brain Res. 1996, 734, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Webley, G.E.; Leidenberger, F. The circadian pattern of melatonin and its positive relationship with progesterone in women. J. Clin. Endocrinol. Metab. 1986, 63, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.F.; Won, C.H.; Bourjeily, G. Sleep in Women Across the Life Span. Chest 2018, 154, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mohler-kuo, M.; Dzemaili, S.; Foster, S.; Werlen, L.; Walitza, S. Stress and Mental Health among Children/Adolescents, Their Parents, and Young Adults during the First COVID-19 Lockdown in Switzerland. Int. J. Environ. Res. Public Health 2021, 18, 4668. [Google Scholar] [CrossRef]
- Ali, N.; Nitschke, J.P.; Cooperman, C.; Baldwin, M.W.; Pruessner, J.C. Systematic manipulations of the biological stress systems result in sex-specific compensatory stress responses and negative mood outcomes. Neuropsychopharmacology 2020, 45, 1672–1680. [Google Scholar] [CrossRef]
- Hermann, D.M.; Bassetti, C.L. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology 2009, 73, 1313–1322. [Google Scholar] [CrossRef]
- Baylan, S.; Griffiths, S.; Grant, N.; Broomfield, N.M.; Evans, J.J.; Gardani, M. Incidence and prevalence of post-stroke insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101222. [Google Scholar] [CrossRef]
- Laaboub, N.; Dubath, C.; Ranjbar, S.; Sibailly, G.; Grosu, C.; Piras, M.; Délessert, D.; Richard-Lepouriel, H.; Ansermot, N.; Crettol, S.; et al. Insomnia disorders are associated with increased cardiometabolic disturbances and death risks from cardiovascular diseases in psychiatric patients treated with weight-gain-inducing psychotropic drugs: Results from a Swiss cohort. BMC Psychiatry 2022, 22, 342. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Cohen, B.E.; Neylan, T.C.; Whooley, M.A. Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: Findings from the Heart and Soul Study. J. Psychiatr. Res. 2013, 47, 1228–1235. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors, Comorbidities, and Consequences of Insomnia in Adults-UpToDate. Available online: https://www.uptodate.com/contents/risk-factors-comorbidities-and-consequences-of-insomnia-in-adults (accessed on 14 November 2023).
- Vgontzas, A.N.; Liao, D.; Pejovic, S.; Calhoun, S.; Karataraki, M.; Basta, M.; Fernández-Mendoza, J.; Bixler, E.O. Insomnia with Short Sleep Duration and Mortality: The Penn State Cohort. Sleep 2010, 33, 1159. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Innominato, P.F.; Rol, M.A.; Lévi, F.; Madrid, J.A. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer 2016, 16, 285. [Google Scholar] [CrossRef]
- Duarte, L.; Menna-Barreto, L.; Miguel, M.; Louzada, F.; Araújo, J.; Alam, M.; Areas, R.; Pedrazzoli, M. Chronotype ontogeny related to gender. Braz. J. Med. Biol. Res. 2014, 47, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A.; RhythmsBoivin, B. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef]
- Rabstein, S.; Burek, K.; Lehnert, M.; Beine, A.; Vetter, C.; Harth, V.; Putzke, S.; Kantermann, T.; Walther, J.; Wang-Sattler, R.; et al. Differences in twenty-four-hour profiles of blue-light exposure between day and night shifts in female medical staff. Sci. Total Environ. 2019, 653, 1025–1033. [Google Scholar] [CrossRef]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607–608, 1073–1084. [Google Scholar] [CrossRef]

| Author(s), Year | Country | Study Design | Aims | Study Population | Main Findings |
|---|---|---|---|---|---|
| Höglund et al., 2020 [14] | Sweden | CSS 1 |
| N = 3046 (females—1898, males—1508) | Women, in general, presented a higher risk for insomnia than men, with the highest risk for younger women and a lower risk for older women. For men, the highest risk for insomnia was in the 30–39 year age group. Insomnia symptom severity was higher in men than in women in the age group of 30–39 years and was lower in both men and women in those aged 50–69 years. |
| Salfi et al., 2020 [15] | Italy | LS 2 | Investigate the changes in sleep and mental health changes during the prolonged lockdown due to the COVID-19 outbreak. | N = 2701 (females—2210, males—491) | Women seemed to be more resilient than men in the long-run, exhibiting a slight trend toward improvement in insomnia, depression, anxiety, and distress at the end of the seven weeks covered by the present research. On the other hand, men showed an exacerbation of insomnia symptoms and a deterioration of sleep quality during the lockdown. Furthermore, male participants reported a substantial increment of perceived stress at the end of the study. In addition, although women reported a higher prevalence of clinical conditions, such as insomnia and depression, in the first part of the lockdown, the sex gap was narrowed after four weeks. |
| Liu et al., 2021 [16] | China | CSS |
| N = 2858 (females—1532, males—1326) | There are no significant differences between sex and age in the prevalence of insomnia. Among all 2858 participants, 19.6% are found to have insomnia. |
| de Almondes et al., 2022 [17] | Greece | CSS | To evaluate the prevalence of sleep disorders in older adults, to describe their sleep patterns, as well as to analyze if there were any changes, in comparison with the pre-pandemic period. | N = 914 (females—372, males—542) | The COVID-19-related lockdown altered sleep habits and worsened sleep disorders among older adults. Sex comparisons were also made considering the worsening of each pre-existent sleep disorder. Significant changes were present in insomnia and restless leg syndrome in women and in sleep apnea in men. Women exhibited higher sleep latency, higher frequency of awakenings during sleep, lower sleep efficiency, lower sleep quality, and lower sleep awakening quality when compared to men. |
| Jeon et al., 2023 [18] | Korea | CSS | To assess perceived changes in the prevalence of insomnia due to the pandemic in in Korea and identify the factors associated with sleep changes during the pandemic outbreak. | N = 4000 (females—1965, males—2035) | The prevalence of insomnia was significantly higher in women than in men across all age groups. Insomnia prevalence was associated with female sex, night workers, being unmarried, and smoking. Among men, the prevalence was the highest in those aged 20–29 years, while among women, it was the highest in those aged 30–39 years and showed a decreasing tendency with age. Moderate to severe insomnia was reported by 12.9% of the participants. Among men, the 20–29 years age group had the highest prevalence, whereas among women, the 60–69 years age group had the highest prevalence. |
| Matas et al., 2022 [19] | Portugal | CS 3 | Identify the features of patients who develop insomnia after an ischemic stroke and characterize them. | N = 157 (females—81, males—74) | Male sex and previous minor vascular events were significantly associated with the development of insomnia after ischemic stroke. |
| Silva-Costa et al., 2022 [20] | Brazil | CSS | Evaluate sex-specific associations between sleep problems and cardiometabolic risk factors. | N = 13,723 (females—7491, males—6232) | Obesity, hypertension, and high glycated hemoglobin were associated with self-reported sleep duration and insomnia symptoms (either separately or linked to short sleep duration) in women, but not in men. Cardiometabolic risk factors were associated with insomnia symptoms plus short sleep duration only in women. In relation to hypertriglyceridemia, statistically significant associations with insomnia symptoms were observed among both women and men. |
| Pudlo et al., 2022 [21] | Poland | CS | To assess the prevalence of insomnia in the early post-COVID-19 recovery period and explore differences in the results acquired from the Athens Insomnia Scale by sex and selected infection severity parameters. | N = 200 (females—99, males—101) | Women achieved a higher score in overall AIS. The analysis of the results obtained by all participants in the AIS shows a significant correlation with the duration of symptoms (days). The results suggest a higher risk of insomnia among women. |
| Huang et al., 2023 [22] | Australia | CS | Estimate the differences in CVD-free life expectancy between people with different sleep profiles. | N = 308,683 (females—173,546, males—135,137) | The study observed a gradual loss in CVD-free life expectancy toward poor sleep. Compared with healthy sleepers, female and male poor sleepers lost 1.80 and 2.31 CVD-free years, respectively, while female and male intermediate sleepers lost 0.48 and 0.55 years, respectively. Among men, those with clinical insomnia or sleep-related breathing disorders lost CVD-free life by 3.84 or 6.73 years, respectively. Among women, sleep-related breathing disorders or other sleep disorders were associated with 7.32 or 1.43 years lost, respectively. |
| Li et al., 2021 [23] | USA | CSS | To describe rest-activity rhythm (RAR) patterns among general adults and to explore variations by sex, age, and race/ethnicity. | N = 8200 (females—4224, males—3976) | Women had higher RAR amplitude and mesor and also more robust (pseudo-F statistic), more stable (higher interdaily stability), and less fragmented (lower intradaily variability) RAR than men. Women were also more likely have a normal acrophase than men. |
| Rodríguez-González-moro et al., 2020 [24] | Spain | CSS | To determine the prevalence of self-reported sleep quality and to investigate those factors that may predict the risk of suffering poor sleep quality in a large sample of public workers from Murcia. | N = 476 (females—232, males—244) | No significant differences were found according to sex in the overall sleep quality scores, but there were differences in the duration of sleep. The mean score in the reduced scale of the Morningness–Eveningness Questionnaire was similar between females and males. According to the scale, 62.0% of public workers had morning chronotypes, and 38.0% were classified as having an evening-intermediate chronotype. Fixed morning shifts and evening chronotypes were independent predictors of suffering from poor sleep quality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajėdienė, E.; Urbonavičiūtė, V.; Ramanauskaitė, V.; Strazdauskas, L.; Stefani, A. Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review. Medicina 2024, 60, 474. https://doi.org/10.3390/medicina60030474
Pajėdienė E, Urbonavičiūtė V, Ramanauskaitė V, Strazdauskas L, Stefani A. Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review. Medicina. 2024; 60(3):474. https://doi.org/10.3390/medicina60030474
Chicago/Turabian StylePajėdienė, Evelina, Viltė Urbonavičiūtė, Vita Ramanauskaitė, Lukas Strazdauskas, and Ambra Stefani. 2024. "Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review" Medicina 60, no. 3: 474. https://doi.org/10.3390/medicina60030474
APA StylePajėdienė, E., Urbonavičiūtė, V., Ramanauskaitė, V., Strazdauskas, L., & Stefani, A. (2024). Sex Differences in Insomnia and Circadian Rhythm Disorders: A Systematic Review. Medicina, 60(3), 474. https://doi.org/10.3390/medicina60030474







