A Narrative Review of Stroke of Cortical Hand Knob Area
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overview
3.2. Epidemiology
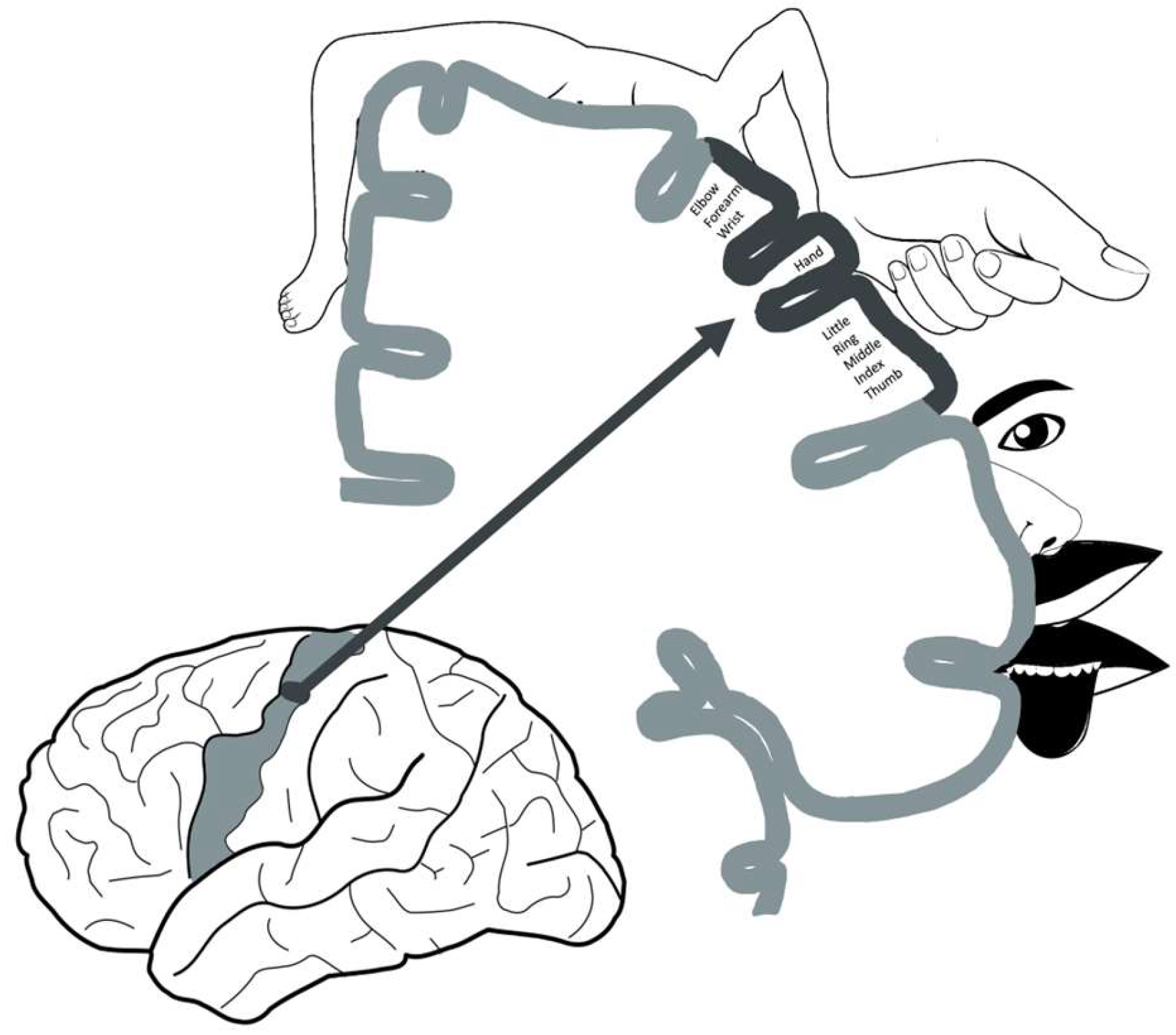
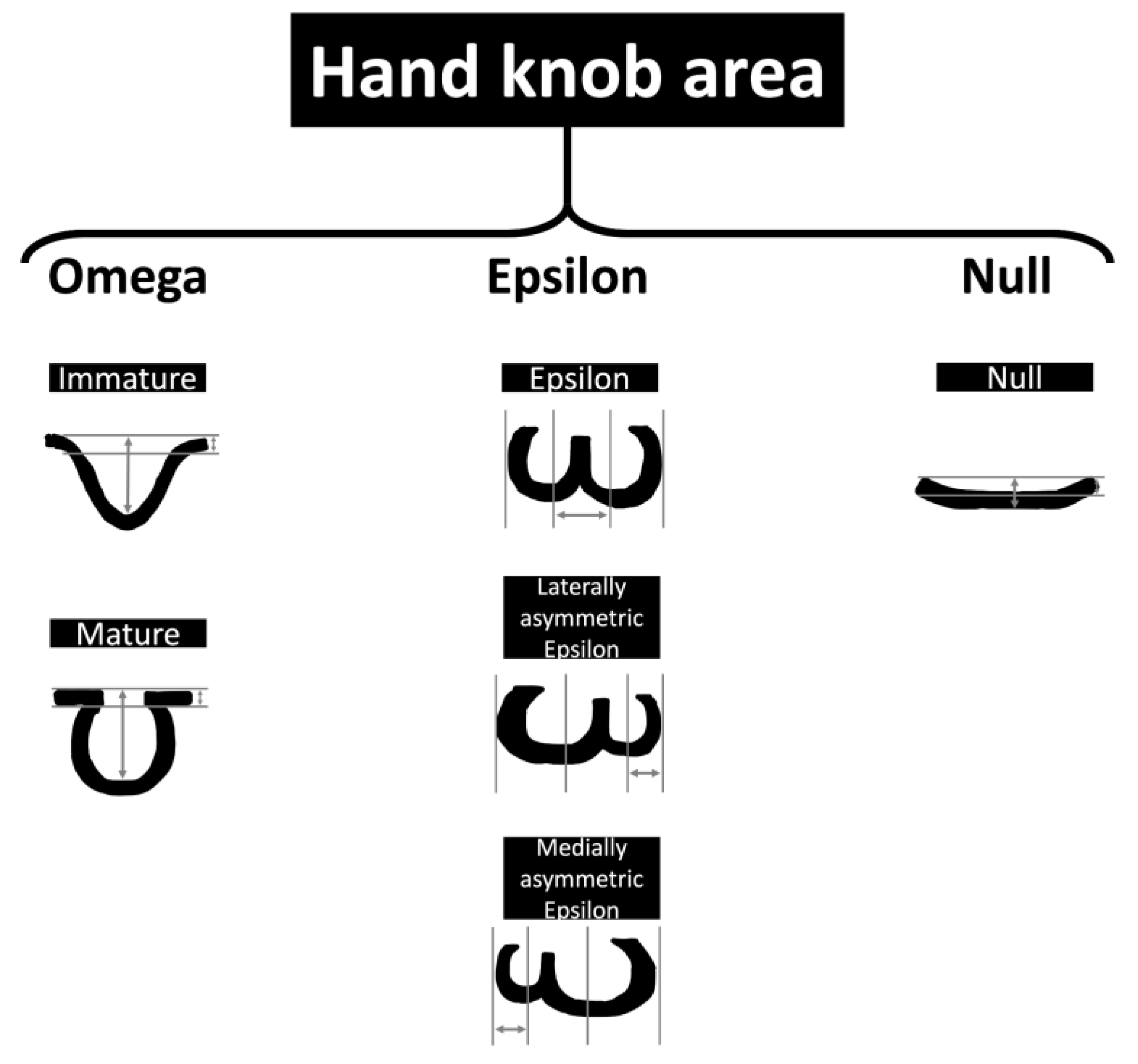
3.3. Neuroanatomy and Neuroimaging
3.3.1. Neuroanatomy Development
3.3.2. Changes in Motor Function after Stroke
4. Clinical Assessment, Management, and Prognosis
4.1. Clinical Assessment
4.2. Management Subsection
4.3. Prognosis
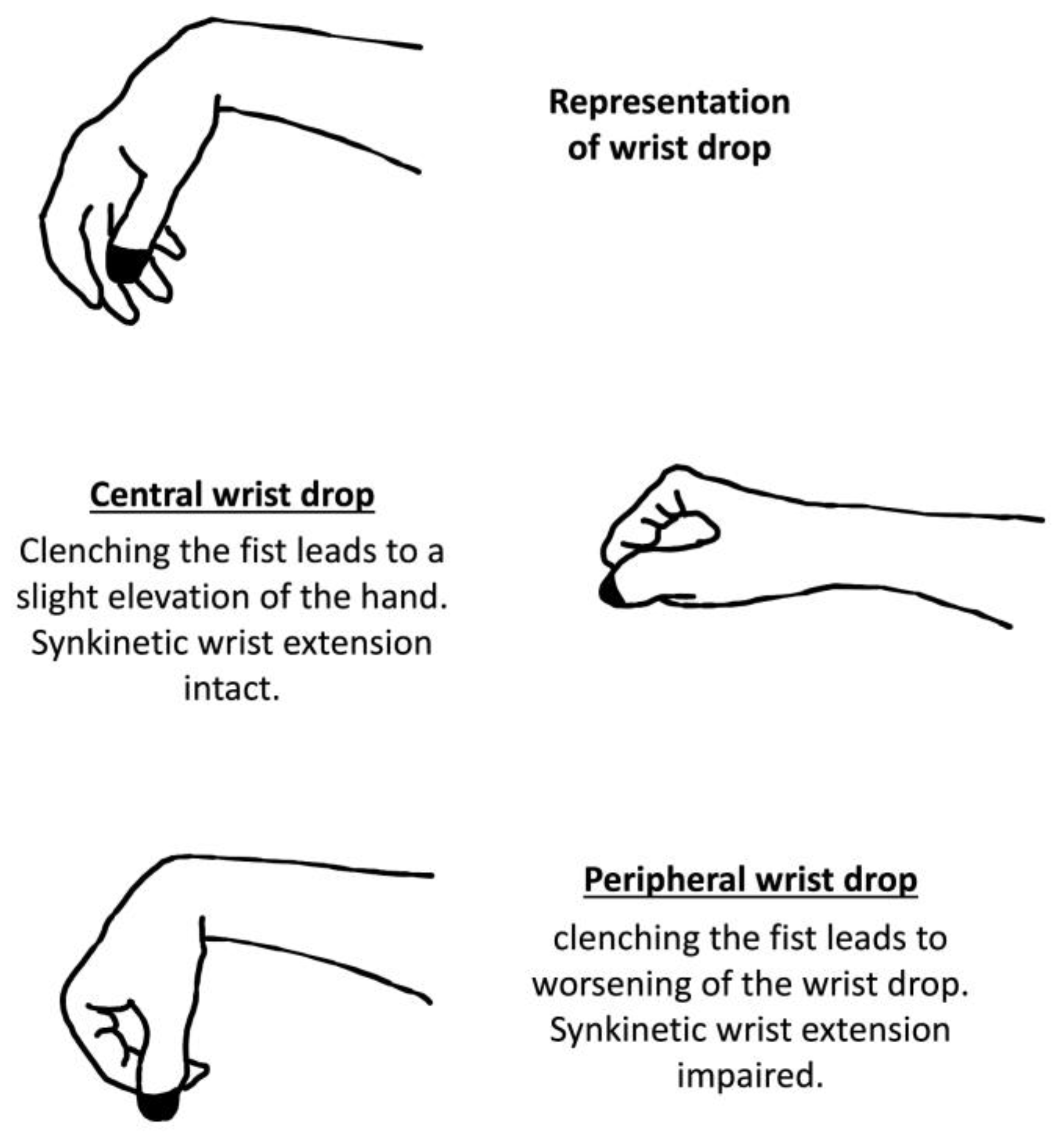
5. Peripheral Versus Central Wrist Drop
Other Unusual Presentations
6. Limitations and Future Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tei, H. Monoparesis of the right hand following a localised infarct in the left “precentral knob”. Neuroradiology 1999, 41, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Back, T.; Mrowka, M. Infarction of the “hand knob” area. Neurology 2001, 57, 1143. [Google Scholar] [CrossRef] [PubMed]
- Gass, A.; Szabo, K.; Behrens, S.; Rossmanith, C.; Hennerici, M. A diffusion-weighted MRI study of acute ischemic distal arm paresis. Neurology 2001, 57, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Granziera, C.; Kuntzer, T.; Vingerhoets, F.; Cereda, C. Small cortical stroke in the" hand knob" mimics anterior interosseous syndrome. J. Neurol. 2008, 255, 1423–1424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, J.; Flint, A. “Hand knob” infarction. J. Neurol. Neurosurg. Psych. 2008, 79, 406. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Müller-Schunk, S.; Freilinger, T.; Düring, M.; Pfefferkorn, T.; Dichgans, M. Ischemic stroke of the cortical “hand knob” area: Stroke mechanisms and prognosis. J. Neurol. 2009, 256, 1146–1151. [Google Scholar] [CrossRef]
- Kitamura, E.; Hamada, J.; Suzuki, K.; Akutsu, T.; Kan, S.; Mochizuki, H. A case of pure motor isolated finger palsy due to cerebral infarction. Rinsho Shinkeigaku 2010, 50, 572–577. [Google Scholar] [CrossRef][Green Version]
- Alstadhaug, K.B.; Sjulstad, A. Isolated hand paresis: A case series. Cerebrovasc. Dis. Extra 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Dekeyzer, S.; Acou, M.; Hemelsoet, D. Right hand knob infarction. Acta Neurol. Bel. 2014, 114, 135–136. [Google Scholar] [CrossRef]
- Jusufovic, M.; Lygren, A.; Aamodt, A.H.; Nedregaard, B.; Kerty, E. Pseudoperipheral palsy: A case of subcortical infarction imitating peripheral neuropathy. BMC Neurol. 2015, 15, 151. [Google Scholar] [CrossRef][Green Version]
- De Medeiros, F.C.; Viana, D.C.R.; Cunha, M.N.; Hatasa, C.C.; Araújo, R.V. Pure motor monoparesis due to infarction of the" hand knob" area: Radiological and morphological features. Neurol. Sci. 2017, 38, 1877–1879. [Google Scholar] [CrossRef]
- Dijkstra, J.; Timmerman, M.; van Rooij, F. Isolated monoparesis of hand or foot caused by cortical ischaemic stroke. Ned. Tijdschr. Geneeskd. 2017, 161, D2121. [Google Scholar] [PubMed]
- Kim, H.W.; Lee, D.G. Resting-state metabolism of hand knob area on 18F-FDG PET-CT according to hand function and tractography of corticospinal tract after stroke. Ann. Rehabil. Med. 2017, 41, 171–177. [Google Scholar] [CrossRef]
- Folyovich, A.; Varga, V.; Várallyay, G.; Kozák, L.; Bakos, M.; Scheidl, E.; Béres-Molnár, K.A.; Kajdácsi, Z.; Bereczki, D. A case report of isolated distal upper extremity weakness due to cerebral metastasis involving the hand knob area. BMC Cancer 2018, 18, 947. [Google Scholar] [CrossRef] [PubMed]
- Orosz, P.; Szőcs, I.; Rudas, G.; Folyovich, A.; Bereczki, D.; Vastagh, I. Cortical hand knob stroke: Report of 25 cases. J. Stroke Cerebrovasc. Dis. 2018, 27, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Q.; Li, S.-j.; Hu, W.-l. New clinical characteristics and risk factors of hand knob infarction. Neurol. Sci. 2018, 39, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Finkelsteyn, A.M.; Saucedo, M.A.; Miquelini, L.A.; Chertcoff, A.; Bandeo, L.; Pacha, S.; Cejas, L.L.; Roca, C.U.; Pardal, M.F.; Reisin, R. Ischemic stroke of the “hand knob area”: A case series and literature review. J. Clin. Neurosci. 2019, 65, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, Y.; Tanaka, M.; Tanaka, K. Hand knob stroke from cancer-associated thromboembolism. CMAJ 2019, 191, E1164. [Google Scholar] [CrossRef] [PubMed]
- Shelley, B.P.; Harishchandra, P.; Devadas, A.K. Selective hand motor cortex lesions masquerading as “Pseudoperipheral Nerve Palsy”. Ann. Indian Acad. Neurol. 2020, 23, 688. [Google Scholar] [CrossRef]
- Daneasa, A.I.; Heckmann, J.G. My oldest stroke patient. Practic. Neurol. 2021, 21, 463. [Google Scholar] [CrossRef]
- Davies, M.L.; Harrison, M. ‘Cortical Hand’in the Emergency Department: Two Case Reports. J. Emerg. Med. 2022, 62, e13–e15. [Google Scholar] [CrossRef]
- Nicolini, P.; Arighi, A.; Gherbesi, E.; Lo Russo, F.M.; Mandelli, C.; Schinco, G.; Carugo, S.; Lucchi, T. Ischaemic Stroke of the “Hand-Knob” Area Due to Paradoxical Cerebral Air Embolism after Central Venous Catheterization—A Doubly Rare Occurrence: A Case Report and an Overview of Pathophysiology, Diagnosis, and Treatment. Brain Sci. 2022, 12, 772. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, X.; Liu, X.; Wang, L.; Zhu, R. Clinical features, etiology, and prognosis of hand knob stroke: A case series. BMC Neurol. 2022, 22, 331. [Google Scholar] [CrossRef]
- Alshanqiti, M.A.; Alharbi, N.; Althobaiti, F.A.; Alzahrani, S.S.; Alwadai, M.; Asiri, M.; Alshareef, F.; Alqahtani, M.; Bugshan, T.F. Hand Knob Syndrome Secondary to Ipsilateral Concomitant Carotid Fibromuscular Dysplasia and Proximal Atherosclerotic Disease. Cureus 2023, 15, e40072. [Google Scholar] [CrossRef]
- Celebisoy, M.; Özdemirkiran, T.; Tokucoglu, F.; Kaplangi, D.N.; Arici, S. Isolated hand palsy due to cortical infarction: Localization of the motor hand area. Neurologist 2007, 13, 376–379. [Google Scholar] [CrossRef]
- Yousry, T.; Schmid, U.; Alkadhi, H.; Schmidt, D.; Peraud, A.; Buettner, A.; Winkler, P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 1997, 120, 141–157. [Google Scholar] [CrossRef]
- Kaneko, O.; Fischbein, N.; Rosenberg, J.; Wintermark, M.; Zeineh, M. The “white gray sign” identifies the central sulcus on 3T high-resolution T1-weighted images. Am. J. Neurorad. 2017, 38, 276–280. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, H.; Zhang, Y.; Wang, M.; Liu, C.; Wang, X.; Cheng, Y.; Jin, C.; Yang, J.; Li, X. Morphologic variants of the hand motor cortex in developing brains from neonates through childhood assessed by MR imaging. Am. J. Neurorad. 2022, 43, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Willett, F.R.; Deo, D.R.; Avansino, D.T.; Rezaii, P.; Hochberg, L.R.; Henderson, J.M.; Shenoy, K.V. Hand knob area of premotor cortex represents the whole body in a compositional way. Cell 2020, 181, 396–409.e326. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Fornia, L.; Rossi, M.; Howells, H.; Leonetti, A.; Puglisi, G.; Nibali, M.C.; Bellacicca, A.; Grimaldi, M.; Bello, L. Anatomo-functional characterisation of the human “hand-knob”: A direct electrophysiological study. Cortex 2019, 113, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-L.; Hsu, H.-Y.; Wang, P.-Y. Isolated hand weakness in cortical infarctions. J. Formos. Med. Assoc. 2006, 105, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, T.; Parikh, S.; Bruno, M.K.; Hallett, M. Finger and face representations in the ipsilateral precentral motor areas in humans. J. Neurophysiol. 2005, 93, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Ahdab, R.; Ayache, S.S.; Hosseini, H.; Mansour, A.G.; Kerschen, P.; Farhat, W.H.; Chalah, M.A.; Lefaucheur, J.P. Precise finger somatotopy revealed by focal motor cortex injury. Neurophysiol. Clin. 2020, 50, 27–31. [Google Scholar] [CrossRef]
- Hamzei, F.; Liepert, J.; Dettmers, C.; Weiller, C.; Rijntjes, M. Two different reorganization patterns after rehabilitative therapy: An exploratory study with fMRI and TMS. Neuroimage 2006, 31, 710–720. [Google Scholar] [CrossRef]
- Strick, P.L.; Dum, R.P.; Rathelot, J.A. The cortical motor areas and the emergence of motor skills: A neuroanatomical perspective. Annu. Rev. Neurosci. 2021, 44, 425–447. [Google Scholar] [CrossRef]
- Griffin, D.M.; Strick, P.L. The motor cortex uses active suppression to sculpt movement. Sci. Adv. 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Feydy, A.; Carlier, R.; Roby-Brami, A.; Bussel, B.; Cazalis, F.; Pierot, L.; Burnod, Y.; Maier, M.A. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002, 33, 1610–1617. [Google Scholar] [CrossRef]
- Jang, S.H.; Cho, S.H.; Kim, Y.H.; Kwon, Y.H.; Byun, W.M.; Lee, S.J.; Park, S.M.; Chang, C.H. Cortical activation changes associated with motor recovery in patients with precentral knob infarct. Neuroreport 2004, 15, 395–399. [Google Scholar] [CrossRef]
- Jang, S.H.; Ahn, S.H.; Yang, D.S.; Lee, D.K.; Kim, D.K.; Son, S.M. Cortical reorganization of hand motor function to primary sensory cortex in hemiparetic patients with a primary motor cortex infarct. Arch. Phys. Med. Rehabil. 2005, 86, 1706–1708. [Google Scholar] [CrossRef]
- Jang, S.H.; Ahn, S.H.; Lee, J.; Cho, Y.W.; Son, S.M. Cortical reorganization of sensori-motor function in a patient with cortical infarct. NeuroRehabilitation 2010, 26, 163–166. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, Y.H.; Lee, M.Y.; Lee, D.Y.; Hong, J.H. Difference of neural connectivity for motor function in chronic hemiparetic stroke patients with intracerebral hemorrhage. Neurosci. Lett. 2012, 531, 80–85. [Google Scholar] [CrossRef]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012, 59, 2771–2782. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P. Limb-kinetic apraxia due to injury of corticofugal tracts from secondary motor area in patients with corona radiata infarct. Acta Neurol. Belg. 2016, 116, 467–472. [Google Scholar] [CrossRef]
- Plantin, J.; Pennati, G.V.; Roca, P.; Baron, J.C.; Laurencikas, E.; Weber, K.; Godbolt, A.K.; Borg, J.; Lindberg, P.G. Quantitative Assessment of Hand Spasticity After Stroke: Imaging Correlates and Impact on Motor Recovery. Front. Neurol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Giuffre, A.; Kahl, C.K.; Zewdie, E.; Wrightson, J.G.; Bourgeois, A.; Condliffe, E.G.; Kirton, A. Reliability of robotic transcranial magnetic stimulation motor mapping. J. Neurophysiol. 2021, 125, 74–85. [Google Scholar] [CrossRef]
- van der Cruijsen, J.; Dooren, R.F.; Schouten, A.C.; Oostendorp, T.F.; Frens, M.A.; Ribbers, G.M.; van der Helm, F.C.T.; Kwakkel, G.; Selles, R.W. Addressing the inconsistent electric fields of tDCS by using patient-tailored configurations in chronic stroke: Implications for treatment. Neuroimage Clin. 2022, 36, 103178. [Google Scholar] [CrossRef]
- Maeder-Ingvar, M.; van Melle, G.; Bogousslavsky, J. Pure monoparesis: A particular stroke subgroup? Arch. Neurol. 2005, 62, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Lambercy, O.; Dovat, L.; Gassert, R.; Burdet, E.; Teo, C.L.; Milner, T. A haptic knob for rehabilitation of hand function. IEEE Trans. Neural. Syst. Rehabil. Eng. 2007, 15, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Harjpal, P.; Kovela, R.K.; Vardhan, V. Positive Outcomes of Early Task-Specific Training and Action Observation Mirror Therapy Following Infarction of Hand Knob Area: A Case Report. Cureus 2022, 14, e29819. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Ephraim Joseph, G.J.; Kuah, C.W.; Chua, K.S. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Kaeser, M.; Savidan, J.; Fregosi, M.; Rouiller, E.M.; Schmidlin, E. Assessment of the effect of continuous theta burst stimulation of the motor cortex on manual dexterity in non-human primates in a direct comparison with invasive intracortical pharmacological inactivation. Eur. J. Neurosci. 2019, 50, 3599–3613. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, J.; Lee, H.J.; Na, Y.; Chang, W.H.; Kim, Y.H. Comparison of hemodynamic changes after repetitive transcranial magnetic stimulation over the anatomical hand knob and hand motor hotspot: A functional near-infrared spectroscopy study. Restor. Neurol. Neurosci. 2020, 38, 407–417. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Lee, H.J.; Lee, J.; Na, Y.; Chang, W.H.; Kim, Y.H. Optimal stimulation site for rTMS to improve motor function: Anatomical hand knob vs. hand motor hotspot. Neurosci. Lett. 2021, 740, 135424. [Google Scholar] [CrossRef]
- Hoei, T.; Kawahira, K.; Shimodozono, M.; Fukuda, H.; Shigenobu, K.; Ogura, T.; Matsumoto, S. Repetitive facilitative exercise under continuous electrical stimulation for recovery of pure motor isolated hand palsy after infarction of the "hand knob" area: A case report. Physiother. Theory Pract. 2023, 39, 1545–1552. [Google Scholar] [CrossRef]
- Gharabaghi, A.; Naros, G.; Walter, A.; Grimm, F.; Schuermeyer, M.; Roth, A.; Bogdan, M.; Rosenstiel, W.; Birbaumer, N. From assistance towards restoration with epidural brain-computer interfacing. Restor. Neurol. Neurosci. 2014, 32, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Badoud, S.; Borgognon, S.; Cottet, J.; Chatagny, P.; Moret, V.; Fregosi, M.; Kaeser, M.; Fortis, E.; Schmidlin, E.; Bloch, J.; et al. Effects of dorsolateral prefrontal cortex lesion on motor habit and performance assessed with manual grasping and control of force in macaque monkeys. Brain Struct. Funct. 2017, 222, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Wasson, P.; Prodoehl, J.; Coombes, S.A.; Corcos, D.M.; Vaillancourt, D.E. Predicting grip force amplitude involves circuits in the anterior basal ganglia. Neuroimage 2010, 49, 3230–3238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turton, A.J.; Butler, S.R. A multiple case design experiment to investigate the performance and neural effects of a programme for training hand function after stroke. Clin. Rehabil. 2004, 18, 754–763. [Google Scholar] [CrossRef] [PubMed]
- List, J.; Kübke, J.C.; Lindenberg, R.; Külzow, N.; Kerti, L.; Witte, V.; Flöel, A. Relationship between excitability, plasticity and thickness of the motor cortex in older adults. Neuroimage 2013, 83, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Carlson, H.L.; Zewdie, E.; Kirton, A. Developmental Remodelling of the Motor Cortex in Hemiparetic Children with Perinatal Stroke. Pediatr. Neurol. 2020, 112, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhang, R.; Jiang, W.; Fu, Z. Integrity of The Hand Fibers of The Corticospinal Tract Shown by Diffusion Tensor Imaging Predicts Hand Function Recovery After Hemorrhagic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105447. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Ragnedda, G.; Canu, P.; Nardone, R. Synkinetic wrist extension in distinguishing cortical hand from radial nerve palsy. Pract. Neurol. 2018, 18, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Evans, B.A.; Huston, J. Pseudoulnar palsy from a small infarct of the precentral knob. Neurology 2000, 54, 2185. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, P.; Lentini, A.; Baima, C.; Grasso, M.; Azzaro, C.; Bosco, G.; Bergamasco, B.; Benna, P. Pseudo-ulnar sensory loss in a patient from a small cortical infarct of the postcentral knob. Neurology 2005, 64, 1981–1982. [Google Scholar] [CrossRef]
- Kakinuma, K.; Nakajima, M.; Hieda, S.; Ichikawa, H.; Kawamura, M. Progressive cerebral infraction initially presenting with pseudo-ulnar nerve palsy in a patient with severe internal carotid artery stenosis. Rinsho Shinkeigaku 2010, 50, 666–668. [Google Scholar] [CrossRef][Green Version]
- Lee, S.J. Recurrent Patent Foramen Ovale-Related Cerebral Infarcts Alternately Causing Bilateral Hand Paresis. Case Rep. Neurol. 2017, 9, 210–215. [Google Scholar] [CrossRef]
- Kawabata, Y.; Miyaji, Y.; Joki, H.; Seki, S.; Mori, K.; Kamide, T.; Tamase, A.; Nomura, M.; Kitamura, Y.; Tanaka, F. Isolated index finger palsy due to cortical infarction. J. Stroke Cerebrovasc. Dis. 2014, 23, e475–e476. [Google Scholar] [CrossRef]
| Reference | Demographic Data (Sex/Age) | Ischemic Stroke Type (TOAST Criteria) | Outcome | Note |
|---|---|---|---|---|
| Tei et al. (1999) [1] | 75/M | Likely small vessel occlusion of hand knob area | Weakness of distal right arm, especially with fine movements. Stereognosis, position sense, and sensation were intact | Cranial computed tomography did not reveal findings of the acute stroke |
| Back et al. (2001) [2] | 81/F | Likely small vessel occlusion of cortical precentral hand knob area | Flaccid paralysis of the right arm without loss of sensation or reflexes | - |
| Gass et al. (2001) [3] | 73/M | 70% stenosis of right ICA, LVO | DAP + facial and wrist palsy, mild sensory loss | Weakness was measured according to the medical research council scale |
| 44/F | Dissection of left ICA, other determined etiology | DAP + ulnar involvement | ||
| 62/F | Undetermined etiology- likely cardioembolic as workup revealed cardiac arrhythmia | DAP + facial palsy and mild sensory loss | ||
| 74/M | Right RCA stenosis 80%, LVO | DAP | ||
| 43/M | Large PFO; other determined etiology | DAP + radial nerve symptoms | ||
| 59/M | Left ICA stenosis 80%, LVO | DAP + facial, wrist, elbow palsy with mild sensory loss | ||
| 79/F | 80% stenosis of right ICA, LVO | DAP + ulnar nerve palsy symptoms | ||
| 75/M | No occlusion, undetermined etiology | DAP + ulnar nerve palsy | ||
| 49/F | L MCA stenosis, LVO | DAP | ||
| 69/F | Undetermined etiology | DAP | ||
| 76/M | Likely cardioembolic as the patient had PFO | DAP + radial nerve palsy symptoms | ||
| 75/F | Likely cardioembolic, as the patient had cardiac arrhythmia | DAP + radial symptoms | ||
| 66/M | Other undetermined etiology | DAP + ulnar nerve symptoms | ||
| 77/M | Likely cardioembolic in the setting of PFO | DAP + wrist and elbow palsy | ||
| Granziera et al. (2008) [4] | 67/M | Cardioembolism | Isolated left-hand paresis with paresis of thumb and index flexion | - |
| Hall et al. (2008) [5] | 67/M | Right internal carotid plaque (large artery atherosclerosis) | Plegia of left-hand flexion and extension | - |
| Peters et al. (2009) [6] | 70.8 (mean)/29 subjects (22M and 7F) | Atherosclerosis of the carotid artery (27/29 subjects), large artery atherosclerosis. Cardioembolic source (4/29 subjects) | All 29 subjects with contralateral arm paresis were detected on diffusion-weighted imaging. 16 patients had right-sided infarcts, and 13 had left-sided infarcts | 79% of the individuals had full recovery within 25 months |
| Kitamura et al. (2010) [7] | 73/M | Cardioembolism | Pure motor isolated finger palsy of the left thumb and index finger | Some peripheral nerve palsies could be isolated motor strokes |
| Alstadhaug et al. (2013) [8] | 62.61 (mean)/13 subjects (11M and 2F) | LVO noted in 11/13 subjects Two subjects had cardioembolism | Acute isolated hand paresis of contralateral arm | - |
| Dekeyzer et al. (2014) [9] | 69/M | Stenosis of cervical and cavernous segment of R ICA, large vessel atherosclerosis | Paresis of the left arm, mild hyperreflexia, and lack of sensory symptoms | Non-contrast cranial CT scan demonstrated blurring of right-hand knob area |
| Jusufovic et al. (2015) [10] | 44/F | Right proximal MCA stenosis, large vessel atherosclerosis | Motor deficits of left arm—claw hand deformity, impairment of adduction and abduction, brisk deep tendon reflexes | - |
| De Medeiros et al. (2017) [11] | 71/F | Cardioembolic source as the patient had atrial fibrillation | Right-hand weakness, fine motor impairment, preserved reflexes, lack of sensory involvement | - |
| Dijkstra et al. (2017) [12] | 78/M | - | Weakness of right thumb and index finger | - |
| Kim et al. (2017) [13] | 60 (mean)/17 subjects (8M and 9F) | - | - | - |
| Folyovich et al. (2018) [14] | 70/M | Pulmonary adenocarcinoma metastasis to precentral gyrus, stroke of other determined etiology | Left upper extremity weakness without sensory loss | Left arm weakness was the presenting sign of the malignancy |
| Orosz et al. (2018) [15] | 67 (mean)/25 subjects (12M and 13F) | 12/25 were secondary to large artery atherosclerosis. Nine were strokes of undetermined etiology, four were strokes of determined etiology including anus carcinoma, carotid artery dissection, lupus anticoagulant, and factor V Leiden | Arm paresis contralateral to the area affected | One subject had bilateral hand paresis secondary to bilateral hand knob infarctions |
| Wang et al. (2018) [16] | 67 (mean)/9 subjects (4F and 5M) | Three subjects developed large artery atherosclerosis. Three subjects developed stroke of undetermined etiology. Two subjects developed cardioembolism. One subject developed stroke of other determined etiology (Moya Moya affecting bilateral MCA) | Distal arm weakness, one subject with radial features, one subject with ulnar features and the rest of them with uniform features | Lesions of the lateral hand knob area affected radial nerve distribution, and lesions of the medial hand knob area affected ulnar nerve distribution indicating a topographic representation. This study is the first report of Moya Moya disease causing hand knob infarction |
| Finkelsteyn et al. (2019) [17] | 60 (mean)/12 subjects (9M and 3F) | Two subjects developed large artery atherosclerosis (ipsilateral ICA stenosis greater than 50%). Two subjects developed cardioembolism (one with intracardiac thrombus and one with AV block). One subject developed a stroke of other determined etiology (thrombophilia). Seven developed strokes of undetermined etiology | Contralateral arm paralysis without sensory findings | Largest cohort of patients with hand knob area stroke in Latin America |
| Tomoda et al. (2019) [18] | 72/M | Thromboembolism secondary to pancreatic adenocarcinoma resulting in hypercoagulable state, stroke of other determined etiology | Paresis of left arm extension | Cancer-associated embolism has a high rate of recurrence |
| Shelley et al. (2020) [19] | 59/M | Hyperhomocysteinemia, stroke of other determined etiology | Inability to extend right wrist and finger (isolated right pseudo radial paralysis) | - |
| 47/M | Hyperhomocystinemia resulting in common carotid artery thrombus, stroke of other determined etiology | Left pseudo-radial paralysis | - | |
| 30/F | Metastatic lesion to the hand knob area, from primary adenocarcinoma of the lung, stroke of other determined etiology | Left pseudo-median paralysis | - | |
| 60/F | Giant cell arteritis, stroke of other determined etiology | Left pseudo-median paralysis | - | |
| 35/M | Common carotid artery thrombosis in the setting of iron deficiency anemia, thrombocytosis, stroke of other determined etiology | Right pseudo-radial paralysis | - | |
| 65/F | Left ventricle thrombus that embolized to left ICA (artery to artery embolism), stroke of other determined etiology | Right pseudo-median palsy | First known case of artery-to-artery embolism resulting in hand knob infarct | |
| Daneasa et al. (2021) [20] | 106/M | Stenosis of the left internal carotid artery, large artery atherosclerosis | Distal paralysis of the right hand | The oldest patient reported in the literature with hand knob infarct |
| Davies et al. (2022) [21] | 59/M | Unknown mechanism in both cases, possible large artery atherosclerosis | Sudden onset of left-hand grip strength loss | Highlights the importance of recognition of stroke symptoms by emergency department physicians and discusses the risk of future stroke |
| 88/M | Sudden painless weakness of left hand | |||
| Nicolini et al. (2022) [22] | 83/M | Cerebral air embolism in the setting of central venous catheterization that traveled through a PFO, stroke of other determined etiology | Sudden, transient loss of consciousness, left arm weakness upon waking up | First ever reported case of cerebral air embolism resulting in hand knob infarct |
| Zhang et al. (2022) [23] | 62 (mean)/9 subjects (5M and 4F) | Six subjects with large artery atherosclerosis, six subjects with small artery occlusion, one subject with cardioembolism, two subjects with a stroke of undetermined etiology, and two with strokes of other determined etiology | Contralateral hand paresis in all 19 subjects | Largest cohort of patients with hand knob infarct in China |
| Alshanqiti et al. (2023) [24] | 62/F | Fibromuscular dysplasia and severe atherosclerotic stenosis of the right internal carotid artery (large artery atherosclerosis versus stroke of other determined etiology) | Wrist extension drop of the left hand, no sensory deficits | First known report of hand knob infarct secondary to fibromuscular dysplasia |
| First Author et al./Year | Country | Incidence | Number of Individuals Affected | Number of Individuals in the Study |
|---|---|---|---|---|
| Finkelsteyn et al. (2019) [17] | Argentina | 3.53% | 12 | 339 |
| Alstadhaug et al. (2013) [8] | Norway | 1.5% | 13 | 866 |
| Celebisoy et al. (2007) [25] | Turkey | 0.98% | 8 | 815 |
| Zhang et al. (2022) [23] | China | 0.9% | 19 | 2224 |
| Peters et al. (2009) [6] | Germany | 0.83% | 29 | 3499 |
| Orosz et al. (2018) [15] | Hungary | 0.35% | 25 | 720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Byroju, V.V.; Mukkamalla, S.; Caprara, A.L.F. A Narrative Review of Stroke of Cortical Hand Knob Area. Medicina 2024, 60, 318. https://doi.org/10.3390/medicina60020318
Rissardo JP, Byroju VV, Mukkamalla S, Caprara ALF. A Narrative Review of Stroke of Cortical Hand Knob Area. Medicina. 2024; 60(2):318. https://doi.org/10.3390/medicina60020318
Chicago/Turabian StyleRissardo, Jamir Pitton, Vishnu Vardhan Byroju, Sushni Mukkamalla, and Ana Letícia Fornari Caprara. 2024. "A Narrative Review of Stroke of Cortical Hand Knob Area" Medicina 60, no. 2: 318. https://doi.org/10.3390/medicina60020318
APA StyleRissardo, J. P., Byroju, V. V., Mukkamalla, S., & Caprara, A. L. F. (2024). A Narrative Review of Stroke of Cortical Hand Knob Area. Medicina, 60(2), 318. https://doi.org/10.3390/medicina60020318







