Case Study of a Complex Neurovascular Disorder: Choroidal Arteriovenous Malformation
Abstract
1. Introduction
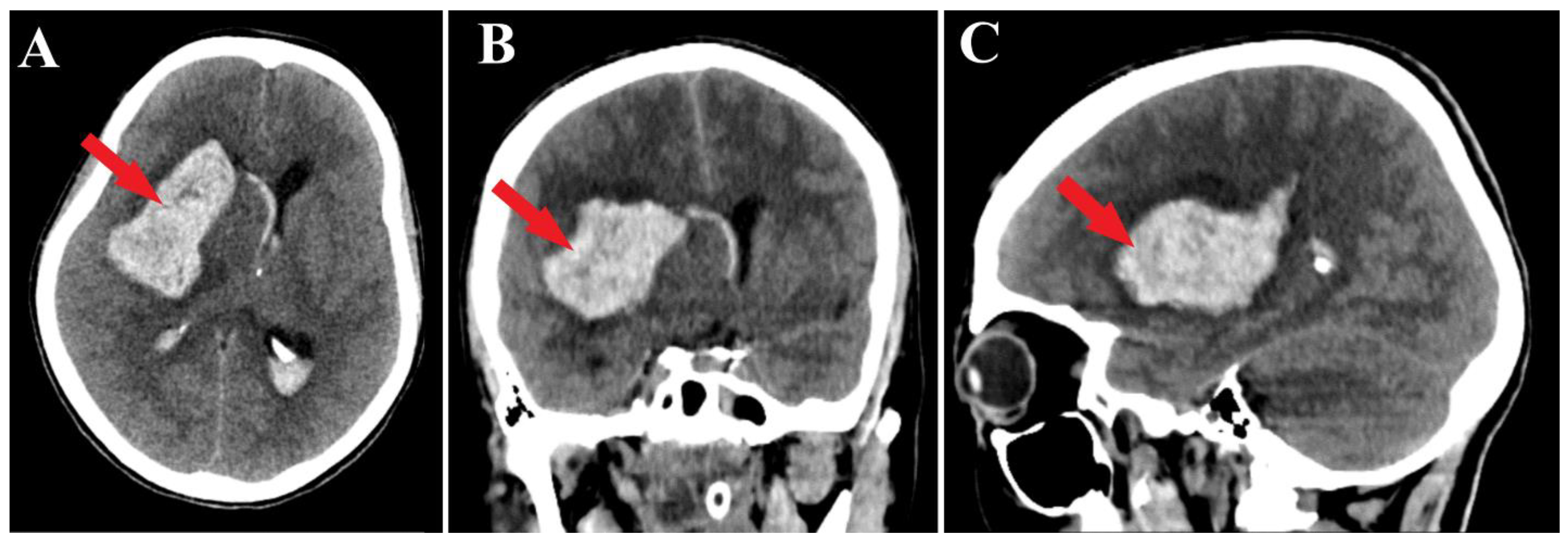
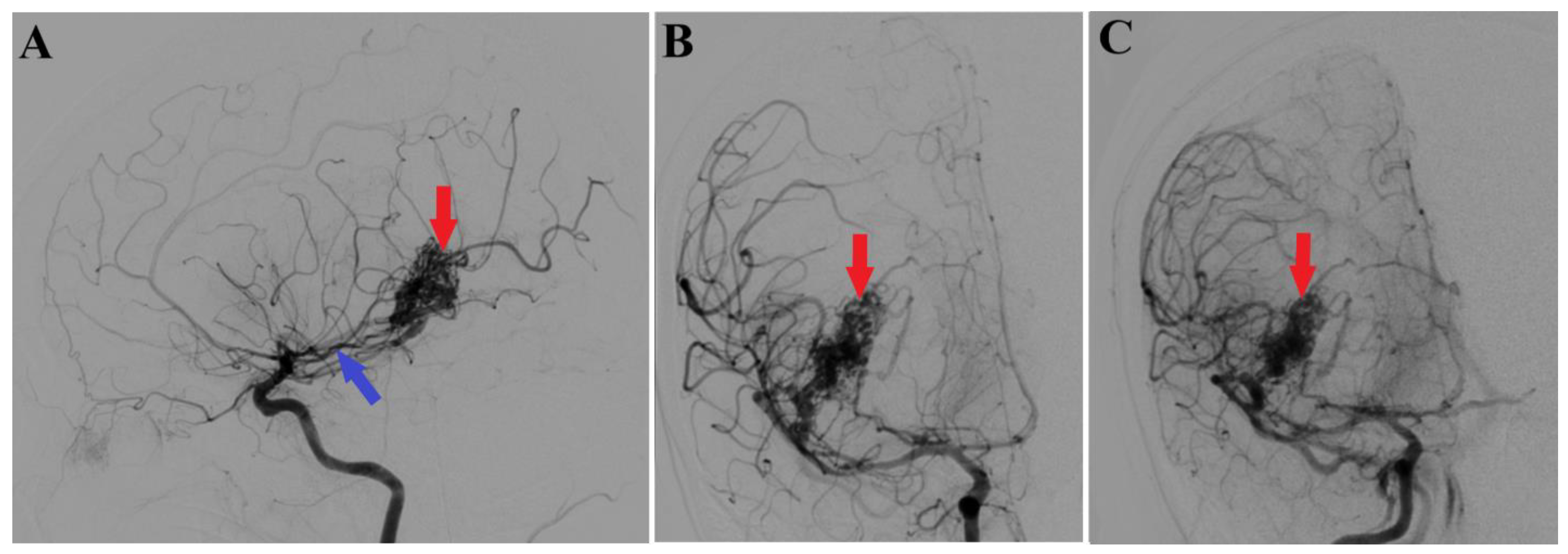
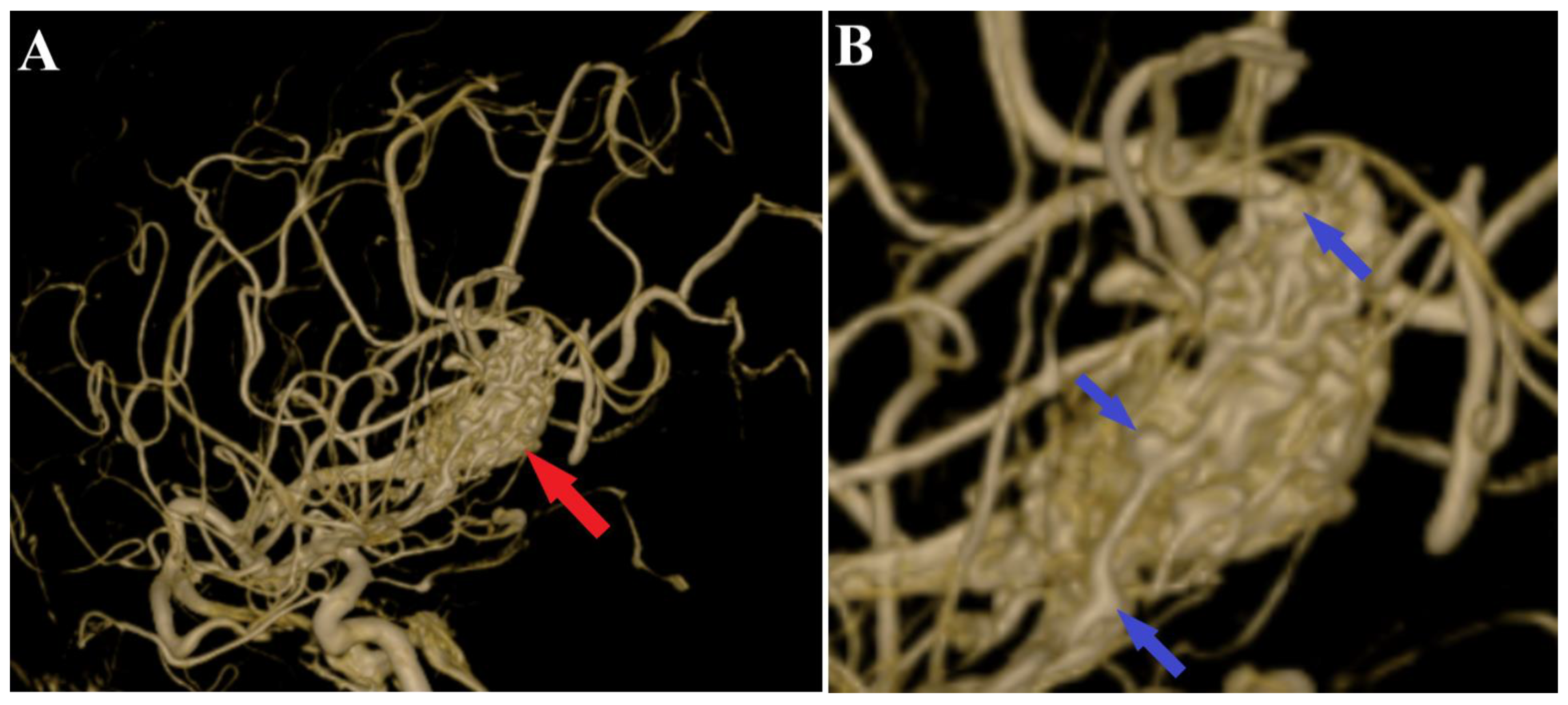
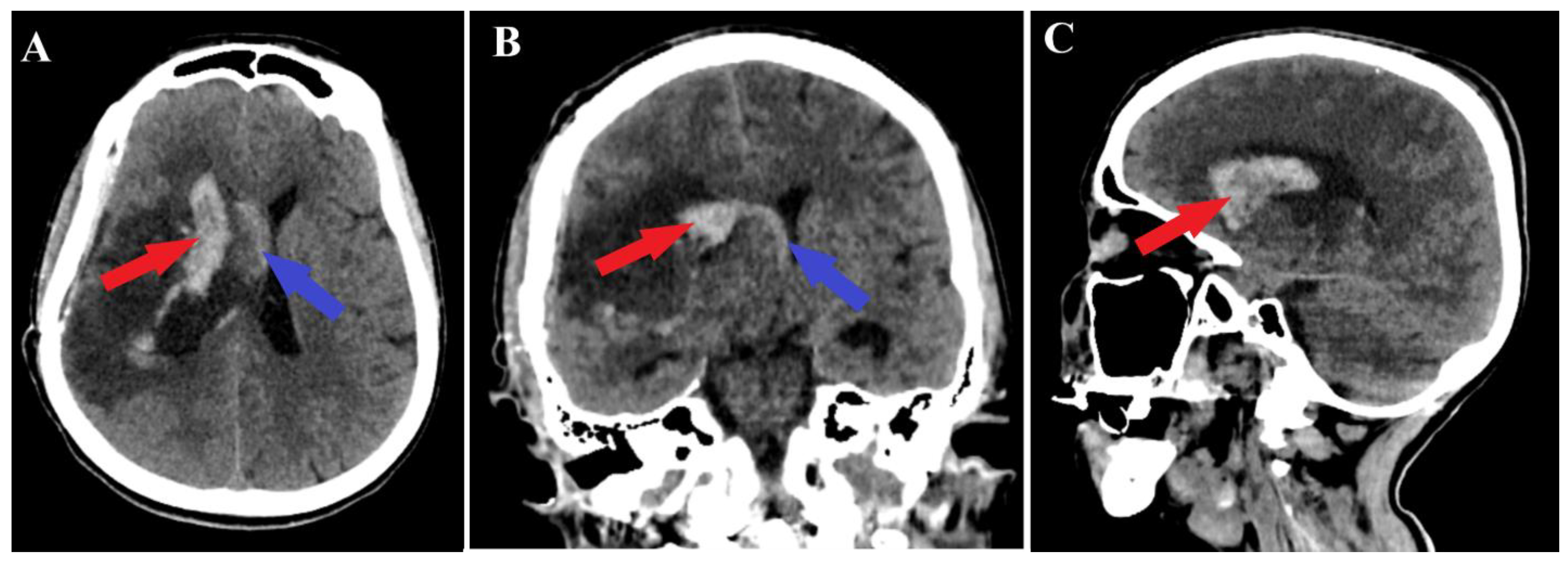
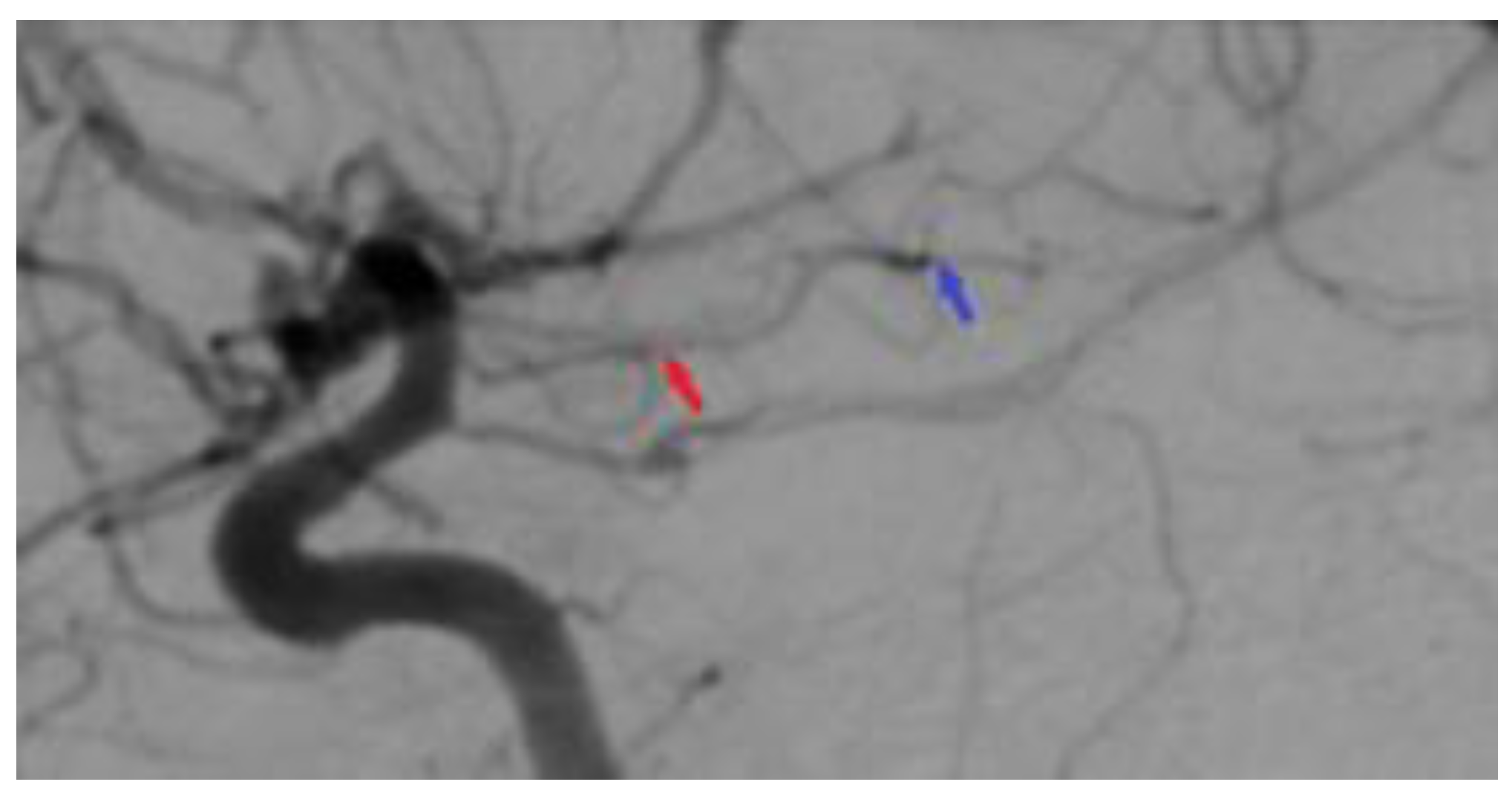
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abecassis, I.J.; Xu, D.S.; Batjer, H.H.; Bendok, B.R. Natural history of brain arteriovenous malformations: A systematic review. Neurosurg. Focus 2014, 37, E7. [Google Scholar] [CrossRef] [PubMed]
- Stapf, C.; Mast, H.; Sciacca, R.R.; Choi, J.H.; Khaw, A.V.; Connolly, E.S.; Pile-Spellman, J.; Mohr, J.P. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006, 66, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Su, L.; Wang, D.; Fan, X. Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods. J. Interv. Med. 2023, 6, 170–175. [Google Scholar] [CrossRef]
- Ricolfi, F.; Manelfe, C.; Meder, J.F.; Arrué, P.; Decq, P.; Brugiéres, P.; Cognard, C.; Gaston, A. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology 1999, 41, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, Y.; Yang, X.; Sheng, A.; Lu, J. Case report: A choroidal fissure pial arteriovenous malformation inducing venous congestive edema of the medulla oblongata and cervicothoracic spinal cord presented with proximal arm predominant weakness. Front. Neurol. 2023, 14, 1128366. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, C.; Stapf, C.; Hartmann, A.; Sciacca, R.R.; Mansmann, U.; Terbrugge, K.; Lasjaunias, P.; Mohr, J.P.; Mast, H.; Meisel, J. Demographic, Morphological, and Clinical Characteristics of 1289 Patients With Brain Arteriovenous Malformation. Stroke 2000, 31, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Gadol, A.; Conger, A.; Kulwin, C.; Lawton, M. Diagnosis and evaluation of intracranial arteriovenous malformations. Surg. Neurol. Int. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; González-López, P.; Daniel, R.T. The transcallosal transchoroidal approach to the diencephalic-mesencephalic junction: How I do it. Acta Neurochir. 2019, 161, 2329–2334. [Google Scholar] [CrossRef]
- Baldoncini, M.; Campero, A.; Cruz, J.C.P.; Recalde, R.; Parraga, R.; Gonzalez, F.J.S.; Fortte, M.; López, P.G. Microsurgical Anatomy and Approaches to the Cerebral Central Core. World Neurosurg. 2019, 129, e23–e34. [Google Scholar] [CrossRef]
- Costa, M.D.S.; Santos, B.F.d.O.; Guardini, F.B.d.A.; Chaddad-Neto, F. Microsurgical treatment for arteriovenous malformation of the corpus callosum and choroidal fissure. Neurosurg. Focus 2017, 43, V12. [Google Scholar] [CrossRef]
- Domingo, R.A.; Grewal, S.; Tawk, R.G. Interhemispheric Transcallosal Approach for Resection of Choroidal Arteriovenous Malformation: Operative Video. World Neurosurg. 2020, 136, 73. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsumoto, S. Anterior choroidal artery arteriovenous malformation. Its clinical manifestations and surgical treatment. Surg. Neurol. 1984, 22, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Ryu, B.; Sato, S.; Niimi, Y. Successful embolization of ventricular arteriovenous malformation supplied by the choroidal artery: A case report and literature review. Surg. Neurol. Int. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, M.; Satow, T.; Matsushige, T.; Mori, H.; Iihara, K. Superselective Provocative Test with Propofol Using Motor-Evoked Potential Monitoring for Managing Cerebral Arteriovenous Malformations Fed by the Anterior Choroidal Artery. J. Stroke Cerebrovasc. Dis. 2016, 25, e153–e157. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Jiang, C.; Li, Y.; Yang, X.; Wu, Z. Endovascular treatment for cerebral perforating artery aneurysms. Neurol. Res. 2011, 33, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Hu, X.; Li, W.; He, H.; Jiang, C.; Li, Y. Curative and adjunctive AVM Onyx embolization of AVMs through the choroidal arteries. Interv. Neuroradiol. 2017, 23, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Ogilvy, C.S.; Stieg, P.E.; Awad, I.; Brown, R.D.; Kondziolka, D.; Rosenwasser, R.; Young, W.L.; Hademenos, G.; Khaw, A.; Mohr, J.; et al. AHA Scientific Statement: Recommendations for the management of intracranial arteriovenous malformations: A statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 2001, 32, 1458–1471. [Google Scholar] [CrossRef]
- Starke, R.M.; Komotar, R.J.; Hwang, B.Y.; Fischer, L.E.; Garrett, M.C.; Otten, M.L.; Connolly, E.S. Treatment guidelines for cerebral arteriovenous malformation microsurgery. Br. J. Neurosurg. 2009, 23, 376–386. [Google Scholar] [CrossRef]
- Zuurbier, S.M.; Salman, R.A.-S. Interventions for treating brain arteriovenous malformations in adults. Cochrane Database Syst. Rev. 2019, 9, CD003436. [Google Scholar] [CrossRef]
- Al-Shahi, R.; Stapf, C. The Prognosis and Treatment of Arteriovenous Malformations of the Brain. Pract. Neurol. 2005, 5, 194–205. [Google Scholar] [CrossRef]
- Beijnum, J.; Bhattacharya, J.J.; Counsell, C.E.; Papanastassiou, V.; Ritchie, V.; Roberts, R.C.; Sellar, R.J.; Warlow, C.; Salman, R.A.-S. Patterns of brain arteriovenous malformation treatment: Prospective, population-based study. Stroke 2008, 39, 3216–3221. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Li, C.; Su, H.; Yu, J. Imaging Characteristics and Endovascular Treatment of Brain Arteriovenous Malformations Mainly Fed by the Posterior Cerebral Artery. Front. Neurol. 2021, 11, 609461. [Google Scholar] [CrossRef] [PubMed]
- Elkordy, A.; Endo, H.; Sato, K.; Matsumoto, Y.; Kondo, R.; Niizuma, K.; Endo, T.; Fujimura, M.; Tominaga, T. Embolization of the choroidal artery in the treatment of cerebral arteriovenous malformations. J. Neurosurg. 2017, 126, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Oran, I.; Parildar, M.; Derbent, A. Ventricular/paraventricular small arteriovenous malformations: Role of embolisation with cyanoacrylate. Neuroradiology 2005, 47, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.; Kano, H.; Yang, H.-C.; Niranjan, A.; Flickinger, J.; Lunsford, L.D. Gamma Knife surgery for arteriovenous malformations within or adjacent to the ventricles. J. Neurosurg. 2014, 121, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Nozaki, K.; Takagi, Y.; Kikuta, K.-I.; Mikuni, N. SURGERY OF CEREBRAL ARTERIOVENOUS MALFORMATIONS. Neurosurgery 2007, 61, 389. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Kondziolka, D.; Flickinger, J.C.; Yang, H.-C.; Park, K.-J.; Flannery, T.J.; Liu, X.; Niranjan, A.; Lunsford, L.D. Aneurysms increase the risk of rebleeding after stereotactic radiosurgery for hemorrhagic arteriovenous malformations. Stroke 2012, 43, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Lunsford, L.D.; Flickinger, J.C.; Yang, H.-C.; Flannery, T.J.; Awan, N.R.; Niranjan, A.; Novotny, J.; Kondziolka, D. Stereotactic radiosurgery for arteriovenous malformations, Part 1: Management of Spetzler-Martin Grade I and II arteriovenous malformations. J. Neurosurg. 2012, 116, 11–20. [Google Scholar] [CrossRef]
- Crowley, R.W.; Ducruet, A.F.; McDougall, C.G.; Albuquerque, F.C. Endovascular advances for brain arteriovenous malformations. Neurosurgery 2014, 74 (Suppl. S1), S74–S82. [Google Scholar] [CrossRef]
- Kalani, M.Y.S.; Albuquerque, F.C.; Fiorella, D.; McDougall, C.G. Endovascular Treatment of Cerebral Arteriovenous Malformations. Neuroimaging Clin. N. A. 2013, 23, 605–624. [Google Scholar] [CrossRef]
- Flores, B.C.; Klinger, D.R.; Rickert, K.L.; Barnett, S.L.; Welch, B.G.; White, J.A.; Batjer, H.H.; Samson, D.S. Management of intracranial aneurysms associated with arteriovenous malformations. Neurosurg. Focus 2014, 37, E11. [Google Scholar] [CrossRef] [PubMed]
- Xuan, N.T.; Tuong, P.N.; Khoa, T.; Hiep, P.N.; Van Thanh, N.; Bao, D.H. Volume-Staged Radiosurgery for Large Arteriovenous Malformation. Case Rep. Neurol. 2020, 12, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, J.C.; Kondziolka, D.; Lunsford, L.; Pollock, B.E.; Yamamoto, M.; Gorman, D.A.; Schomberg, P.J.; Sneed, P.; Larson, D.; Smith, V.; et al. A multi-institutional analysis of complication outcomes after arteriovenous malformation radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N. Choroid plexus tumors in children. Neurosurg. Clin. N. A. 2003, 14, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ben Nsir, A.; Gdoura, Y.; Thai, Q.-A.; Kassar, A.Z.; Hattab, N.; Jemel, H. Intraventricular Glioblastomas. World Neurosurg. 2016, 88, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W. Intraventricular Tumors. World Neurosurg. 2013, 79, S17.e15–S17.e19. [Google Scholar] [CrossRef] [PubMed]
- James, R.F.; Kramer, D.R.; Page, P.S.; Gaughen, J.R.; Martin, L.B.; Mack, W.J. Strategic and Technical Considerations for the Endovascular Embolization of Intracranial Meningiomas. Neurosurg. Clin. N. A. 2016, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lin, F.; Wu, J.; Li, H.; Chen, X.; Li, Z.; Ma, J.; Cao, Y.; Wang, S.; Zhao, J. Brain Arteriovenous Malformations Supplied by the Anterior Choroidal Artery: Treatment Outcomes and Risk Factors for Worsened Muscle Strength After Surgical Resection. World Neurosurg. 2017, 104, 567–574. [Google Scholar] [CrossRef]
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; Terbrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 2015, 1, 15008. [Google Scholar] [CrossRef]
- Fernández-Miranda, J.C.; de Oliveira, E.; Rubino, P.A.; Wen, H.T.; Rhoton, A.L. Microvascular anatomy of the medial temporal region: Part 1: Its application to arteriovenous malformation surgery. Neurosurg. 2010, 67, ons237–ons276. [Google Scholar] [CrossRef]
- Yu, J.; Xu, N.; Zhao, Y.; Yu, J. Clinical importance of the anterior choroidal artery: A review of the literature. Int. J. Med. Sci. 2018, 15, 368–375. [Google Scholar] [CrossRef]
- Hou, K.; Li, G.; Luan, T.; Xu, K.; Yu, J. The prospects and pitfalls in the endovascular treatment of moyamoya disease–associated intracranial aneurysms. Neurosurg. Rev. 2021, 44, 261–271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, C.; Covache-Busuioc, R.-A.; Bratu, B.-G.; Glavan, L.A.; Corlatescu, A.D.; Ciurea, A.V. Case Study of a Complex Neurovascular Disorder: Choroidal Arteriovenous Malformation. Medicina 2024, 60, 302. https://doi.org/10.3390/medicina60020302
Toader C, Covache-Busuioc R-A, Bratu B-G, Glavan LA, Corlatescu AD, Ciurea AV. Case Study of a Complex Neurovascular Disorder: Choroidal Arteriovenous Malformation. Medicina. 2024; 60(2):302. https://doi.org/10.3390/medicina60020302
Chicago/Turabian StyleToader, Corneliu, Razvan-Adrian Covache-Busuioc, Bogdan-Gabriel Bratu, Luca Andrei Glavan, Antonio Daniel Corlatescu, and Alexandru Vlad Ciurea. 2024. "Case Study of a Complex Neurovascular Disorder: Choroidal Arteriovenous Malformation" Medicina 60, no. 2: 302. https://doi.org/10.3390/medicina60020302
APA StyleToader, C., Covache-Busuioc, R.-A., Bratu, B.-G., Glavan, L. A., Corlatescu, A. D., & Ciurea, A. V. (2024). Case Study of a Complex Neurovascular Disorder: Choroidal Arteriovenous Malformation. Medicina, 60(2), 302. https://doi.org/10.3390/medicina60020302




