Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues
Abstract
1. Introduction
2. Technical Overview and Mechanistic Explanation of Irreversible Electroporation
3. Patient Selection
4. Procedural Considerations
5. Safety and Complications
6. Clinical Outcomes
7. Post-IRE Immunological Responses
8. Challenges for New Centers to Start Using IRE
9. Post-IRE Pathologic-Radiologic Aspects
10. Comparison between IRE and Thermal Ablation Methods
11. Future Directions with IRE for Liver Cancers
12. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| High-Focused Ultrasound (HIFU) | Non-invasive technique that uses focused ultrasound to induce cell death |
| Microwave Ablation (MWA) | Thermal ablative technique that uses heat energy generated from microwaves to induce cell death |
| Irreversible Electroporation (IRE) | Non-thermal ablative technique that utilizes direct current to induce cell death |
| Cryoablation (CRYO) | Thermal ablative technique that uses |
| Radiofrequency ablation (RFA) | Thermal ablative technique that uses alternating current to induce cell death |
| Nanoknife | Commercial name of irreversible electroporation device |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; González-Flores, E.; Rubio, C.; Urbano, J.; Camps, M.V.; Ciampi-Dopazo, J.J.; Rincón, J.O.; Macías, V.M.; Braco, M.A.G.; Suarez-Artacho, G. Multidisciplinary management of liver metastases in patients with colorectal cancer: A consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin. Transl. Oncol. 2019, 22, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef] [PubMed]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; Van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; De Jong, M.C.; De Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colo rectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Benson, A.B.; D’angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef]
- Edeline, J.; Raoul, J.-L.; Vauleon, E.; Guillygomac’h, A.; Boudjema, K.; Boucher, E. Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic liver: A retrospective study. World J. Gastroenterol. 2009, 15, 713–716. [Google Scholar] [CrossRef]
- Merle, P.; Subic, M. Comparison and analysis of the efficacy of drug therapy for liver cancer. Hepatoma Res. 2020, 6, 60. [Google Scholar] [CrossRef]
- Uhlig, J.; Lukovic, J.; Dawson, L.A.; Patel, R.A.; Cavnar, M.J.; Kim, H.S. Locoregional Therapies for Colorectal Cancer Liver Metastases: Options Beyond Resection. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 133–146. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Thomas, S.; Kim, K.R. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin. Interv. Radiol. 2014, 31, 138–148. [Google Scholar] [CrossRef]
- Foltz, G. Image-guided percutaneous ablation of hepatic malignancies. Semin. Interv. Radiol. 2014, 31, 180–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villard, C.; Soler, L.; Gangi, A.; Mutter, D.; Marescaux, J. Toward realistic radiofrequency ablation of hepatic tumors 3D simulation and planning. In Medical Imaging 2004: Visualization, Image-Guided Procedures, and Display; SPIE: Bellingham, WA, USA, 2004; Volume 5367, p. 586. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G. Irreversible Electroporation. Semin. Interv. Radiol. 2015, 32, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.R.; Cheung, W.; Ellis, S.J.; Federman, D.; Kavnoudias, H.; Loader-Oliver, D.; Roberts, S.; Evans, P.; Ball, C.; Haydon, A. Investigation of the safety of irreversible electroporation in humans. J. Vasc. Interv. Radiol. 2011, 22, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ruarus, A.H.; Barabasch, A.; Catalano, O.; Leen, E.; Narayanan, G.; Nilsson, A.; Padia, S.A.; Wiggermann, P.; Scheffer, H.J.; Meijerink, M.R. Irreversible Electroporation for Hepatic Tumors: Protocol Standardization Using the Modified Delphi Technique. J. Vasc. Interv. Radiol. 2020, 31, 1765–1771.e15. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.; Cheung, W.; Kavnoudias, H.; Majeed, A.; Kemp, W.; Roberts, S.K. Irreversible Electroporation For Hepatocellular Carcinoma: Longer-Term Outcomes At A Single Centre. Cardiovasc. Interv. Radiol. 2021, 44, 247–253. [Google Scholar] [CrossRef]
- Cannon, R.; Ellis, S.; Hayes, D.; Narayanan, G.; Martin, R.C. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 2012, 107, 544–549. [Google Scholar] [CrossRef]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions—Part II: Recommendations. J. Vasc. Interv. Radiol. 2019, 30, 1168–1184.e1. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, D.S.; Hwang, S.W.; Easa, J.E.; Resnick, D.K.; Baisden, J.L.; Bess, S.; Cho, C.H.; DePalma, M.J.; Dougherty, P.; Fernand, R.; et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014, 14, 180–191. [Google Scholar] [CrossRef]
- Deodhar, A.; Dickfeld, T.; Single, G.W.; Hamilton, W.C.; Thornton, R.H.; Sofocleous, C.T.; Maybody, M.; Gónen, M.; Rubinsky, B.; Solomon, S.B. Irreversible electroporation near the heart: Ventricular arrhythmias can be prevented with ECG synchronization. Am. J. Roentgenol. 2011, 196, W330–W335. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goldman, D.A.; D’Angelica, M.I.; DeMatteo, R.P.; Allen, P.J.; Balachandran, V.P.; Jarnagin, W.R.; Kingham, T.P. Recurrence patterns following irreversible electroporation for hepatic malignancies. J. Surg. Oncol. 2017, 115, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Scheffer, H.J.; Vieveen, J.M.; van Tilborg, A.A.J.M.; Meijer, S.; van Kuijk, C.; Tol, M.P.v.D.; Meijerink, M.R.; Bouwman, R.A. Anaesthetic management during open and percutaneous irreversible electroporation. Br. J. Anaesth. 2014, 113, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible Electroporation for nonthermal tumor ablation in the clinical setting: A systematic review of safety and efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011. [Google Scholar] [CrossRef]
- Narayanan, G.; Hosein, P.J.; Arora, G.; Barbery, K.J.; Froud, T.; Livingstone, A.S.; Franceschi, D.; Lima, C.M.R.; Yrizarry, J. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J. Vasc. Interv. Radiol. 2012, 23, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, H.J.; Melenhorst, M.C.; Echenique, A.M.; Nielsen, K.; van Tilborg, A.A.; van den Bos, W.; Vroomen, L.G.; van den Tol, P.M.; Meijerink, M.R. Irreversible Electroporation for Colorectal Liver Metastases. Tech. Vasc. Interv. Radiol. 2015, 18, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Froud, T.; Venkat, S.R.; Barbery, K.J.; Gunjan, A.; Narayanan, G. Liver Function Tests Following Irreversible Electroporation of Liver Tumors: Experience in 174 Procedures. Tech. Vasc. Interv. Radiol. 2015, 18, 140–146. [Google Scholar] [CrossRef]
- Narayanan, G.; Bhatia, S.; Echenique, A.; Suthar, R.; Barbery, K.; Yrizarry, J. Vessel Patency Post Irreversible Electroporation. Cardiovasc. Interv. Radiol. 2014, 37, 1523–1529. [Google Scholar] [CrossRef]
- Distelmaier, M.; Barabasch, A.; Heil, P.; Kraemer, N.A.; Isfort, P.; Keil, S.; Kuhl, C.K.; Bruners, P. Midterm safety and efficacy of irreversible electroporation of malignant liver tumors located close to major portal or hepatic veins. Radiology 2017, 285, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, M.; Zeman, F.; Niessen, C.; Lang, S.A.; Beyer, L.P.; Müller, M.; Stroszczynski, C.; Wiggermann, P. Bile duct injury after irreversible electroporation of hepatic malignancies: Evaluation of mr imaging findings and laboratory values. J. Vasc. Interv. Radiol. 2016, 27, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Pedersoli, F.; Schulze-Hagen, M.; Zimmerman, M.; Isfort, P.; Kuhl, C.K.; Schmitz-Rode, T.; Bruners, P. Predictors of Occlusion of Hepatic Blood Vessels after Irreversible Electroporation of Liver Tumors. J. Vasc. Interv. Radiol. 2020, 31, 2033–2042.e1. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Crocetti, L.; Narayanan, G. Irreversible Electroporation in the Treatment of Hepatocellular Carcinoma. Tech. Vasc. Interv. Radiol. 2015, 18, 135–139. [Google Scholar] [CrossRef]
- Hosein, P.J.; Echenique, A.; Loaiza-Bonilla, A.; Froud, T.; Barbery, K.; Lima, C.M.R.; Yrizarry, J.M.; Narayanan, G. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J. Vasc. Interv. Radiol. 2014, 25, 1233–1239.e2. [Google Scholar] [CrossRef] [PubMed]
- Verloh, N.; Jensch, I.; Lürken, L.; Haimerl, M.; Dollinger, M.; Renner, P.; Wiggermann, P.; Werner, J.M.; Zeman, F.; Stroszczynski, C.; et al. Similar complication rates for irreversible electroporation and thermal ablation in patients with hepatocellular tumors. Radiol. Oncol. 2019, 53, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, M.; Beyer, L.P.; Haimerl, M.; Niessen, C.; Jung, E.-M.; Zeman, F.; Stroszczynski, C.; Wiggermann, P. Adverse effects of irreversible electroporation of malignant liver tumors under CT fluoroscopic guidance: A single-center experience. Diagn. Interv. Radiol. 2015, 21, 471–475. [Google Scholar] [CrossRef]
- Niessen, C.; Thumann, S.; Beyer, L.; Pregler, B.; Kramer, J.; Lang, S.; Teufel, A.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci. Rep. 2017, 7, 43687. [Google Scholar] [CrossRef]
- Stillström, D.; Beermann, M.; Engstrand, J.; Freedman, J.; Nilsson, H. Initial experience with irreversible electroporation of liver tumours. Eur. J. Radiol. Open 2019, 6, 62–67. [Google Scholar] [CrossRef]
- Frühling, P.; Nilsson, A.; Duraj, F.; Haglund, U.; Norén, A. Single-center nonrandomized clinical trial to assess the safety and efficacy of irreversible electroporation (IRE) ablation of liver tumors in humans: Short to mid-term results. Eur. J. Surg. Oncol. 2017, 43, 751–757. [Google Scholar] [CrossRef]
- Mafeld, S.; Wong, J.J.; Kibriya, N.; Stenberg, B.; Manas, D.; Bassett, P.; Aslam, T.; Evans, J.; Littler, P. Percutaneous Irreversible Electroporation (IRE) of Hepatic Malignancy: A Bi-institutional Analysis of Safety and Outcomes. Cardiovasc. Interv. Radiol. 2019, 42, 577–583. [Google Scholar] [CrossRef]
- Sutter, O.; Calvo, J.; N’kontchou, G.; Nault, J.-C.; Ourabia, R.; Nahon, P.; Ganne-Carrié, N.; Bourcier, V.; Zentar, N.; Bouhafs, F.; et al. Safety and efficacy of irreversible electroporation for the treatment of hepatocellular carcinoma not amenable to thermal ablation techniques: A retrospective single-center case series. Radiology 2017, 284, 877–886. [Google Scholar] [CrossRef]
- Mazal, N.; West, D. Novel Therapy for Unresectable Hilar Cholangiocarcinoma ‘Klatskin Tumor’ Utilizing Percutaneous Irreversible Electroporation: A Case Report. OMICS J. Radiol. 2017, 6, 258. [Google Scholar] [CrossRef]
- Martin, E.K.; Bhutiani, N.; Egger, M.E.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Kelly, L.R.; Vitale, G.C.; Martin, R.C. Safety and efficacy of irreversible electroporation in the treatment of obstructive jaundice in advanced hilar cholangiocarcinoma. HPB 2018, 20, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.C.; van Veldhuisen, E.; Ruarus, A.H.; Coelen, R.J.; Roos, E.; van Delden, O.M.; Besselink, M.G.; Klümpen, H.-J.; van Lienden, K.P.; van Gulik, T.M.; et al. Outcomes of Irreversible Electroporation for Perihilar Cholangiocarcinoma: A Prospective Pilot Study. J. Vasc. Interv. Radiol. 2022, 33, 805–813.e1. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, Z.; Lei, K.; Liao, J.; Peng, Z.; Lin, M.; Liang, P.; Yu, J.; Peng, S.; Chen, S.; et al. Irreversible electroporation induces CD8+ T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 2021, 503, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Kakimi, K.; Takeuchi, H.; Fujieda, N.; Saito, K.; Sato, E.; Sakamaki, K.; Moriyasu, F.; Itoi, T. Irreversible Electroporation versus Radiofrequency Ablation: Comparison of Systemic Immune Responses in Patients with Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2019, 30, 845–853.e6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Beicos, A.; Venkat, S.; Songrug, T.; Poveda, J.; Garcia-Buitrago, M.; Mohan, P.P.; Narayanan, G. Irreversible Electroporation of Hepatic and Pancreatic Malignancies: Radiologic-Pathologic Correlation. Tech. Vasc. Interv. Radiol. 2015, 18, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.G.; Bhattacharya, R.; Yeh, M.M.; Padia, S.A. Irreversible Electroporation Can Effectively Ablate Hepatocellular Carcinoma to Complete Pathologic Necrosis. J. Vasc. Interv. Radiol. 2015, 26, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Philips, P.; Scoggins, C.R.; McMasters, K.M.; Potts, M.H.; Martin, R.C. Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child-Pugh B (7/8) hepatocellular carcinoma (HCC). HPB 2016, 18, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Fan, Y.; Wang, Z.; Lang, X. Irreversible electroporation versus radiofrequency ablation for malignant hepatic tumor: A prospective single-center double-arm trial. J. Interv. Med. 2022, 5, 89–94. [Google Scholar] [CrossRef]
- Wada, T.; Sugimoto, K.; Sakamaki, K.; Takahashi, H.; Kakegawa, T.; Tomita, Y.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. Comparisons of Radiofrequency Ablation, Microwave Ablation, and Irreversible Electroporation by Using Propensity Score Analysis for Early Stage Hepatocellular Carcinoma. Cancers 2023, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Procissi, D.; Tyler, P.; Omary, R.A.; Larson, A.C.; Lo, L.-W.; Chen, S.-A.; Young, M.-L.; Dawes, T.R.; et al. Rapid dramatic alterations to the tumor microstructure in pancreatic cancer following irreversible electroporation ablation. Nanomedicine 2014, 9, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef]
- SWhite, B.; Zhang, Z.; Chen, J.; Gogineni, V.R.; Larson, A.C. Early Immunologic Response of Irreversible Electroporation versus Cryoablation in a Rodent Model of Pancreatic Cancer. J. Vasc. Interv. Radiol. 2018, 29, 1764–1769. [Google Scholar]
- Partridge, B.R.; O’brien, T.J.; Lorenzo, M.F.; Coutermarsh-Ott, S.L.; Barry, S.L.; Stadler, K.; Muro, N.; Meyerhoeffer, M.; Allen, I.C.; Davalos, R.V.; et al. High-Frequency Irreversible Electroporation for Treatment of Primary Liver Cancer: A Proof-of-Principle Study in Canine Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2020, 31, 482–491.e4. [Google Scholar] [CrossRef]
- Gish, R.G.; Marrero, J.A.; Benson, A.B. A multidisciplinary approach to the management of hepatocellular carcinoma. Gastroenterol. Hepatol. 2010, 6 (Suppl. S6), 1–16. [Google Scholar]
- Siddique, O.; Yoo, E.R.; Perumpail, R.B.; Perumpail, B.J.; Liu, A.; Cholankeril, G.; Ahmed, A. The importance of a multidisciplinary approach to hepatocellular carcinoma. J. Multidiscip. Healthc. 2017, 10, 95–100. [Google Scholar] [CrossRef] [PubMed]
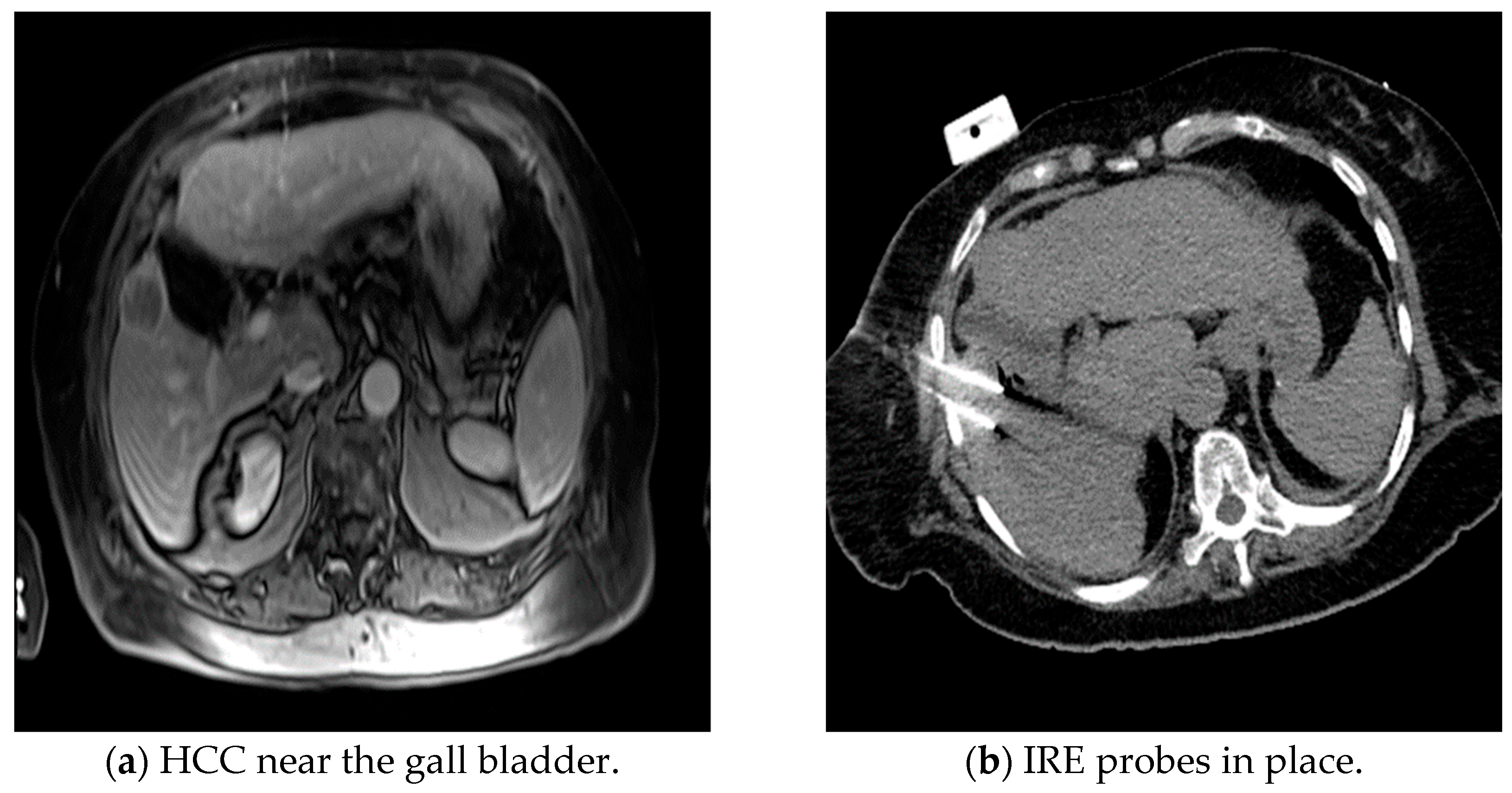
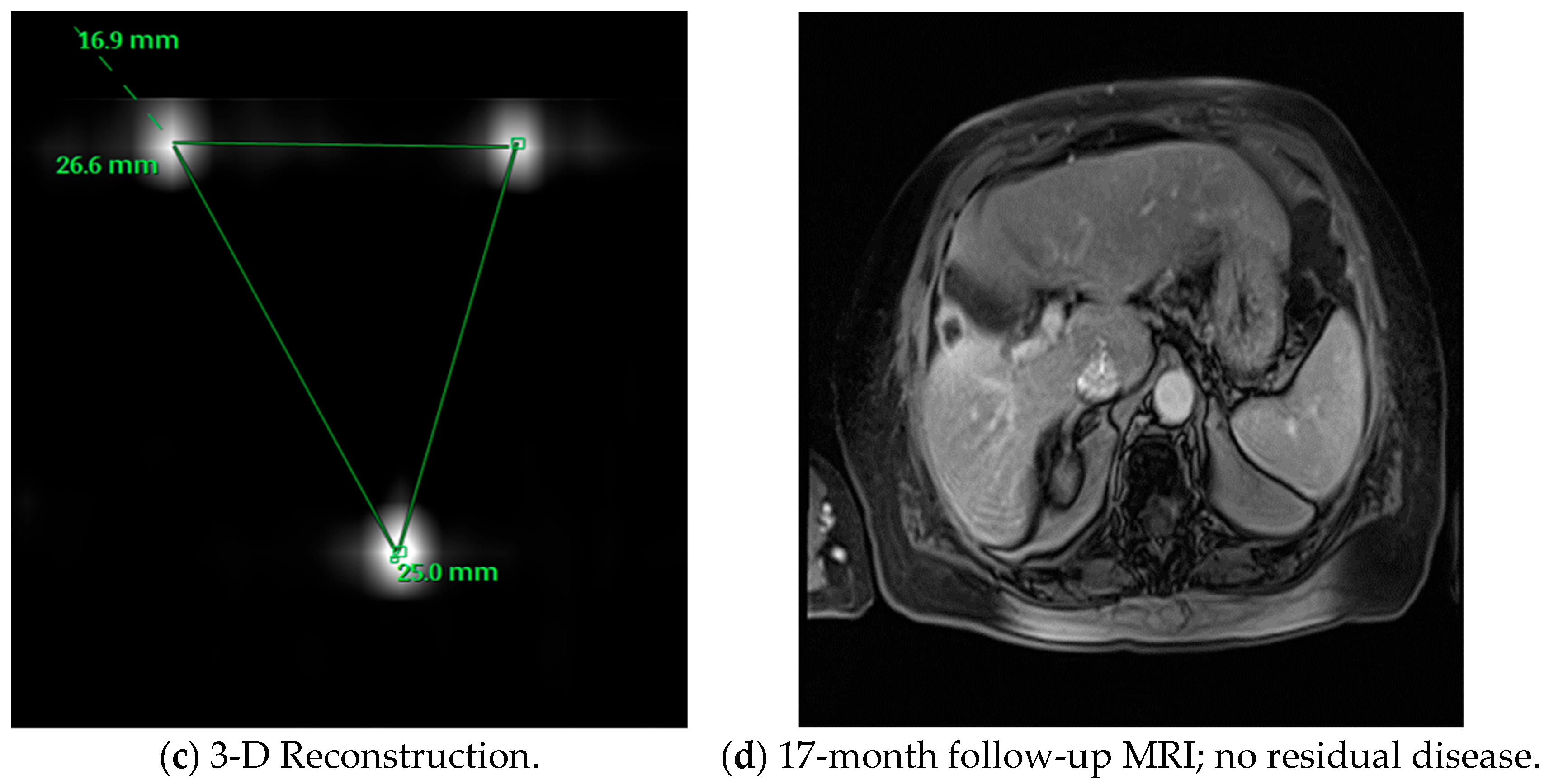

| Technology | Mode of Action | Advantages | Limitations |
|---|---|---|---|
| Radiofrequency ablation (RFA) | Thermally induced coagulation necrosis generated by high-frequency alternating current. | Widely available. Relatively inexpensive. Effective. | Potential for inadequate treatment in proximity to large vessels. Risk of thermal damage to adjacent structures. Tissue charring. Larger tumors (>2.5 cm) require multiple electrodes. |
| Microwave ablation (MWA) | Thermal ablation induced through agitation of water molecules. | Does not require grounding pads. More predictable lesion as compared to RFA. Can treat larger tumors. Fast acting. | Potential for inadequate treatment in proximity to large vessels. Risk of thermal damage to adjacent structures. |
| Cryoablation | Changes in argon gas pressure generate freeze-thaw cycles. Probe tip temperatures of −185° can be reached. | Relatively lower postoperative pain as compared to RFA and MWA. | Potential for inadequate treatment in proximity to large vessels. Risk of thermal damage to adjacent structures. |
| High Intensity Focused Ultrasound (HIFU) | Thermal coagulation combined with cavitation. | No percutaneous probes. | Potential for inadequate treatment in proximity to large vessels. Risk of thermal damage to adjacent structures. |
| Irreversible electroporation (IRE) | Electrical pulses which create permanent pores in cell membranes leading to apoptosis. | Non-thermal. Can be used near vessels and ducts. Preservation of extracellular matrix and parenchymal structures. | Risk of arrhythmia. Requires general anesthesia, muscle relaxation, and cardiac synchronization. Technically challenging and time consuming. |
| Indications | Relative Contraindication | Absolute Contraindication |
|---|---|---|
| Patient | ||
|
|
|
| Anatomic | ||
|
|
|
| Study | Patient Characteristics | Tumor Types | IRE Parameters | Major Outcomes |
|---|---|---|---|---|
| Narayanan et al. [30] | 101 patients, ages 24–83, 129 lesions,158 vessels examined for patency on follow-up | Liver (100), Pancreas (18), Kidney (3), Pelvis (1), Aorto-caval lymph nodes (2), Adrenal (2), Lung (1), Retroperitoneal (1), Surgical bed of prior Whipple (1) | 90 high-voltage (1500–3000 V) direct current (25–45 A) electrical pulses were delivered, in nine sets of 10 pulses between paired unipolar electrodes or a single bipolar electrode. | Vascular changes in 4.4% (7/158) after IRE of hepatic tumors with 50 tumors abutting and 10 surrounding vessels |
| Distelmaier et al. [31] | 29 patients, mean age 63 years ± 12 | 8 primary, 35 secondary malignant liver tumors located immediately adjacent to major hepatic veins, portal veins or both | 70–90 pulses per probe pair, pulse length 90 μs, max voltage 3000 V w/electrocardiographic triggering | No occlusion or narrowing of vessels post-IRE procedure for 43 hepatic tumors |
| Tamura et al. [33] | 39 patients, mean age 57.8 years ± 11.8 | Colon (27), Intrahepatic (2), HCC (1), Hilar (1), Esophageal (2), Pancreatic (1), Mammary (1), Ewing sarcoma (1); Primary (1), Metastatic (38) | 70–90 pulses per probe pair; Pulse length 90 μs; Max voltage 3000 V with electrocardiographic triggering | 33 portal veins and 64 hepatic veins analyzed; Occlusions of hepatic/portal veins were subclinical w/out ramifications w/most being less than 4 mm; No hepatic veins larger than 4 mm became occluded |
| Froud et al. [29] | 174 ablation procedures in 124 patients, mean age | Liver lesions included metastatic disease (62), with colorectal making up 31/62; Primary liver cancer (62), HCC (53), Cholangiocarcinoma (8) and (1) unknown diagnosis | 70–90 pulses per pair using between 1500–3000 V | In most post-IRE cases, abnormalities in liver functions resolved without intervention, did not prevent treatment, and showed similar results to those found after RFA or cryoablation |
| Dollinger et al. [32] | 24 patients, mean age 59.3 years, 53 hepatic lesions in 35 ablation procedures | 53 hepatic tumors w/14 primary; Segment IV (20), Segment V (10), Segment VI (1), Segment VII (6), Segment VIII (16) | Two to six monopolar 18-gauge IRE probes were placed parallel to each other in or around the target tumor; 70 pulses per cycle; 90 µs pulse length; Voltage-to-distance ratio, 1500 V/cm of needle distance | Successful ablation of 53 tumors adjacent to 55 major bile ducts; Biliary ductal changes, including mild stenosis or dilatation, were observed on imaging in 15 out of 55 ducts, only 3 patients developed transient cholestasis that resolved without intervention |
| Scheffer et al. [26] | 16 studies, 221 patients with 325 treated tumors | Patients presenting with lesions in liver (129), Pancreas (69), Kidney (14), Lung (6), Lesser pelvis (1), Lymph node (2) | Heterogeneity of reporting details, i.e., interelectrode distance, applied voltage + resulting current, pulse duration, number of electrodes, and probe repositioning, did not allow for detailed review of parameters | 16% complication rate (Grade I and II); Higher risk of complications associated with placement of more electrodes, i.e., probe-related punctures such as hemothorax, pneumothorax, and pleural effusions; 3 cases of biliary obstructions with 2 being a result of local tumor progression as opposed to ablation-induced biliary stenosis |
| Dollinger et al. [37] | 85 IRE procedures in 56 patients; Patient group consisted of 42 men and 14 women with a median age of 61 years (range, 22–81 years) | 28 patients with 52 lesions of primary liver tumors; HCC (45), CCA (7); 28 patients with 62 lesions of secondary liver tumors; Colorectal tumor (44), Breast carcinoma (6), Neuroendocrine tumor (3), Pancreatic tumor (3), Other (6) | Voltage 1650–3000 V; 90 µs pulse length; 70 pulses per cycle | 7.1% (6/58) experienced major complications with hepatic abscess in 4.7% (4 patients), bleeding in 2.4% (2 patients), 1 patient needing arterial embolization and 1 a blood transfusion; Minor complications in 18.8% (16/85), minor hemorrhage in 5.9% (5), portal vein branch thrombosis in 5.9% (5), pneumothorax with no chest drain in 3.5% (3), hepatic arteriovenous shunt in 3.5% (3), and temporary neurologic deficits due to peri-interventional positioning in 2.3% (2) |
| Study | Patient Characteristics | Tumor Types | Ablation Parameters | Major Outcomes |
|---|---|---|---|---|
| Niessen et al. [38] | 71 patients, median age 63.5 ± 10.8 years | 103 liver tumors, 35 patients had primary liver tumors, 36 had liver metastases; 43.7% HCC, 5.6% Cholangiocarcinoma, 38% Colorectal, 12.7% other metastases | 1650–3000 V; Pulse length 90 µs; 70 pulses per cycle under constant EKG monitoring | Median overall survival (OS) was 26.3 months, no difference in median OS between patients with primary and metastatic disease (26.8 vs. 19.9 months; p = 0.41). Patients with a tumor diameter >3 cm (p < 0.001) or more than 2 lesions (p < 0.005) had a lower overall median OS |
| Stillström et al. [39] | 42 patients had 50 treatments, 59 tumors | 59 tumors, 51% colorectal liver metastases, 34% HCC | 10–20 test pulses delivered b/t each electrode pair, minimum of 70 treatment pulses delivered b/t each electrode pair | No local recurrence within 12 months for 61% of the patients; Local recurrence rates for the entire group were 26% at 6 months and 37% at 1 year; Local recurrence for the CRCLM and HCC groups at 1 year was 38% and 17% respectively |
| Cannon et al. [20] | 44 patients, 48 IRE ablations | 20 colorectal lesions, 14 HCC, 10 other metastasis | 3000 V pulses, 90 pulses delivered lasting 20–100 µs each; Nanoknife system | Overall local recurrence-free survival (LRFS) at 6 months was 94.6% and 59.5% at 12 months; Higher recurrence rates was seen for tumors greater than 4 cm in size (HR 3.236, 95% CI: 0.585–17.891; p = 0.178) |
| Frühling et al. [40] | 30 patients with 38 lesions treated with IRE between September 2011 and September 2014; Mean age 63 years | 23 CRLM (colorectal cancer w/liver metastasis), 8 HCC, 7 other metastases | Minimum 90 treatment pulses delivered b/t each adequate electrode pair (distance not exceeding 25 mm); Current 40 A, no less than 30 A | Ablation success 78.9% at 3 months, 65.8% at 6 months; 6 minor complications, 1 major complication; No mortality at 30 days |
| Mafeld et al. [41] | 52 patients, 59 lesions, mean age of 64 years (range from 28–94), primary or secondary hepatic malignancy | Primary: HCC (20), Cholangiocarcinoma (3); Secondary: Colorectal (28), Neuroendocrine (1), Pancreatic (1), Breast (1), Gastrointestinal stromal tumor (1), Malignant thymoma (1); Mean diameter 2.4 cm | 90 pulses, 1500 v/cm applied b/t each electrode pair (including test pulses); Range of 20–50 A; Electrodes placed in parallel 1–2 cm apart | 12 months, 44% were progression-free (95% CI 30–66%); Lesions larger than 2 cm were associated with shorter time to progression and patients with CRCLM had a more rapid time to progression compared to HCC; Median OS was 38 months with a 90% (95% CI: 72%,97%) patient survival at 12 months and 65% (95% CI: 40%,81%) survival at 24 months and 52% (95% CI 22%, 75%) survival at 36 months |
| Sutter et al. [42] | 58 patients, median age 65.4 years, range of 41.6–90 years, 75 HCC lesions | 75 HCC tumors, median lesion diameter 24 mm (range of 6–90 mm) | Nanoknife IRE; Max 3000 V and 50 A; 2–6 19-gauge electrodes w/adjustable exposure of length of active tip (5–40 mm); Cardiac synchronization; Electrodes placed parallel w/max distance of 2.5 cm | 77.3% achieved complete tumor ablation after single IRE procedure with 92.0% tumor ablation after 3 procedures; 6 and 12-month overall local tumor progression free survival (PFS) was 87% (95% CI: 77%, 93%) and 70% (95% CI: 56%, 81%) |
| Hosein et al. [35] | 29 patients, 58 lesions, 36 IRE procedures, median age of 62 years | 58 tumors, median number of lesions treated was 2, median lesion size was 2.7 cm | Nanoknife IRE; 70-ms, 1500–3000 V, 25–45 A | 2 years after procedure, median OS was 62% (95% CI: 37%, 87%) and median PFS 18% (95% CI: 0%,35%); 36% of patients had complete response, 21% partial response, 25% stable disease, and 18% progressive disease at median follow up 11 months |
| Martin et al. [44] | 26 patients with obstructive jaundice due to advanced hilar cholangiocarcinoma treated with IRE with median age of 63 years, 137 patients with no ablation (control) with median age of 61 years | Advanced stage 3 or 4 hilar cholangiocarcinoma causing obstructive jaundice, IRE patients had 26 total lesions, 137 non-IRE (control) patients | Goal to perform 100 electrical pulses in groups of 10, pulse duration 70–90 µs, pulse interval of 250 ms | After percutaneous transhepatic biliary drainage (PTBD), 2 patients had ≥grade 3 complications; IRE resulted in relief of biliary obstruction and let patients live w/out PTBD for median 10 months |
| Franken et al. [45] | 12 patients, mean age of 63 years ± 12 | Unresectable locally advanced perihilar cholangiocarcinoma or N2 lymph node involvement | Active tip length 1.5–2 cm w/interelectrode distance of 10–24 mm w/5 mm margin around lesion; 90 treatment pulses, 9 sets of 10 pulses b/t paired unipolar electrodes; Voltage setting 1500 V/cm | 6 patients had major adverse events (CTCAE grade ≥ 3); No 90-day mortality; Technical success in 100% of cases; No intraprocedural events related to IRE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, G.; Koethe, Y.; Gentile, N. Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues. Medicina 2024, 60, 251. https://doi.org/10.3390/medicina60020251
Narayanan G, Koethe Y, Gentile N. Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues. Medicina. 2024; 60(2):251. https://doi.org/10.3390/medicina60020251
Chicago/Turabian StyleNarayanan, Govindarajan, Yilun Koethe, and Nicole Gentile. 2024. "Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues" Medicina 60, no. 2: 251. https://doi.org/10.3390/medicina60020251
APA StyleNarayanan, G., Koethe, Y., & Gentile, N. (2024). Irreversible Electroporation of the Hepatobiliary System: Current Utilization and Future Avenues. Medicina, 60(2), 251. https://doi.org/10.3390/medicina60020251





