The Benefit of Hydrogen Gas as an Adjunctive Therapy for Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Adjunctive Hydrogen Inhalation
2.3. Instrumentation and Interpretation of Possible Side-Effects
2.4. Clinical and Laboratory Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Available online: https://goldcopd.org/2023-gold-report-2 (accessed on 27 November 2023).
- Jia, G.; Yu, S.; Sun, W.; Yang, J.; Wang, Y.; Qi, Y.; Chen, Y. Hydrogen Sulfide Attenuates Particulate Matter-Induced Emphysema and Airway Inflammation through Nrf2-Dependent Manner. Front. Pharmacol. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Q.; Wang, D.; Feng, S.; Zhao, Y.; Shi, Y.; Liu, Q. Protective Effects of Hydrogen-Rich Saline on Rats with Smoke Inhalation Injury. Oxid. Med. Cell. Longev. 2015, 2015, 106836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-G.; Sun, W.-Z.; Hu, J.-Y.; Jie, Z.-J.; Xu, J.-F.; Cao, J.; Song, Y.-L.; Wang, C.-H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Castelblanco, M.; Lugrin, J.; Ehirchiou, D.; Nasi, S.; Ishii, I.; So, A.; Martinon, F.; Busso, N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 2018, 293, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Q.; Mao, Y.; Xu, S.; Xia, C.; Shi, X.; Zhang, J.H.; Yuan, H.; Sun, X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem. Res. 2010, 35, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Dong, Z.; Dimitropoulou, C.; Su, Y. Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid. Redox Signal. 2011, 15, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Lu, J.; Zhang, Y.; Sun, X.; Huang, Q.; Wang, Q. Hydrogen-rich saline improves non-alcoholic fatty liver disease by alleviating oxidative stress and activating hepatic PPARα and PPARγ. Mol. Med. Rep. 2017, 15, 1305–1312. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Z.; Wu, X.; Zhang, J. Hydrogen-Rich Saline Inhibits Lipopolysaccharide-Induced Acute Lung Injury and Endothelial Dysfunction by Regulating Autophagy through mTOR/TFEB Signaling Pathway. J. Biomed. Res. Int. 2020, 2020, 9121894. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Z.; Cai, J.; Liu, W.; Liu, Y.; Zhang, J.H.; Denoble, P.J.; Tao, H.; Sun, X. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp. Biol. Med. 2009, 234, 1212–1219. [Google Scholar] [CrossRef]
- Fang, Y.; Fu, X.J.; Gu, C.; Xu, P.; Wang, Y.; Yu, W.R.; Sun, Q.; Sun, X.J.; Yao, M. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J. Burn Care Res. 2011, 32, e82–e91. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, X.; Dai, Q.; Fan, Y.; Huang, X. Hydrogen-rich saline alleviates lung injury associated with cecal ligation and puncture-induced sepsis in rats. Exp. Mol. Pathol. 2015, 98, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Maekawa, Y.; Kawamura, A.; Abe, T.; Ohta, S.; Fukuda, K.; et al. H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J. Am. Heart Assoc. 2012, 1, e003459. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, D.; Hu, J.; Mei, H.; Shu, J.; Long, Z.; Yuan, L.; Li, D.; Guan, R.; Li, Y.; et al. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J. Thorac. Dis. 2018, 10, 3232–3243. [Google Scholar] [CrossRef]
- Liu, S.L.; Liu, K.; Sun, Q.; Liu, W.W.; Tao, H.Y.; Sun, X.J. Hydrogen therapy may be a novel and effective treatment for COPD. Front. Pharmacol. 2020, 11, 562268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Geng, W.; Jiang, C.; Zhao, S.; Liu, Y.; Zhang, Y.; Qin, S.; Li, C.; Zhang, X.; Si, Y. Hydrogen-rich saline inhibits tobacco smoke-induced chronic obstructive pulmonary disease by alleviating airway inflammation and mucus hypersecretion in rats. Exp. Biol. Med. 2017, 242, 1534–1541. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Qian, L.; Wu, Z.; Cen, J.; Pasca, S.; Tomuleasa, C. Medical Application of Hydrogen in Hematological Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3917393. [Google Scholar] [CrossRef]
- Takeuchi, S.; Wada, K.; Nagatani, K.; Osada, H.; Otani, N.; Nawashiro, H. Hydrogen may inhibit collagen-induced platelet aggregation: An ex vivo and in vivo study. Intern. Med. 2012, 51, 1309–1313. [Google Scholar] [CrossRef]
- Luisetti, M.; Ma, S.; Iadarola, P.; Stone, P.J.; Viglio, S.; Casado, B.; Lin, Y.Y.; Snider, G.L.; Turino, G.M. Desmosine as a biomarker of elastin degradation in COPD: Current status and future directions. Eur. Respir. J. 2008, 32, 1146–1157. [Google Scholar] [CrossRef]
- Viglio, S.; Stolk, J.; Luisetti, M.; Ferrari, F.; Piccinini, P.; Iadarola, P. From micellar electrokinetic chromatography to liquid chromatography-mass spectrometry: Revisiting the way of analyzing human fluids for the search of desmosines, putative biomarkers of chronic obstructive pulmonary disease. Electrophoresis 2014, 35, 109–118. [Google Scholar] [CrossRef]
- Gudmann, N.S.; Manon-Jensen, T.; Sand, J.M.B.; Diefenbach, C.; Sun, S.; Danielsen, A.; Karsdal, M.A.; Leeming, D.J. Lung tissue destruction by proteinase 3 and cathepsin G mediated elastin degradation is elevated in chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 2018, 503, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
| Inhaled Hydrogen Adjuvant Therapy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | ||||||
| Age | 77 | 58 | 87 | 65 | 59 | 67 | ||||||
| Gender (M/F) | M | F | M | M | M | M | ||||||
| Smoking status | former | active | former | active | active | never | ||||||
| Pack-years | 20 | 45 | 30 | 30 | 15 | 0 | ||||||
| treatment | pre | post | pre | post | pre | post | pre | post | pre | post | pre | post |
| Post-BD FVC (L) | 2.49 | 2.73 | 2.59 | 2.54 | 1.28 | 1.36 | 2.9 | 2.9 | 4.5 | 4.31 | 1.05 | 1.01 |
| Post-BD FEV1 (L) | 1.11 | 1.19 | 1.16 | 1.17 | 0.68 | 0.73 | 1.55 | 1.57 | 2.65 | 2.62 | 0.6 | 0.63 |
| Post-BD FEV1/FVC (%) | 44 | 44 | 45 | 46 | 53 | 54 | 53.25 | 54 | 59 | 61 | 56 | 63 |
| DLCO (%) mL/mHg/mim/L | 52.23 | 65.53 | 42.11 | 34.85 | 32.9 | 33.8 | 17.66 | 17.37 | 50.29 | 50.47 | 56 | 66.29 |
| DLCO/VA (%) mL/mHg/mim/L | 73.6 | 79.23 | 38.26 | 43.81 | 96.47 | 96.45 | 23.2 | 21.82 | 61.18 | 60.76 | 118 | 130.99 |
| CAT | 9 | 9 | 11 | 3 | 25 | 21 | 17 | 11 | 14 | 3 | 18 | 8 |
| mMRC | 2 | 2 | 1 | 0 | 4 | 4 | 4 | 3 | 1 | 0 | 3 | 2 |
| Sleep quality | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 |
| 6 MWT Rest SpO2% | 97 | 97 | 98 | 96 | 95 | 96 | 87 | 92 | 98 | 97 | 91 | 92 |
| Lowest SpO2% | 93 | 93 | 92 | 92 | 90 | 92 | 72 | 79 | 92 | 92 | 79 | 87 |
| 6 MWD (m) | 241 | 241 | 425 | 485 | 137 | 152 | 245.5 | 137.5 | 362.5 | 443.25 | 463.5 | 384 |
| MIP (cmH2O) | 70 | 70 | 60 | 110 | 80 | 90 | 70 | 70 | 120 | 90 | 60 | 50 |
| MEP (cmH2O) | 120 | 120 | 90 | 100 | 90 | 90 | 100 | 70 | 80 | 130 | 150 | 120 |
| Inhaled Hydrogen Adjuvant Therapy | |||
|---|---|---|---|
| Factor | Pre-Treatment Median (IQR) | Post-Treatment Median (IQR) | p-Value |
| Post-BD FVC (L) | 2.54 (1.22–3.30) | 2.64 (1.27–3.25) | 0.893 |
| Post-BD FEV1 (L) | 1.14 (0.66–1.83) | 1.18 (0.71–1.83) | 0.141 |
| FEV1/FVC (%) | 53.12 (45–56.75) | 54 (45.5–61.5) | 0.112 |
| DLCO | 46.2 (29.09–53.17) | 42.66 (29.69–65.72) | 0.345 |
| DLCO/VA | 67.39 (34.50–101.85) | 69.99 (38.31–105.08) | 0.345 |
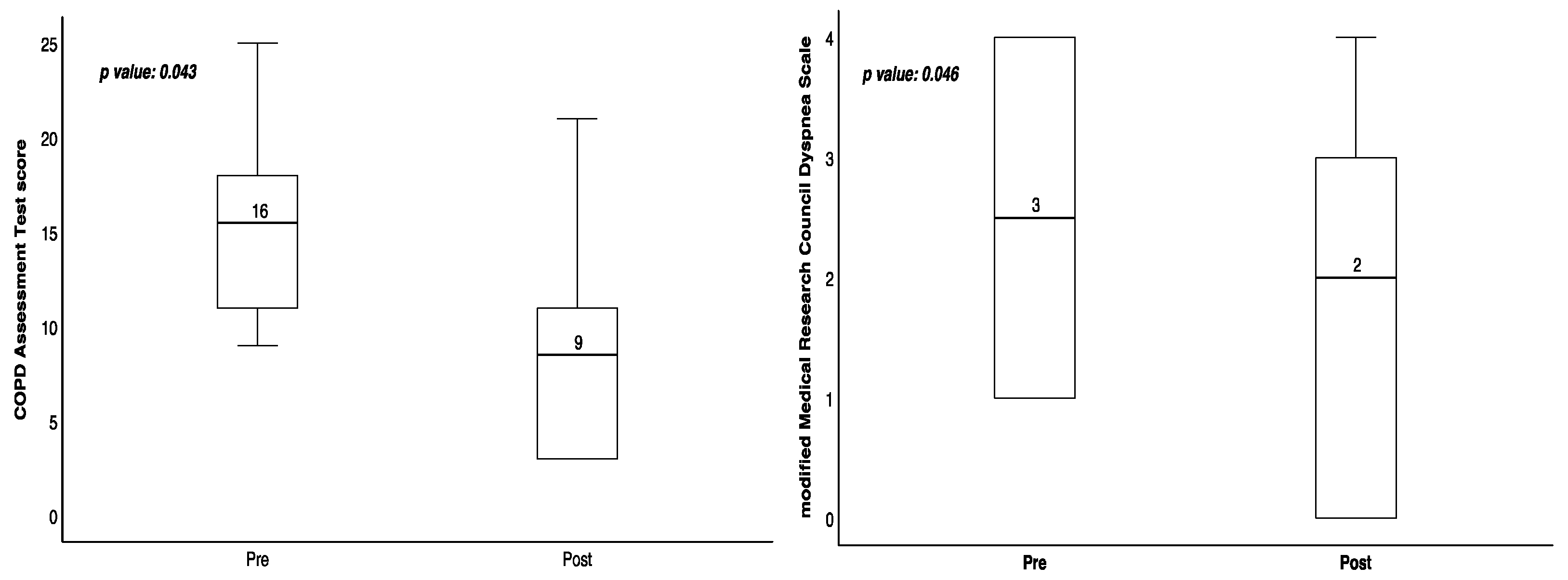
| CAT | 15.5 (10.5–19.75) | 8.5 (3–13.5) | 0.043 |
| mMRC | 2.5 (1–4) | 2 (0–3.25) | 0.046 |
| Sleep quality | 0 (0–1) | 0 (0–1) | 1.000 |
| Lowest SpO2% | 91 (77.25–92.25) | 92 (85–92.25) | 0.109 |
| 6 MWD | 304 (215–434.63) | 312.5 (148.38–452.94) | 0.893 |
| MIP (cmH2O) | 70 (60–90) | 80 (65–95) | 0.854 |
| MEP (cmH2O) | 95 (87.5–127.5) | 110 (85–122.5) | 1.000 |
| Inhaled Hydrogen Adjuvant Therapy | |||
|---|---|---|---|
| Factor | Pre-Treatment Median (IQR) | Post-Treatment Median (IQR) | p-Value |
| WBC | 6.8 (6.55–7.65) | 6.8 (6.05–10.05) | 0.500 |
| RBC | 4.70 (4.61–4.94) | 4.69 (4.50–5.24) | 0.892 |
| Hb | 14.65 (14.3–15.43) | 14.8 (13.9–16.4) | 1.000 |
| HCT | 43.75 (41.98–48.1) | 44.9 (41.83–47.88) | 0.498 |
| MCV | 92.4 (89.05–96.35) | 92.1 (88.85–98.45) | 0.892 |
| MCH | 31.05 (29.98–32.28) | 31.1 (30–32.73) | 0.500 |
| MCHC | 33.35 (32.83–34.03) | 33.7 (32.73–34.33) | 0.279 |
| RDW-SD | 44.4 (43.4–51.03) | 43.9 (42.65–50.25) | 0.138 |
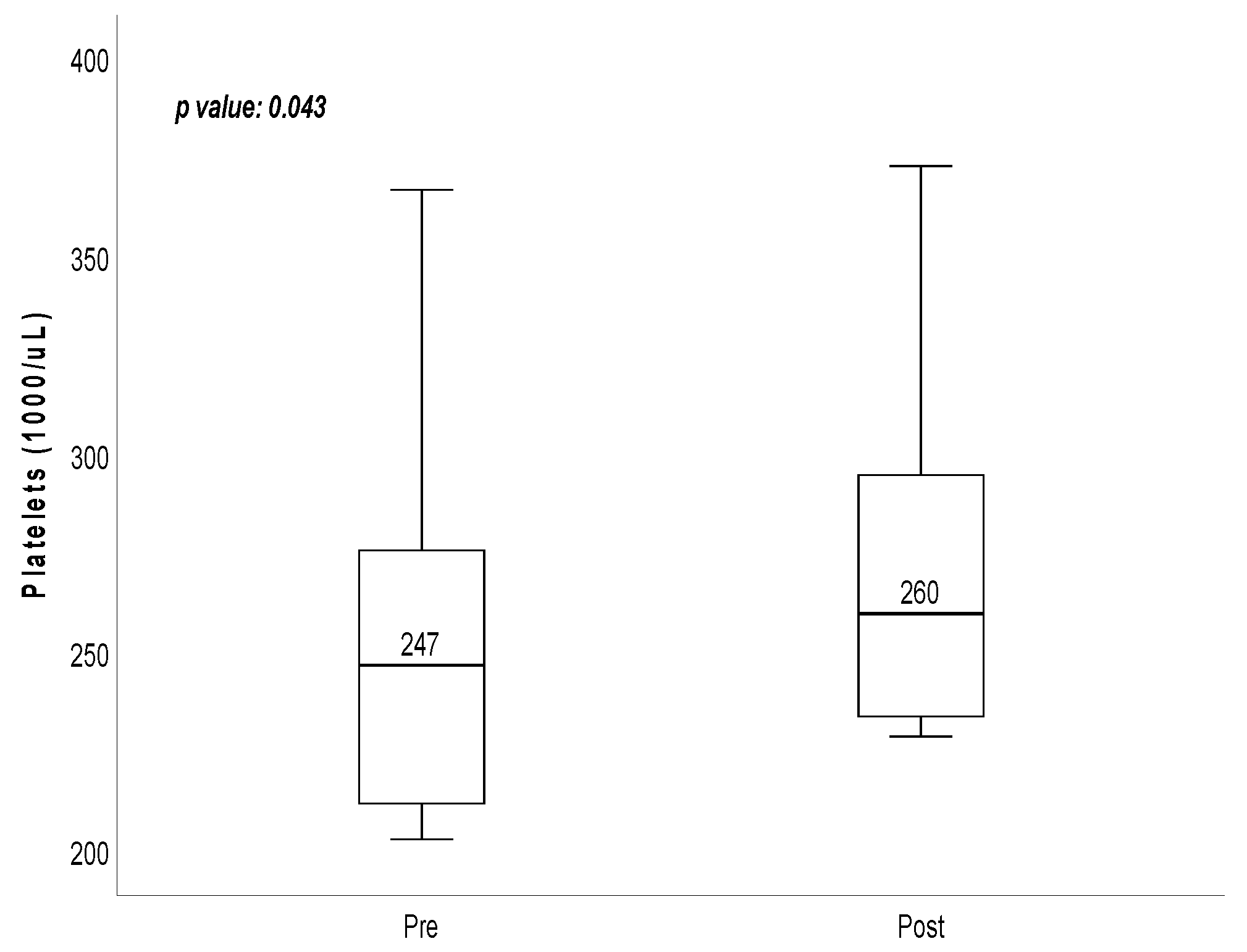
| Platelets | 247 (209.75–298.75) | 260 (232.75–314.5) | 0.043 |
| RDW-CV | 13.35 (12.5–14.33) | 13.1 (12.43–14.1) | 0.109 |
| PDW | 11.45 (10.53–13.2) | 10.65 (10.18–11.48) | 0.080 |
| MPV | 10.1 (9.75–10.75) | 9.85 (9.5–10.25) | 0.131 |
| Eosinophil | 1.35 (1.03–1.58) | 1.95 (1.2–2.95) | 0.116 |
| TAC | 475 (410–522.25) | 513 (432–587) | 0.225 |
| GPX | 102.6 (85.58–122.7) | 104.9 (89.88–121.4) | 0.686 |
| MPO | 75.1 (55.45–110) | 62.05 (48.4–101.9) | 0.500 |
| 8-OHdG | 6.69 (4.09–8.59) | 7.32 (3.48–8.38) | 0.500 |
| T-CHOL | 214 (179.25–241.5) | 217.5 (178–263) | 0.416 |
| TG | 110.5 (69.25–177) | 108 (82.75–158.75) | 0.686 |
| LDL-C | 130.5 (84–165.75) | 121 (88–169.25) | 0.893 |
| HDL-C | 51 (45.75–61.25) | 49 (40.75–57.75) | 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-F.; Li, C.-L.; Lee, H.-C.; Chang, H.-C.; Liu, J.-F.; Kuo, H.-C. The Benefit of Hydrogen Gas as an Adjunctive Therapy for Chronic Obstructive Pulmonary Disease. Medicina 2024, 60, 245. https://doi.org/10.3390/medicina60020245
Liu S-F, Li C-L, Lee H-C, Chang H-C, Liu J-F, Kuo H-C. The Benefit of Hydrogen Gas as an Adjunctive Therapy for Chronic Obstructive Pulmonary Disease. Medicina. 2024; 60(2):245. https://doi.org/10.3390/medicina60020245
Chicago/Turabian StyleLiu, Shih-Feng, Chin-Ling Li, Hui-Ching Lee, Hui-Chuan Chang, Jui-Fang Liu, and Ho-Chang Kuo. 2024. "The Benefit of Hydrogen Gas as an Adjunctive Therapy for Chronic Obstructive Pulmonary Disease" Medicina 60, no. 2: 245. https://doi.org/10.3390/medicina60020245
APA StyleLiu, S.-F., Li, C.-L., Lee, H.-C., Chang, H.-C., Liu, J.-F., & Kuo, H.-C. (2024). The Benefit of Hydrogen Gas as an Adjunctive Therapy for Chronic Obstructive Pulmonary Disease. Medicina, 60(2), 245. https://doi.org/10.3390/medicina60020245







