Abstract
Background: Regenerative medicine is evolving with discoveries like the stromal vascular fraction (SVF), a diverse cell group from adipose tissue with therapeutic promise. Originating from fat cell metabolism studies in the 1960s, SVF’s versatility was recognized after demonstrating multipotency. Comprising of cells like pericytes, smooth muscle cells, and, notably, adipose-derived stem cells (ADSCs), SVF offers tissue regeneration and repair through the differentiation and secretion of growth factors. Its therapeutic efficacy is due to these cells’ synergistic action, prompting extensive research. Methods: This review analyzed the relevant literature on SVF, covering its composition, action mechanisms, clinical applications, and future directions. An extensive literature search from January 2018 to June 2023 was conducted across databases like PubMed, Embase, etc., using specific keywords. Results: The systematic literature search yielded a total of 473 articles. Sixteen articles met the inclusion criteria and were included in the review. This rigorous methodology provides a framework for a thorough and systematic analysis of the existing literature on SVF, offering robust insights into the potential of this important cell population in regenerative medicine. Conclusions: Our review reveals the potential of SVF, a heterogeneous cell mixture, as a powerful tool in regenerative medicine. SVF has demonstrated therapeutic efficacy and safety across disciplines, improving pain, tissue regeneration, graft survival, and wound healing while exhibiting immunomodulatory and anti-inflammatory properties.
1. Introduction
The field of regenerative medicine is perpetually evolving, constantly being shaped by ground-breaking discoveries that promise to revolutionize the way we approach various medical conditions. One of the key players in this landscape is the stromal vascular fraction (SVF), characterized by its diverse cellular composition extracted from the adipose tissue that has demonstrated significant therapeutic potential across multiple medical disciplines [1].
Understanding the journey of SVF in medicine necessitates a glimpse into its historical context. The 1960s marked the advent of the SVF narrative when Rodbell initiated his studies into fat cell metabolism, a pursuit that ultimately led to the identification of an ‘active’ fraction of non-adipocyte cells, a collective later known as SVF [1]. The term, however, came into mainstream usage only after the pivotal work by Zuk et al., which unearthed the multipotency of adipose-derived stromal cells, a vital component of SVF [2].
SVF are a heterogeneous mixture of cells, pericytes, smooth muscle cells, and, most importantly, adipose-derived stem cells (ADSCs) [1]. These cells play a crucial role in tissue regeneration and repair, primarily due to their ability to differentiate into various cell types and release angiogenic and anti-inflammatory factors [3]. Among these, the ADSCs are particularly notable for their multipotency, enabling them to differentiate into various cell types such as adipocytes, osteoblasts, and chondrocytes under appropriate conditions [4]. Moreover, these cells are known for their angiogenic and immunomodulatory capabilities, primarily due to their secretion of growth factors and cytokines [5]. The therapeutic efficacy of SVF can be attributed to these diverse cell types acting in synergy. While the regenerative and reparative capacities can be traced back to the ADSCs, the immune cells within SVF contribute to the immunomodulatory effects, essential for tissue repair and regeneration. Over the following decades, this recognition spiraled into a flurry of research investigating the regenerative potential of SVF, spurred by its accessibility and abundant stem cell content. SVF began garnering attention across diverse disciplines [6].
SVF has been employed in various clinical settings due to its regenerative, immunomodulatory, and anti-inflammatory properties. In the field of plastic and reconstructive surgery, SVF-enriched fat grafting has been shown to improve graft survival and wound healing [7]. In orthopedics, SVF has been used to treat osteoarthritis, with studies reporting improvement in pain scores and joint function [8]. In cardiology, SVF therapy is being explored for myocardial ischemia, with encouraging results in pre-clinical and early-phase clinical trials [9]. These varied applications underscore the versatile nature of SVF and its potential to revolutionize the landscape of regenerative medicine. However, the current understanding of SVF is not without limitations and challenges, necessitating further investigation to unlock its full therapeutic potential [10].
The objective of this comprehensive review is to meticulously scrutinize the breadth and depth of contemporary clinical literature concerning SVF, from its inception to its present-day applications, spotlighting potential future trajectories. By compiling and critically examining a wide array of studies, we endeavor to offer a panoramic view of SVF’s potential, thereby contributing to the foundational knowledge that propels the field of regenerative medicine forward.
2. Materials and Methods
In this review, we employed a systematic approach to gather and analyze the relevant literature concerning SVF. The objective was to provide a comprehensive overview of SVF, including its biological composition, mechanisms of action, clinical applications, and potential future directions.
2.1. Search Strategy
An extensive literature search was conducted using several databases: PubMed, Embase, Scopus, Web of Science, and the Cochrane Library. The search period was from January 2018 to June 2023 as this period of time represents the most recent five years, ensuring that the data and research findings are current. This is particularly important in fields where advancements happen rapidly, as older studies might become outdated or less relevant. The primary keywords used were ‘stromal vascular fraction’, ‘adipose-derived stromal cells’, ‘adipose tissue’, ‘regenerative medicine’, and ‘clinical applications’. These keywords were used in combination with other terms relevant to the specific sections of this review. For example, for the section on clinical applications, terms like ‘wound healing’, ‘osteoarthritis’, ‘myocardial ischemia’, etc., were used in conjunction with the primary keywords.
2.2. Selection Criteria
Inclusion criteria comprised original research articles. The selected studies included prospective and/or retrospective case series, and review articles written in English that explored the composition, mechanism of action, therapeutic applications, and future perspectives of SVF. Studies that were not peer-reviewed, such as preprints, were excluded. Likewise, articles not available in English, letters to the editor, studies that did not provide explicit data on SVF, and duplicate studies were also omitted from this review. For clinical trials, only those that reported clear methodologies, patient outcomes, and statistical analyses were considered. Articles had to be explicitly centered on the stromal vascular fraction (SVF). This ensured that the study provided specific insights into SVF, rather than a peripheral or broad overview of adipose tissue or regenerative medicine. The paper had to showcase a sound research methodology, which is a testament to the credibility, reliability, and replicability of the study’s findings. Those with ambiguous or poorly defined methodologies were not considered appropriate for inclusion. Research studies with a limited sample size, specifically those with fewer than 5 participants, might not provide the robust evidence that this review aims to collate. Non-original research articles, such as commentaries, editorials, and opinion papers were excluded to maintain the integrity and objective of our study.
2.3. Data Extraction
For each selected article, data were extracted by two independent reviewers (ENG and NM). The data comprised the year of publication, study type, the number of participants (for clinical trials), main findings, and conclusions and complications. Any discrepancies between the reviewers were resolved through discussion until a consensus was reached.
3. Results
The systematic literature search yielded a total of 787 articles. After removing duplicates and screening titles and abstracts, 84 full-text articles were assessed for eligibility. Of these, 16 articles met the inclusion criteria and were included in the review (Table 1) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. The selected studies included prospective and/or retrospective case series, randomized controlled clinical trials, and reviews. This rigorous methodology provides a framework for a thorough and systematic analysis of the existing literature on SVF, offering robust insights into the potential of this important cell population in regenerative medicine.

Table 1.
Data Synthesis and Analysis.
4. Discussion
The reviewed studies collectively demonstrate the potential therapeutic applications of SVF in various medical disciplines. Containing a diverse array of cells such as adipose-derived stem cells (ADSCs), pericytes, and smooth muscle cells, the SVF has demonstrated promising regenerative, immunomodulatory, and anti-inflammatory effects (Figure 1). Characterizing the purification of SVF is of paramount importance, especially when considering its application in therapeutic contexts. The purification process ensures that unwanted components, potentially harmful contaminants, or non-functional elements are removed, leaving behind a highly enriched fraction that can be safely and effectively used for regenerative purposes [3]. The purification and analysis of SVF entail a comprehensive evaluation of its cellular and molecular constituents. First, cellular composition is often deciphered using flow cytometry, which uses specific markers to quantify cell types, such as ASCs (CD34+, CD31−, CD45−), endothelial cells (CD31+), and immune cells (CD45+). Additionally, microscopy, such as histological or fluorescent examinations, visually presents cellular composition [3,4,27,28,29,30,31,32,33,34] (Table 2).
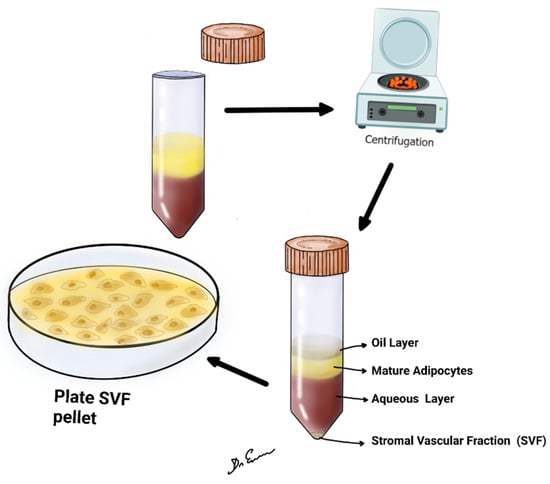
Figure 1.
The figure shows lipoaspirate, centrifuged at 2500 or 3000 rpm, for 4 min at room temperature. After centrifugation, upper oil fraction, middle condensed lipoaspirate, lower aqueous fraction, and the stromal vascular fraction were observed.

Table 2.
SVF cell content isolated from the aqueous portion.
In the field of orthopedics, SVF has been investigated for the treatment of osteoarthritis (OA). In a retrospective study by Kim et al. [12] with 43 participants, SVF implantation was found to improve pain and cartilage regeneration in knee OA. Garza et al. [15] reported improved pain scores and cartilage regeneration in patients with knee OA following SVF implantation, and both studies underscored the absence of complications. Furthermore, Zhang et al. [13] demonstrated that SVF treatment resulted in better clinical outcomes compared to hyaluronic acid therapy in knee OA patients. In the retrospective study conducted by Brian et al. [20], which involved 350 participants, a pivotal focus was placed on patients with arthritis undergoing SVF cell therapy. This study stands out for its significant findings, which revealed marked improvements in both pain levels and mobility among the treated patients, especially notable in those diagnosed with stage III arthritis. These improvements were not just incremental but substantial, indicating a pronounced therapeutic effect of SVF therapy on the symptoms of arthritis [20]. In a prospective study by Perdomo-Pantoja et al. [17] with 36 participants, SVF was found to be comparable to bone marrow cells (BMCs) in spinal fusion, suggesting its viability in spinal surgeries. Similarly, Choi et al. [18] demonstrated a higher early bone fusion rate when using SVF, pointing towards its potential in enhancing spinal fusion outcomes in their prospective study with 10 participants. In plastic and reconstructive surgery, SVF-enriched fat grafting has shown positive results in improving graft survival and wound healing, as reported by Onoi et al. [11]. The prospective study by Kwon et al. [14], involving 20 participants, reported improved outcomes in scar revision surgery following SVF treatment. The study did not report any complications.
Moon et al. [21] explored the use of SVF in reconstructive surgery, particularly for nasal defect repair. The study demonstrated that tissue-engineered dermis grafts incorporating SVF yielded superior scar quality in the alar zone of the nose compared to traditional artificial dermis grafts. This finding is significant as it highlights SVF’s potential in improving aesthetic outcomes in reconstructive surgery, offering more effective solutions for challenging areas like nasal defects [19,21]. Jeon et al. [24] reported increased fat graft survival rates in breast reconstruction with SVF. These findings suggest that SVF has broad applications in the field of regenerative medicine.
Cardiology is another area where SVF therapy is being explored. Pre-clinical and early-phase clinical trials have shown encouraging results in the use of SVF for myocardial ischemia, as mentioned by Bai et al. [9]. This highlights the potential of SVF in cardiac regenerative medicine, although further research is needed to establish its safety and efficacy in larger clinical trials.
It is worth noting that the reviewed studies have reported generally positive outcomes and a favorable safety profile for SVF therapy. Complications were infrequently reported across the studies, indicating a relatively low risk associated with SVF treatments. However, it is important to interpret these findings with caution due to the limited number of participants in some studies and the lack of long-term follow-up data [25]. Despite the promising results, this review also highlights the need for further research to address the limitations and challenges associated with SVF therapy [35,36,37,38,39] (Table 3).

Table 3.
Effect of stromal vascular fraction on tissues.
For instance, the variability in SVF composition and preparation methods, as well as the optimal dosage and delivery methods, warrant further investigation. The standardization of protocols and rigorous clinical trials will be crucial for establishing the safety and efficacy of SVF therapies. The findings indicate positive outcomes in terms of pain reduction, tissue regeneration, and improved clinical efficacy. However, further research is needed to address the limitations and challenges in order to unlock the full therapeutic potential of SVF and establish its role as a mainstream treatment option in regenerative medicine.
Despite the limitations of some studies due to small sample sizes, the overall findings support the potential of SVF as a versatile tool in regenerative medicine. However, further research is necessary to address challenges such as standardizing SVF isolation and processing methods, optimizing dosage and delivery approaches, and conducting long-term follow-up studies to assess durability and potential side effects. The therapeutic potential of SVF is far from being fully realized, and there are still many avenues to explore in order to unlock its complete potential. As highlighted in this review, the current body of research supports SVF’s role in regenerative medicine, but it also highlights the need for further study to explore its full potential and tackle existing challenges. Future studies should aim to elucidate the precise mechanisms of action of SVF in tissue repair and regeneration. Our understanding of the therapeutic effects of SVF has been primarily attributed to the ADSCs; however, SVF is a heterogeneous cell population, and the roles of the other cellular components remain relatively unexplored. Understanding the individual and synergistic roles of all the cellular components of SVF could lead to the development of targeted and personalized therapeutic strategies [40,41]. The standardization of SVF preparation methods is an important area that requires attention. Current studies have utilized a variety of protocols for the extraction and processing of SVF, leading to a wide variation in the cellular composition and concentrations. Therefore, the standardization of protocols for SVF isolation and processing will be essential to ensure the reproducibility of results across different studies and clinical settings [42].
4.1. Delivery Methods
Dosage and delivery methods are also aspects that need further investigation. The optimal dose and the best route for administration that would maximize the therapeutic effect while minimizing potential adverse reactions are yet to be determined [43]. The route of administration for stromal vascular fraction (SVF) depends on the therapeutic target: it can be directly injected into joints for orthopedic conditions, applied to wounds for healing, introduced intravenously for systemic diseases, delivered into the spinal canal for neurological disorders, or even injected into muscle tissues or brain tissue for specific conditions. The chosen route is always based on the condition in question and ongoing research [44]. Karina et al. [45] showed that the administration of a high dose of SVF up to 10 billion cells in a majority of 421 patients through infusion, spinal, and intra-articular injection was feasible without causing major adverse events and should be further investigated in well-designed phase I-II clinical trials to address the safety and efficacy of the therapy.
Additionally, comprehensive long-term follow-up studies are needed to assess the durability of the therapeutic effects of SVF and to monitor for potential side effects or complications. While the reviewed studies generally report a favorable safety profile for SVF, long-term data will be crucial in solidifying these initial findings [46].
The use of SVF in regenerative medicine signifies a novel and promising approach with potential applications across a broad range of medical disciplines. With its potent regenerative, immunomodulatory, and anti-inflammatory effects, SVF has demonstrated promising outcomes in orthopedics, cardiology, plastic and reconstructive surgery, and more [39]. Importantly, the evidence presented in the reviewed studies suggests a generally favorable safety profile for SVF therapy, marking an encouraging advance in regenerative medicine [47]. These positive outcomes support the potential of SVF as a versatile therapeutic tool, even though further research is needed to fully realize its potential and translate the findings into routine clinical practice.
In a study conducted from 2016 to 2019, Cai et al. [48] evaluated the efficacy of SVF gel in treating chronic wounds. The results highlighted a 100% wound closure rate within an average of 28.3 ± 9.7 days and no recurrences during a 2- to 3-year follow-up. Mechanistic examinations suggested the role of certain growth factors in enhancing cell proliferation and migration, especially in serum-free conditions [49]. Several challenges, however, remain. Standardizing SVF isolation and processing methods, optimizing dosage and delivery methods, and long-term follow-up studies are areas that require further exploration. Zhang et al. [13] investigated the mid-term prognosis of SVF treatment for knee osteoarthritis during a minimum of 5 years, showing that the SVF group had superior VAS and WOMAC scores, and indicated enhanced pain management and knee functionality compared to the HA group. Additionally, SVF showcased a prolonged effectiveness of 61.5 months compared to Na’s 30.3 months. Notably, SVF reduced the risk of clinical failure by 2.6 times, with BML severity and BMI identified as independent prognostic factors. Moreover, while both treatments saw a decline in cartilage volume, the reduction was less pronounced in the SVF group, suggesting potential cartilage protective effects [13]. Additionally, a deeper understanding of the precise mechanisms through which SVF contributes to tissue repair and regeneration is crucial. Different extraction methods and protocols have been developed to harvest SVF, each with its unique strengths and limitations tailored to specific clinical requirements.
Enzymatic Digestion using Collagenase: This technique is particularly known for its efficiency in yielding a high number of SVF cells. By mincing and then digesting adipose tissue with collagenase, the embedded SVF cells are released. Though widely recognized and practiced, this method does raise concerns, especially pertaining to the potential contaminants introduced by animal-derived collagenase. Variations in enzyme quality can also be a bottleneck, sometimes leading to inconsistent outcomes [50]. Given these potential risks, certain regulatory bodies may have reservations about its applicability, especially in human therapeutics [51].
Mechanical Methods: By leveraging physical forces, such as shaking or ultrasonication, SVF cells are extracted from the adipose matrix. This technique’s hallmark is its enzymatic-independent approach, making it favorable in regions with rigorous clinical regulations, as there is no risk of enzyme-related contamination [52]. However, the trade-off includes a comparatively lower cell yield and the potential mechanical stress on the cells, which could compromise their viability.
Water Jet-Assisted Liposuction: A more contemporary method, this technique employs high-pressure water jets to dissociate SVF from adipose tissue. Its minimally invasive nature is its most notable feature, potentially reducing patient discomfort and procedure duration [53]. However, this method comes with caveats, including the need for specialized equipment and expertise. Additionally, the high-pressure jets might inadvertently cause cell damage, raising questions about the viability of the harvested cells [54].
4.2. SVF Preparation Steps
The steps of SVF separation can be summarized as (a) liposuction, (b) mechanical separation or shredding, (c) initial filtration, (d) washing, (e) final filtration, (f) SVF and adipose graft harvesting, and (g) cell counting and/or characterization (Table 4) [51,52,53,54].

Table 4.
Steps of stromal vascular fraction separation.
As we continue to explore and understand the individual and synergistic roles of all cellular components within SVF, the development of targeted and personalized therapeutic strategies becomes a tangible possibility. Considering the rapidly evolving research landscape, large-scale, randomized clinical trials will play a pivotal role in firmly establishing the safety and efficacy of SVF therapies [44]. This will help determine the most effective way to integrate this novel therapeutic approach into mainstream medical practice. While we have only just begun to scratch the surface of the potential applications of SVF in regenerative medicine, the results thus far are encouraging. Medical devices for the preparation of AD-SVF are summarized in Table 5 [43,44,45,46].

Table 5.
Commercial medical products for AD-SVF preparation [43].
The coming years promise to shed more light on this versatile therapeutic tool, and it is our hope that the relentless pursuit of knowledge in this area will usher in a new era of regenerative medicine, leading to improved patient outcomes across a myriad of health conditions [75,76,77].
4.3. Immediate Expectations
Increased clinical trials: An increase in clinical trials is anticipated, targeting the efficacy and safety of SVF across various therapeutic applications. These trials are expected to provide critical data that will inform clinical practice and further research, particularly in areas such as osteoarthritis, wound healing, and myocardial ischemia or neurosurgery.
Technological advancements: The immediate horizon also sees advancements in the technology used for SVF extraction and purification. Efforts will likely be directed toward standardizing protocols to improve the viability and potency of harvested cells, which is essential for ensuring consistent and effective treatment outcomes. Regulatory processes for SVF-based therapies are expected. These advancements will facilitate the transition from laboratory research to clinical applications, ensuring that new treatments are safe and compliant with regulatory standards.
4.4. Long-Term Expectations
Broad-spectrum applications: Over the long term, SVF is expected to find applications in broader medical disciplines. This expansion could offer novel treatments for various chronic diseases and degenerative conditions and in the flourishing field of tissue engineering.
Personalized medicine and integration with other therapies: Future research might enable the use of SVF in personalized regenerative therapies tailored to individual patient needs and specific conditions. This approach could significantly enhance the efficacy of treatments and minimize potential side effects. There is potential for SVF to be combined with other regenerative approaches, such as gene therapy or 3D-bioprinting [78,79,80,81]. This integration could enhance therapeutic outcomes and pave the way for more comprehensive treatment strategies.
4.5. Future Research Directions
Clarifying cellular dynamics: future studies should focus on the specific roles of different cell types within SVF and their synergistic effects in tissue repair and regeneration. Understanding these dynamics is critical to maximizing the therapeutic potential of SVF.
Long-term clinical studies: conducting studies with extended follow-up periods is crucial. These long-term clinical trials are necessary to assess the efficacy and safety of SVF-based therapies over time and to understand the lasting impacts of these treatments.
Dose–response relationship: investigating the optimal dosage and administration routes for SVF in various clinical conditions is essential. This research will help in determining the most effective treatment protocols.
Mechanistic studies: delving into the molecular pathways influenced by SVF can provide deeper insights into its regenerative mechanisms. This knowledge is pivotal for developing targeted therapies to address specific medical conditions more effectively [75].
It is imperative to address ethical concerns and develop comprehensive regulatory guidelines for using SVF in clinical settings. These guidelines will ensure that treatments are practical, ethically sound, and compliant with legal standards.
4.6. Limitations of this Study
Lack of long-term data: many studies may have had short follow-up periods, limiting the ability to draw conclusions about the long-term safety and efficacy of SVF.
Limited sample size: this review may be constrained by the small sample sizes of some included studies, reducing the power to detect significant effects.
Regulatory landscape: differences in regulatory practices across countries may affect the applicability and generalizability of this review’s findings.
5. Conclusions
Our literature review on SVF provides valuable insights into its potential as a powerful tool in regenerative medicine. SVF, composed of a heterogeneous mixture of cells including ADSCs, has demonstrated significant therapeutic efficacy and safety in various medical disciplines. The reviewed studies highlight the positive outcomes of SVF therapy in areas such as orthopedics, plastic and reconstructive surgery, cardiology, and wound healing. SVF has shown promising results in reducing pain, improving tissue regeneration, enhancing graft survival, and promoting wound healing. Moreover, SVF has exhibited immunomodulatory and anti-inflammatory properties, contributing to its regenerative effects. The future of SVF in regenerative medicine holds great promise. Continued research, technological advancements, and regulatory guidelines will contribute to unlocking its full therapeutic potential. The standardization of protocols and large-scale clinical trials will provide robust evidence and establish SVF as a mainstream treatment option. With these developments, SVF has the potential to revolutionize the field of regenerative medicine and offer innovative solutions for a wide range of medical conditions. SVF represents an exciting and evolving field of research that has the potential to transform the landscape of regenerative medicine. By harnessing the regenerative and immunomodulatory properties of SVF, researchers and clinicians can pave the way for innovative treatments that improve patient outcomes and quality of life.
Author Contributions
Conceptualization, E.N.G., O.A.K., E.I.I., K.K.V. and N.M.; methodology, E.N.G., O.A.K., M.D.J.E.R., K.K.V. and N.M.; validation, E.N.G., O.A.K., E.I.I., R.N., K.K.V. and N.M.; formal analysis, E.N.G., O.A.K., and E.I.I.; investigation, E.N.G., K.K.V. and N.M.; resources, E.N.G.; data curation, E.N.G. and E.I.I.; writing—original draft preparation, E.N.G., K.K.V. and N.M.; writing—review and editing, M.D.J.E.R., K.K.V. and N.M.; visualization, O.A.K.; supervision, E.N.G., K.K.V. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodbell, M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Baer, P.C.; Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012, 2012, 812693. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Cho, K.H. Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice. Ann. Dermatol. 2011, 23, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266, Erratum in Stem Cells 2017, 35, 1651–1652. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.C.; Shah, S.; Ogueri, K.S.; Nair, L.S.; Laurencin, C.T. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen. Eng. Transl. Med. 2022, 8, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yan, Y.; Song, Y.H.; Seidensticker, M.; Rabinovich, B.; Metzele, R.; Bankson, J.A.; Vykoukal, D.; Alt, E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur. Heart J. 2010, 31, 489–501. [Google Scholar] [CrossRef]
- Kølle, S.F.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Onoi, Y.; Matsumoto, T.; Sobajima, S.; Tsubosaka, M.; Hayashi, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Clinical use of autologous adipose-derived stromal vascular fraction cell injections for hip osteoarthritis. Regen. Ther. 2023, 24, 94–102. [Google Scholar] [CrossRef]
- Kim, Y.S.; Oh, S.M.; Suh, D.S.; Tak, D.H.; Kwon, Y.B.; Koh, Y.G. Cartilage lesion size and number of stromal vascular fraction (SVF) cells strongly influenced the SVF implantation outcomes in patients with knee osteoarthritis. J. Exp. Orthop. 2023, 10, 28. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; He, B.; Fan, M.; Xiao, M.; Zhang, J.; Chen, D.; Tong, P.; Mao, Q. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Res. Ther. 2022, 13, 105. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, S.; Kim, J.; Song, S.H. Efficacy and safety of stromal vascular fraction on scar revision surgery: A prospective study. J. Dermatolog. Treat. 2023, 34, 2171260. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. Autologous and Allogenic Utilization of Stromal Vascular Fraction and Decellularized Extracellular Matrices in Orthopedic Surgery: A Scoping Review. Arch. Bone Jt. Surg. 2022, 10, 827–832. [Google Scholar] [PubMed]
- Perdomo-Pantoja, A.; Holmes, C.; Cottrill, E.; Rindone, A.; Ishida, W.; Taylor, M.; Tomberlin, C.; Lo, S.F.L.; Grayson, W.L.; Witham, T.F. Comparison of Freshly Isolated Adipose Tissue-derived Stromal Vascular Fraction and Bone Marrow Cells in a Posterolateral Lumbar Spinal Fusion Model. Spine 2021, 46, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Kim, K.T.; Kim, K.G.; Lim, S.H.; Kim, Y.J.; Sonh, S.; Sheen, S.H.; Heo, C.Y.; Han, I. Safety and Tolerability of Stromal Vascular Fraction Combined with β-Tricalcium Phosphate in Posterior Lumbar Interbody Fusion: Phase I Clinical Trial. Cells 2020, 9, 2250. [Google Scholar] [PubMed]
- Rowe, G.; Heng, D.S.; Beare, J.E.; Hodges, N.A.; Tracy, E.P.; Murfee, W.L.; LeBlanc, A.J. Stromal Vascular Fraction Reverses the Age-Related Impairment in Revascularization following Injury. J. Vasc. Res. 2022, 59, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Mehling, B.; Hric, M.; Salatkova, A.; Vetrak, R.; Santora, D.; Ovariova, M.; Mihalyova, R.; Manvelyan, M. A Retrospective Study of Stromal Vascular Fraction Cell Therapy for Osteoarthritis. J. Clin. Med. Res. 2020, 12, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.C.; Chung, H.Y.; Han, S.K.; Jeong, S.H.; Dhong, E.S. Tissue-engineered dermis grafts using stromal vascular fraction cells on the nose: A retrospective case-control study. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Fakin, R.M.; Giovanoli, P.; Calcagni, M. Outcome of Stromal Vascular Fraction-Enriched Fat Grafting Compared to Intramuscular Transposition in Painful End-Neuromas of Superficial Radial Nerve: Preliminary Results. Front. Surg. 2018, 5, 10. [Google Scholar] [CrossRef]
- Calcagni, M.; Zimmermann, S.; Scaglioni, M.F.; Giesen, T.; Giovanoli, P.; Fakin, R.M. The novel treatment of SVF-enriched fat grafting for painful end-neuromas of superficial radial nerve. Microsurgery 2018, 38, 264–269. [Google Scholar] [CrossRef]
- Jeon, H.J.; Choi, D.H.; Lee, J.H.; Lee, J.S.; Lee, J.; Park, H.Y.; Yang, J.D. Prospective Study of the Efficacy of Cell-Assisted Lipotransfer with Stromal Vascular Fraction to Correct Contour Deformities of the Autologous Reconstructed Breast. Aesthetic. Plast. Surg. 2021, 45, 853–863. [Google Scholar] [CrossRef]
- Yin, S.; Yang, X.; Bi, H.; Zhao, Z. Combined Use of Autologous Stromal Vascular Fraction Cells and Platelet-Rich Plasma for Chronic Ulceration of the Diabetic Lower Limb Improves Wound Healing. Int. J. Low. Extrem. Wounds 2021, 20, 135–142. [Google Scholar] [CrossRef]
- Aletto, C.; Giordano, L.; Quaranta, M.; Zara, A.; Notarfrancesco, D.; Maffulli, N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 310. [Google Scholar] [CrossRef]
- Francis, S.L.; Duchi, S.; Onofrillo, C.; Di Bella, C.; Choong, P.F.M. Adipose-Derived Mesenchymal Stem Cells in the Use of Cartilage Tissue Engineering: The Need for a Rapid Isolation Procedure. Stem Cells Int. 2018, 2018, 8947548. [Google Scholar] [CrossRef]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, A.; Riccio, V.; Rioccio, M.; Sbarbati, A. Simple and Rapid Non-Enzymatic Procedure Allows the Isolation of Structurally Preserved Connective Tissue Micro-Fragments Enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359. [Google Scholar] [CrossRef]
- Matsuo, F.S.; de Araújo, P.H.C.; Mota, R.F.; Carvalho, A.J.R.; de Queiroz, M.S.; de Almeida, B.B.; Ferreira, K.C.d.O.S.; Metzner, R.J.M.; Ferrari, G.D.; Alberici, L.C.; et al. RANKL induces beige adipocyte differentiation in preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E866–E877. [Google Scholar] [CrossRef]
- Contreras, G.A.; Kabara, E.; Brester, J.; Neuder, L.; Kiupel, M. Macrophage infiltration in the omental and subcutaneous adipose tissues of dairy cows with displaced abomasum. J. Dairy Sci. 2015, 98, 6176–6187. [Google Scholar] [CrossRef]
- Dey, A.; Ni, Z.; Johnson, M.S.; Sedger, L.M. A multi-colour confocal microscopy method for identifying and enumerating macrophage subtypes and adherent cells in the stromal vascular fraction of human adipose. J. Immunol. Methods 2021, 491, 112988. [Google Scholar] [CrossRef]
- Dulong, J.; Loisel, S.; Rossille, D.; Léonard, S.; Bescher, N.; Bezier, I.; Latour, M.; Monvoisin, C.; Monnier, D.; Bertheuil, N.; et al. CD40L-expressing CD4+ T cells prime adipose-derived stromal cells to produce inflammatory chemokines. Cytotherapy 2022, 24, 500–507. [Google Scholar] [CrossRef]
- Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Genetic and epigenetic control of adipose development. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 3–12. [Google Scholar] [CrossRef]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Orthop. 2017, 4, 33. [Google Scholar] [CrossRef]
- Stachura, A.; Paskal, W.; Pawlik, W.; Mazurek, M.J.; Jaworowski, J. The Use of Adipose-Derived Stem Cells (ADSCs) and Stromal Vascular Fraction (SVF) in Skin Scar Treatment-A Systematic Review of Clinical Studies. J. Clin. Med. 2021, 10, 3637. [Google Scholar] [CrossRef] [PubMed]
- Carstens, M.H.; Zelaya, M.; Calero, D.; Rivera, C.; Correa, D. Adipose-derived stromal vascular fraction (SVF) cells for the treatment of non-reconstructable peripheral vascular disease in patients with critical limb ischemia: A 6-year follow-up showing durable effects. Stem Cell Res. 2020, 49, 102071. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Chae, D.S.; Ryu, H.A.; Kim, S.W. Transplantation of human adipose tissue derived-SVF enhance liver function through high anti-inflammatory property. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 186, 158526. [Google Scholar] [CrossRef]
- Efthymiou, V.; Patti, M.E. It Is Not Just Fat: Dissecting the Heterogeneity of Adipose Tissue Function. Curr. Diab. Rep. 2022, 22, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013, 129, 59–71. [Google Scholar]
- Aguena, M.; Fanganiello, R.D.; Tissiani, L.A.L.; Ishiy, F.A.A.; Atique, R.; Alonso, N.; Passos-Bueno, M.R. Optimization of parameters for a more efficient use of adipose derived stem cells in regenerative medicine therapies. Stem. Cells. Int. 2012, 2012, 303610. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products. Draft Guidance for Industry and Food and Drug Administration Staff; Food and Drug Administration: Moscow, Russia, 2014; Volume 1, p. 1. [Google Scholar]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.; Kocak, P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef] [PubMed]
- Pallua, N.; Grasys, J.; Kim, B.S. Enhancement of progenitor cells by two step centrifugation of emulsified lipoaspirates. Plast. Reconstr. Surg. 2018, 142, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Karina, K.; Rosliana, I.; Rosadi, I.; Schwartz, R.; Sobariah, S.; Afini, I.; Widyastuti, T.; Remelia, M.; Wahyuningsih, K.A.; Pawitan, J.A. Safety of Technique and Procedure of Stromal Vascular Fraction Therapy: From Liposuction to Cell Administration. Scientifica 2020, 2020, 2863624. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, Z.; Li, S.; Wu, Z.; Tan, J.; Yang, W.; Li, G.; Pan, X.; Liu, Y.; Lyu, F.-J.; et al. The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats. Bioengineering 2022, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, J.; Zhang, P.; Liao, Y.; Yuan, Y.; Dong, Z.; Lu, F. Adipose Stromal Vascular Fraction Gel Grafting: A New Method for Tissue Volumization and Rejuvenation. Dermatol. Surg. 2018, 44, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, F.; Feng, J.; Wu, B.; Li, H.; Xiao, S.; Lu, F.; Wei, Z.; Deng, C. Long-term follow-up and exploration of the mechanism of stromal vascular fraction gel in chronic wounds. Stem Cell Res. Ther. 2023, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Hewett, S.; Ross, L.; Delaunay, F.; Goodacre, A.; Ramos, C.; Leong, T.; Saad, A. Regenerative Cells for Facial Surgery: Biofilling and Biocontouring. Aesthetic Surg. J. 2017, 37, S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Mbiine, R.; Wayengera, M.; Ocan, M.; Kiwanuka, N.; Munabi, I.; Muwonge, H.; Lekuya, H.M.; Kawooya, I.; Nakanwagi, C.; Kinengyere, A.A.; et al. Adipose-derived stromal vascular fraction (SVF) in scar treatment: A systematic review proto-col. Am. J. Stem Cells. 2022, 11, 56–63. [Google Scholar]
- Pak, J.; Lee, J.H.; Pak, N.J.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Clinical Protocol of Producing Adipose Tissue-Derived Stromal Vascular Fraction for Potential Cartilage Regeneration. JoVE 2018, 139, 58363. [Google Scholar]
- Zhu, H.; Ge, J.; Chen, X.; Lu, F.; Cai, J. Mechanical Micronization of Lipoaspirates for Regenerative Therapy. J. Vis. Exp. 2019, 15, 145. [Google Scholar]
- Copcu, H.E.; Oztan, S. Not Stromal Vascular Fraction (SVF) or Nanofat, but Total Stromal-Cells (TOST): A New Definition. Systemic Review of Mechanical Stromal-Cell Extraction Techniques. Tissue Eng. Regen. Med. 2021, 18, 25–36. [Google Scholar] [CrossRef]
- Bony, C.; Cren, M.; Domergue, S.; Toupet, K.; Jorgensen, C.; Noël, D. Adipose Mesenchymal Stem Cells Isolated after Manual or Water-jet-Assisted Liposuction Display Similar Properties. Front. Immunol. 2016, 6, 655. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Ellenhorn, J.D.I. Adipose stromal vascular fraction isolation: A head-to-head comparison of four commercial cell separation systems. Plast. Reconstr. Surg. 2013, 132, 932e–939e. [Google Scholar] [CrossRef]
- Packer, J.D.; Chang, W.T.; Dragoo, J.L. The use of vibrational energy to isolate adipose-derived stem cells. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1620. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells from the Infrapatellar Fat Pad. Am. J. Sports Med. 2017, 45, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S.; Birnbaum, Z.E. Adipose stromal vascular fraction isolation: A head-to-head comparison of 4 cell separation systems# 2. Ann. Plast. Surg. 2016, 77, 354–362. [Google Scholar] [PubMed]
- Domenis, R.; Lazzaro, L.; Calabrese, S.; Mangoni, D.; Gallelli, A.; Bourkoula, E.; Manini, I.; Bergamin, N.; Toffoletto, B.; Beltrami, C.A. Adipose tissue derived stem cells: In vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Patel, P.; Li, H.; Huang, L.T.; Wan, H.; Collins, S.; Connell, T.L.; Xu, H. Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast. Reconstr. Surg. 2020, 8, e3010. [Google Scholar] [CrossRef] [PubMed]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose tissue and mesenchymal stem cells: State of the art and Lipogems® technology development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Magnanelli, S.; Screpis, D.; Di Benedetto, P.; Natali, S.; Causero, A.; Zorzi, C. Open-wedge high tibial osteotomy associated with lipogems® intra-articular injection for the treatment of varus knee osteoarthritis–retrospective study. Acta Biomed. Atenei Parm. 2020, 91, e2020022. [Google Scholar]
- Kavala, A.A.; Turkyilmaz, S. Autogenously derived reegenerative cell therapy for venous leg ulcers. Arch. Med. Sci. Atheroscler. Dis. 2018, 3, e156–e163. [Google Scholar] [CrossRef] [PubMed]
- Lobascio, P.; Balducci, G.; Minafra, M.; Laforgia, R.; Fedele, S.; Conticchio, M.; Palasciano, N. Adipose-derived stem cells (MYSTEM® EVO Technology) as a treatment for complex transsphincteric anal fistula. Tech. Coloproctol. 2018, 22, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.P.; van Boxtel, J.; van Dijck, R.; van Dongen, J.A. Platelet Rich STROMA, the Combination of PRP and tSVF and Its Potential Effect on Osteoarthritis of the Knee. Appl. Sci. 2020, 10, 4691. [Google Scholar] [CrossRef]
- Copcu, H.E. Supercharged Mechanical Stromal-cell Transfer (MEST). Plast Reconstr. Surg. Glob. Open 2021, 9, e3552. [Google Scholar] [CrossRef]
- Zocchi, M.L.; Facchin, F.; Pagani, A.; Bonino, C.; Sbarbati, A.; Conti, G.; Vindigni, V.; Bassetto, F. New perspectives in regenerative medicine and surgery: The bioactive composite therapies (BACTs). Eur. J. Plast. Surg. 2022, 45, 1–25. [Google Scholar] [CrossRef]
- Rossi, M.; Roda, B.; Zia, S.; Vigliotta, I.; Zannini, C.; Alviano, F.; Bonsi, L.; Zattoni, A.; Reschiglian, P.; Gennai, A. Characterization of the Tissue and Stromal Cell Components of Micro-Superficial Enhanced Fluid Fat Injection (Micro-SEFFI) for Facial Aging Treatment. Aesthetic Surg. J. 2020, 40, 679–690. [Google Scholar] [CrossRef]
- Cohen, S.R.; Tiryaki, T.; Womack, H.A.; Canikyan, S.; Schlaudraff, K.U.; Scheflan, M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthetic Surg. J. Open Forum 2019, 1, ojz028. [Google Scholar] [CrossRef]
- Tiryaki, K.T.; Cohen, S.; Kocak, P.; Canikyan Turkay, S.; Hewett, S. In-Vitro Comparative Examination of the Effect of Stromal Vascular Fraction Isolated by Mechanical and Enzymatic Methods on Wound Healing. Aesthetic Surg. J. 2020, 40, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef] [PubMed]
- Sesé, B.; Sanmartín, J.M.; Ortega, B.; Matas-Palau, A.; Llull, R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast Reconstr. Surg. 2019, 144, 1079–1088. [Google Scholar] [CrossRef]
- Caforio, M.; Nobile, C. Intra-Articular Administration of Autologous Purified Adipose Tissue Associated with Arthroscopy Ameliorates Knee Osteoarthritis Symptoms. J. Clin. Med. 2021, 10, 2053. [Google Scholar] [CrossRef]
- Ferguson, R.E.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Agaverdiev, M.; Shamsov, B.; Mirzoev, S.; Vardikyan, A.; Ramirez, M.E.; Nurmukhametov, R.; Beilerli, A.; Zhang, B.; Gareev, I.; Pavlov, V. MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications—A systematic review. Noncoding RNA Res. 2022, 8, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, E.N.; Koval, O.A.; Bezuglov, E.; Ramirez, M.d.J.E.; Engelgard, M.; Igorevich, E.I.; Saporiti, A.; Kotenko, K.V.; Montemurro, N. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Medicina 2023, 59, 2090. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7653–7664. [Google Scholar] [PubMed]
- Uhl, J.F.; Sufianov, A.; Ruiz, C.; Iakimov, Y.; Mogorron, H.J.; Ramirez, M.E.; Prat, G.; Lorea, B.; Baldoncini, M.; Goncharov, E.; et al. The Use of 3D Printed Models for Surgical Simulation of Cranioplasty in Craniosynostosis as Training and Education. Brain Sci. 2023, 13, 894. [Google Scholar] [CrossRef]
- Montemurro, N.; Condino, S.; Carbone, M.; Cattari, N.; D’amato, R.; Cutolo, F.; Ferrari, V. Brain Tumor and Augmented Reality: New Technologies for the Future. Int. J. Environ. Res. Public Health 2022, 19, 6347. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.M.; Sigismondi, S.; Nicoletta, M.; Condino, S.; Montemurro, N.; Vozzi, G.; Ferrari, V.; De Maria, C. Analysis of the Robotic-Based In Situ Bioprinting Workflow for the Regeneration of Damaged Tissues through a Case Study. Bioengineering 2023, 10, 560. [Google Scholar] [CrossRef]
- Encarnacion Ramirez, M.; Ramirez Pena, I.; Barrientos Castillo, R.E.; Sufianov, A.; Goncharov, E.; Sanchez, J.A.S.; Colome-Hidalgo, M.; Nurmukhametov, R.; Céspedes, J.R.C.; Montemurro, N. Development of a 3D Printed Brain Model with Vasculature for Neurosurgical Procedure Visualisation and Training. Biomedicines 2023, 11, 330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).