Anti-Platelet Therapy with Cangrelor in Cardiogenic Shock Patients: A Systematic Review and Single-Arm Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
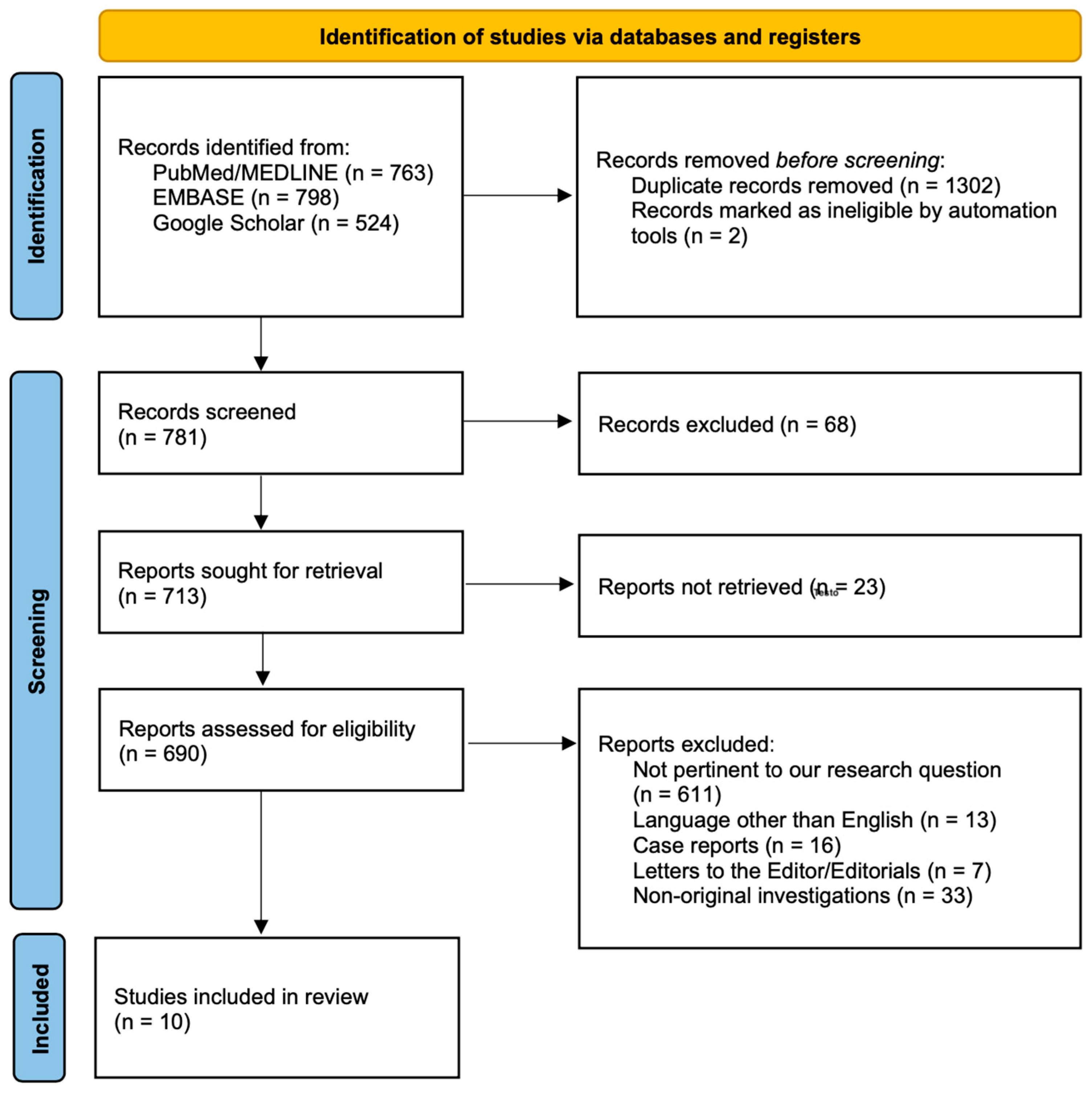
2.2. Study Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction and Study Characteristics
2.4. Statistical Methods
3. Results
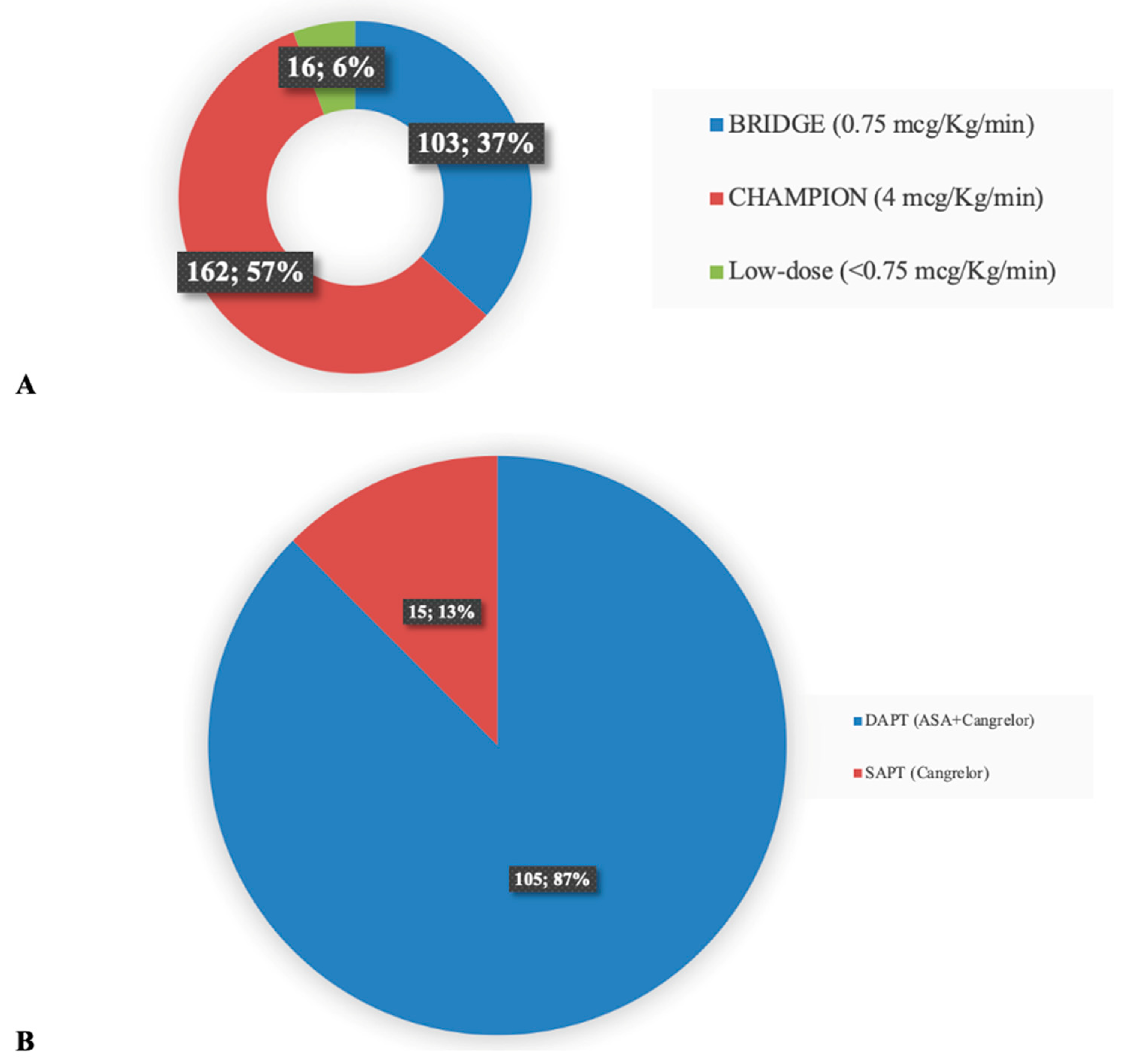
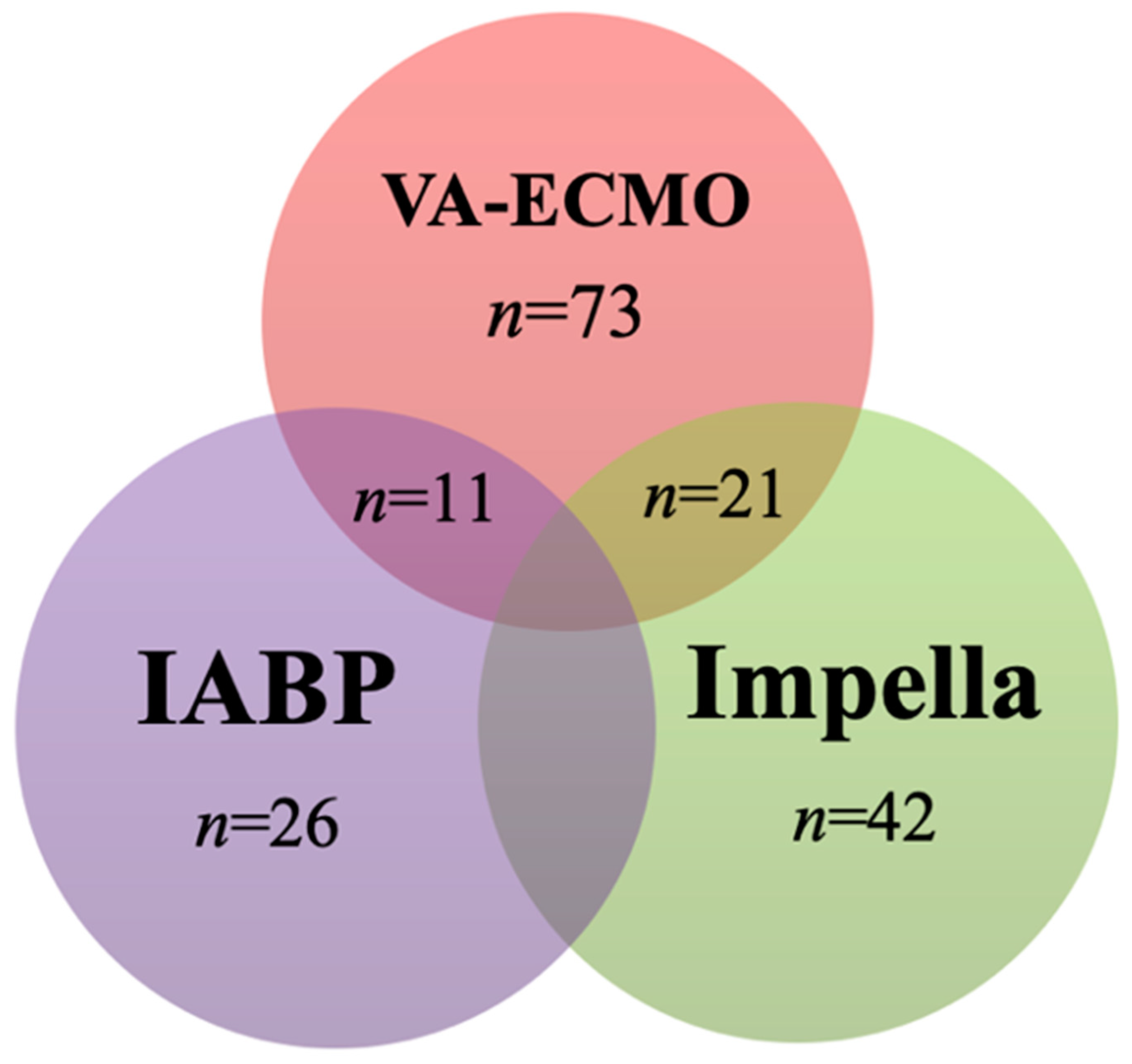
3.1. Characteristics of the Studies
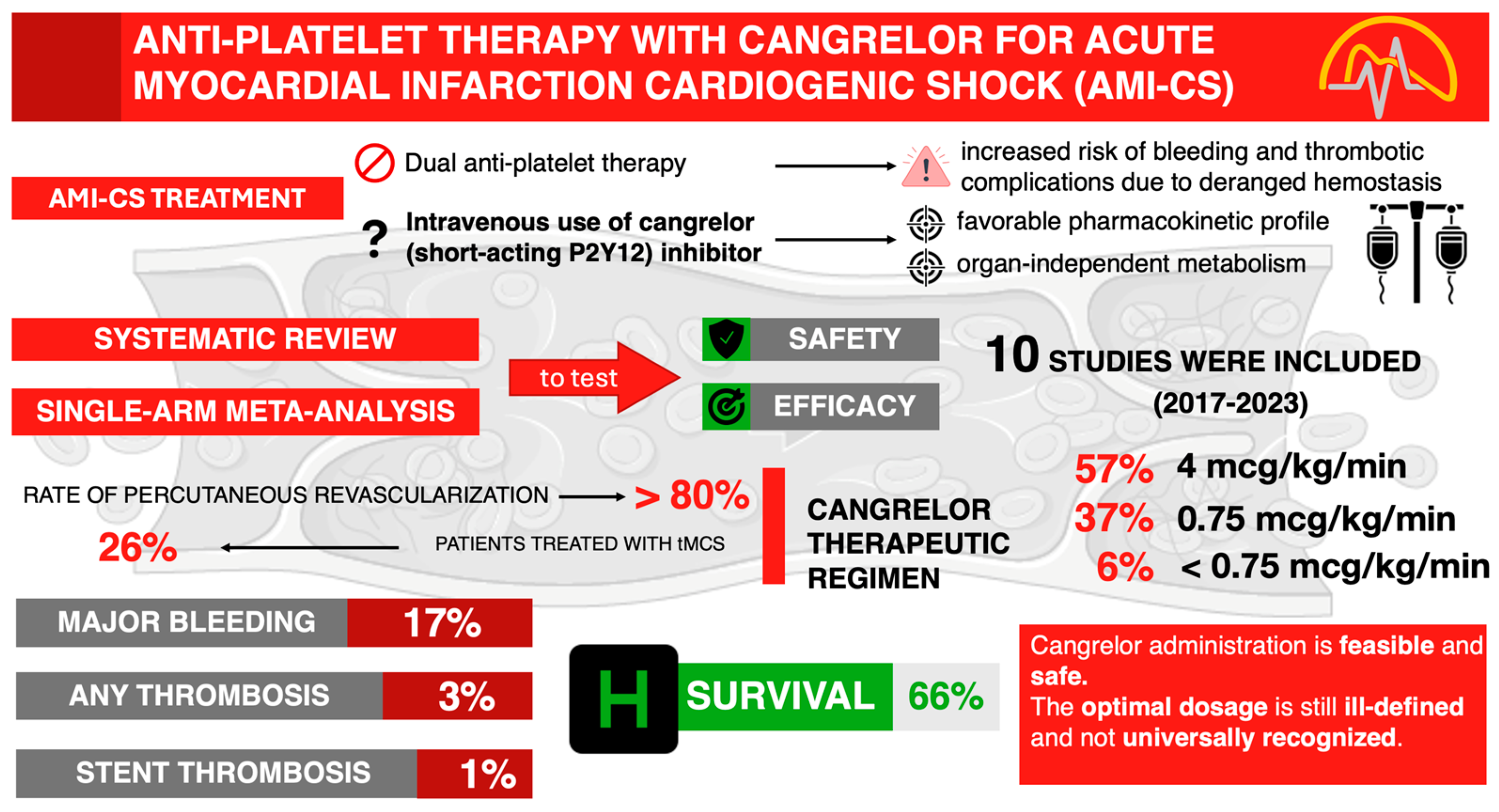
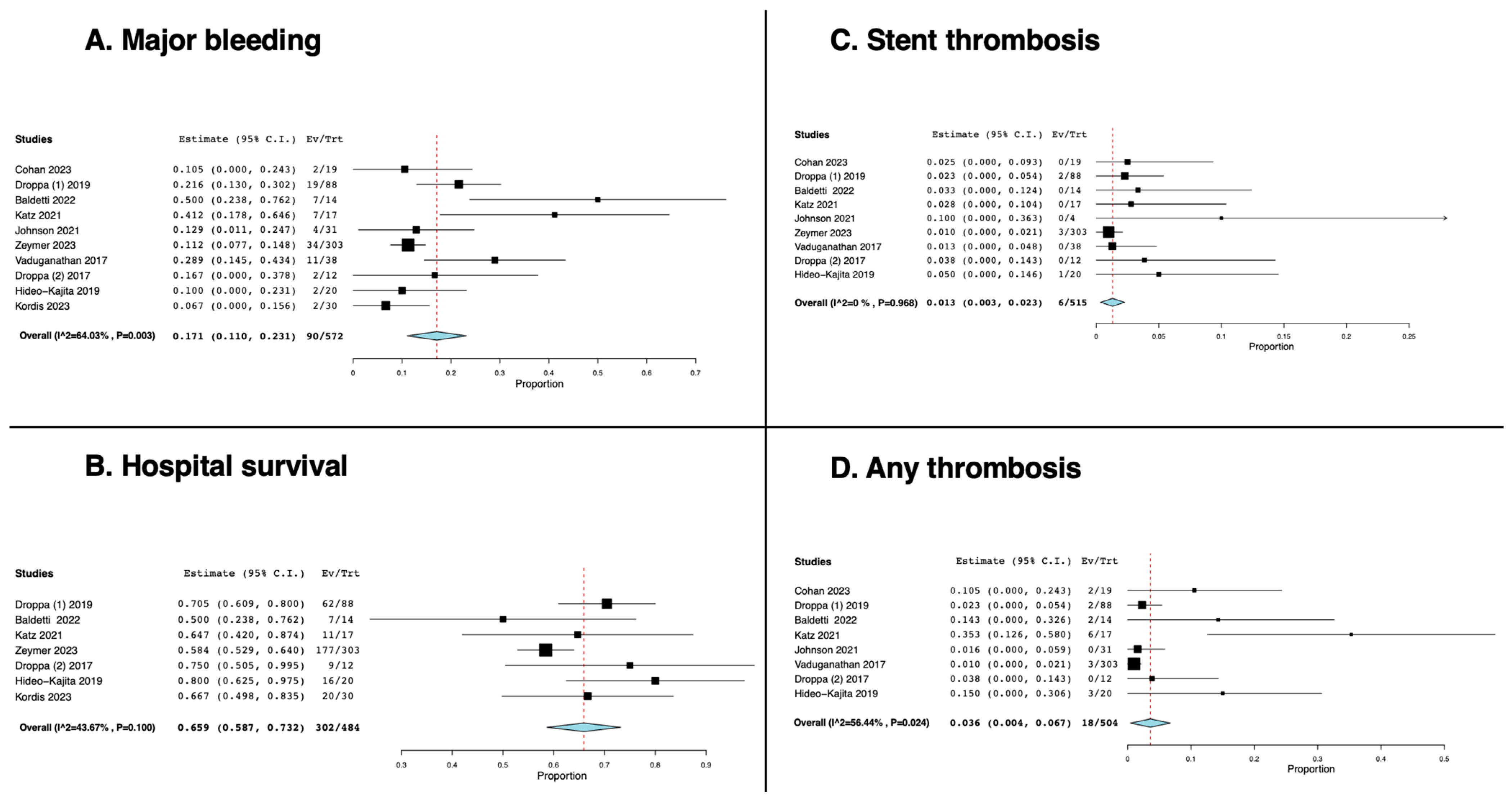
3.2. The Effect of Cangrelor on the Rate of Major Bleeding, Thrombosis, and Hospital Survival
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Bohula, E.A.; van Diepen, S.; Katz, J.N.; Alviar, C.L.; Baird-Zars, V.M.; Barnett, C.F.; Barsness, G.W.; Burke, J.A.; Cremer, P.C.; et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ. Cardiovasc. Qual. Outcomes. 2019, 12, e005618. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Christopher, E.B.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal Life Support in Infarct-Related Cardiogenic Shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Weeks, P.A.; Sieg, A.; Paruthi, C.; Rajapreyar, I. Antiplatelet Therapy Considerations in Ischemic Cardiogenic Shock: Implications of Metabolic Bioactivation. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Qamar, A.; Badreldin, H.A.; Faxon, D.P.; Bhatt, D.L. Cangrelor Use in Cardiogenic Shock: A Single-Center Real-World Experience. JACC Cardiovasc. Interv. 2017, 10, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Marquis-Gravel, G.; Zeitouni, M.; Kochar, A.; Jones, W.S.; Sketch, M.H.; Rao, S.V.; Patel, M.R.; Ohman, E.M. Technical consideration in acute myocardial infarction with cardiogenic shock: A review of antithrombotic and PCI therapies. Catheter. Cardiovasc. Interv. 2020, 95, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Christoph, M.; Schmeinck, S.; Schmieder, K.; Steiding, K.; Schoener, L.; Pfluecke, C.; Quick, S.; Mues, C.; Jellinghaus, S.; et al. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 2014, 85, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Parodi, G.; Bellandi, B.; Xanthopoulou, I.; Capranzano, P.; Capodanno, D.; Valenti, R.; Stavrou, K.; Migliorini, A.; Antoniucci, D.; Tamburino, C.; et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2014, 8, e001593. [Google Scholar] [CrossRef] [PubMed]
- Kubica, J.; Kozinski, M.; Navarese, E.P.; Tantry, U.; Kubica, A.; Siller-Matula, J.M.; Jeong, Y.-H.; Fabiszak, T.; Andruszkiewicz, A.; Gurbel, P.A. Cangrelor: An emerging therapeutic option for patients with coronary artery disease. Curr. Med. Res. Opin. 2014, 30, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Akers, W.S.; Oh, J.J.; Oestreich, J.H.; Ferraris, S.; Wethington, M.; Steinhubl, S.R. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: A direct, parenteral P2Y12 receptor antagonist. J. Clin. Pharmacol. 2010, 50, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Price, S.; Sibbing, D.; Baumbach, A.; Capodanno, D.; Gigante, B.; Halvorsen, S.; Huber, K.; Lettino, M.; Leonardi, S.; et al. Antithrombotic therapy in patients with acute coronary syndrome complicated by cardiogenic shock or out-of-hospital cardiac arrest: A joint position paper from the European Society of Cardiology (ESC) Working Group on Thrombosis, in association with the Acute Cardiovascular Care Association (ACCA) and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Rossini, R.; Masiero, G.; Fruttero, C.; Passamonti, E.; Calvaruso, E.; Cecconi, M.; Carlucci, C.; Mojoli, M.; Guido, P.; Talanas, G.; et al. Antiplatelet Therapy with Cangrelor in Patients Undergoing Surgery after Coronary Stent Implantation: A Real-World Bridging Protocol Experience. TH Open. 2020, 4, e437–e445. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V.J.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Firstenberg, M.S.; Price, M.J.; Tummala, P.E.; Hutyra, M.; Welsby, I.J.; Voeltz, M.D.; Chandna, H.; Ramaiah, C.; Brtko, M.; et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: A randomized controlled trial. JAMA 2012, 307, 265–274. [Google Scholar] [CrossRef]
- Baldetti, L.; Nardelli, P.; Ajello, S.; Melisurgo, G.; Calabrò, M.G.; Pieri, M.; Scandroglio, A.M. Anti-thrombotic Therapy With Cangrelor and Bivalirudin in Venoarterial Extracorporeal Membrane Oxygenation Patients Undergoing Percutaneous Coronary Intervention: A Single-Center Experience. ASAIO J. 2023, 69, e346–e350. [Google Scholar] [CrossRef]
- Bowman, S.; Gass, J.; Weeks, P. Antiplatelet Therapy Bridging with Cangrelor in Patients with Coronary Stents: A Case Series. Ann. Pharmacother. 2019, 53, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Cohan, D.; Uricchio, M.N.; Konopka, C.I.; Montepara, C.A.; Verlinden, N.J. Comparison of clinical outcomes with cangrelor plus aspirin versus oral dual antiplatelet therapy in patients supported with venoarterial extracorporeal membrane oxygenation. Artif. Organs. 2023, 47, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Droppa, M.; Vaduganathan, M.; Venkateswaran, R.V.; Singh, A.; Szumita, P.M.; Roberts, R.J.; Qamar, A.; Hack, L.; Rath, D.; Gawaz, M.; et al. Cangrelor in cardiogenic shock and after cardiopulmonary resuscitation: A global, multicenter, matched pair analysis with oral P2Y12 inhibition from the IABP-SHOCK II trial. Resuscitation 2019, 137, 205–212. [Google Scholar] [CrossRef]
- Katz, A.; Lewis, T.C.; Arnouk, S.; Altshuler, D.; Papadopoulos, J.; Toy, B.; Smith, D.E.; Merchan, C. Clinical Use of Cangrelor After Percutaneous Coronary Intervention in Patients Requiring Mechanical Circulatory Support. Ann. Pharmacother. 2021, 55, 1215–1222. [Google Scholar] [CrossRef]
- Johnson, B.; Horton, E.; Domenico, C.; Nathan, A.; Fanaroff, A.; Acker, M.; Kolansky, D. Safety of Intravenous Cangrelor Administration for Antiplatelet Bridging in Hospitalized Patients: A Retrospective Study. J. Invasive Cardiol. 2021, 33, E998–E1003. [Google Scholar] [CrossRef] [PubMed]
- Zeymer, U.; Lober, C.; Richter, S.; Olivier, C.B.; Huber, K.; Haring, B.; Schwimmbeck, P.; Andrassy, M.; Akin, I.; Cuneo, A.; et al. Cangrelor in patients with percutaneous coronary intervention for acute myocardial infarction after cardiac arrest and/or with cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 462–463. [Google Scholar] [CrossRef]
- Droppa, M.; Borst, O.; Rath, D.; Müller, K.; Gawaz, M.; Bhatt, D.L.; Geisler, T. Impact of Intravenous P2Y12-Receptor Inhibition with Cangrelor in Patients Presenting with Acute Coronary Syndrome and Cardiogenic Shock—A Case Series. Cell Physiol. Biochem. 2017, 42, 1336–1341. [Google Scholar] [CrossRef]
- Hideo-Kajita, A.; Rogers, T.; Buchanan, K.; Iantorno, M.; Gajanana, D.; Ozaki, Y.; Dan, K.; Kolm, P.; Brathwaite, E.; Beyene, S.; et al. Effects of Cangrelor as Adjunct Therapy to Percutaneous Coronary Intervention. Am. J. Cardiol. 2019, 123, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Kordis, P.; Bozic Mijovski, M.; Berden, J.; Steblovnik, K.; Blinc, A.; Noc, M. Cangrelor for comatose survivors of out-of-hospital cardiac arrest undergoing percutaneous coronary intervention: The CANGRELOR-OHCA study. EuroIntervention 2023, 18, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Schneider, D.J.; Bhatt, D.L.; French, W.J.; Price, M.J.; Saucedo, J.F.; Shaburishvili, T.; Huber, K.; Prats, J.; Liu, T.; et al. Pharmacodynamic effects of cangrelor and clopidogrel: The platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J. Thromb. Thrombolysis. 2012, 34, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Xanthopoulou, I.; Gkizas, V.; Kassimis, G.; Theodoropoulos, K.C.; Makris, G.; Koutsogiannis, N.; Damelou, A.; Tsigkas, G.; Davlouros, P.; et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2012, 5, 797–804. [Google Scholar] [CrossRef]
- Radu, R.I.; Ben Gal, T.; Abdelhamid, M.; Antohi, E.; Adamo, M.; Ambrosy, A.P.; Geavlete, O.; Lopatin, Y.; Lyon, A.; Miro, O.; et al. Antithrombotic and anticoagulation therapies in cardiogenic shock: A critical review of the published literature. ESC Heart Fail. 2021, 8, 4717–4736. [Google Scholar] [CrossRef] [PubMed]
- Van Edom, C.J.; Gramegna, M.; Baldetti, L.; Beneduce, A.; Castelein, T.; Dauwe, D.; Frederiks, P.; Giustino, G.; Jacquemin, M.; Janssens, S.P.; et al. Management of Bleeding and Hemolysis During Percutaneous Microaxial Flow Pump Support: A Practical Approach. JACC Cardiovasc. Interv. 2023, 16, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
| 1st Author, Country | Journal, Year [Reference] | Sample Size | Study Design | Age, y | Male, Sex | Percutaneous Coronary Intervention Rate, % | Systemic Anticoagulation (Drug) | Concomitant Antithrombotic Drug(s) Administered with Cangrelor |
|---|---|---|---|---|---|---|---|---|
| Cohan, USA | Artif Organs, 2023 [24] | 37 | Retrospective | 64 ± 11 | 62% | 100% | heparin | aspirin |
| Droppa, Germany | Resuscitation, 2019 [25] | 176 | Propensity matching from trial | 71 (59–77) | 72% | 93% | heparin or bivalirudin | aspirin |
| Baldetti, Italy | ASAIO J., 2022 [21] | 14 | Retrospective | 58 (54–67) | 93% | 86% | bivalirudin | none |
| Katz, USA | Ann Pharmacother., 2021 [26] | 17 | Retrospective | 65 (54–71) | 82% | 100% | heparin | aspirin |
| Johnson, USA | J Invasive Cardiol., 2021 [27] | 31 | Retrospective | 62 ± 10 | 74% | not specified | heparin | not specified |
| Zeymer, Germany | Eur Heart J Acute Cardiovasc Care, 2023 [28] | 303 | Retrospective | 65 ± 13 | 78% | 100% | not specified | not specified |
| Vaduganathan, USA | JACC Cardiovasc Interv., 2017 [29] | 38 | Retrospective | 65 (56–76) | 63% | 82% | heparin or bivalirudin | aspirin |
| Droppa, Germany | Cell Physiol Biochem., 2017 [30] | 12 | Retrospective | 65 ± 3 | 83% | 100% | bivalirudin | aspirin |
| Hideo-Kajita, USA | Am J Cardiol., 2019 [31] | 223 | Retrospective | 63 ± 11 | 65% | 100% | heparin or bivalirudin | aspirin |
| Kordis, Slovenia | EuroIntervention, 2023 [32] | 91 | Randomized controlled trial | not specified | not specified | 100% | heparin | aspirin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Andria Ursoleo, J.; Baldetti, L.; Pieri, M.; Nardelli, P.; Altizio, S.; Ajello, S.; Scandroglio, A.M. Anti-Platelet Therapy with Cangrelor in Cardiogenic Shock Patients: A Systematic Review and Single-Arm Meta-Analysis. Medicina 2024, 60, 2092. https://doi.org/10.3390/medicina60122092
D’Andria Ursoleo J, Baldetti L, Pieri M, Nardelli P, Altizio S, Ajello S, Scandroglio AM. Anti-Platelet Therapy with Cangrelor in Cardiogenic Shock Patients: A Systematic Review and Single-Arm Meta-Analysis. Medicina. 2024; 60(12):2092. https://doi.org/10.3390/medicina60122092
Chicago/Turabian StyleD’Andria Ursoleo, Jacopo, Luca Baldetti, Marina Pieri, Pasquale Nardelli, Savino Altizio, Silvia Ajello, and Anna Mara Scandroglio. 2024. "Anti-Platelet Therapy with Cangrelor in Cardiogenic Shock Patients: A Systematic Review and Single-Arm Meta-Analysis" Medicina 60, no. 12: 2092. https://doi.org/10.3390/medicina60122092
APA StyleD’Andria Ursoleo, J., Baldetti, L., Pieri, M., Nardelli, P., Altizio, S., Ajello, S., & Scandroglio, A. M. (2024). Anti-Platelet Therapy with Cangrelor in Cardiogenic Shock Patients: A Systematic Review and Single-Arm Meta-Analysis. Medicina, 60(12), 2092. https://doi.org/10.3390/medicina60122092






