Prognostic Value of Neutrophil, Monocyte, Lymphocyte, and Platelet/High-Density Lipoprotein Ratios in Ischemic Heart Disease: An NHANES Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Design
2.2. Ethical Statement
2.3. Participants
2.4. Mortality Data
2.5. Neutrophil, Monocyte, Lymphocyte, Platelet, and TC/HDL Ratios
2.6. Other Variables
2.7. Statistical Analysis
3. Results
3.1. Patient Selection
3.2. Characteristics of the Study Participants
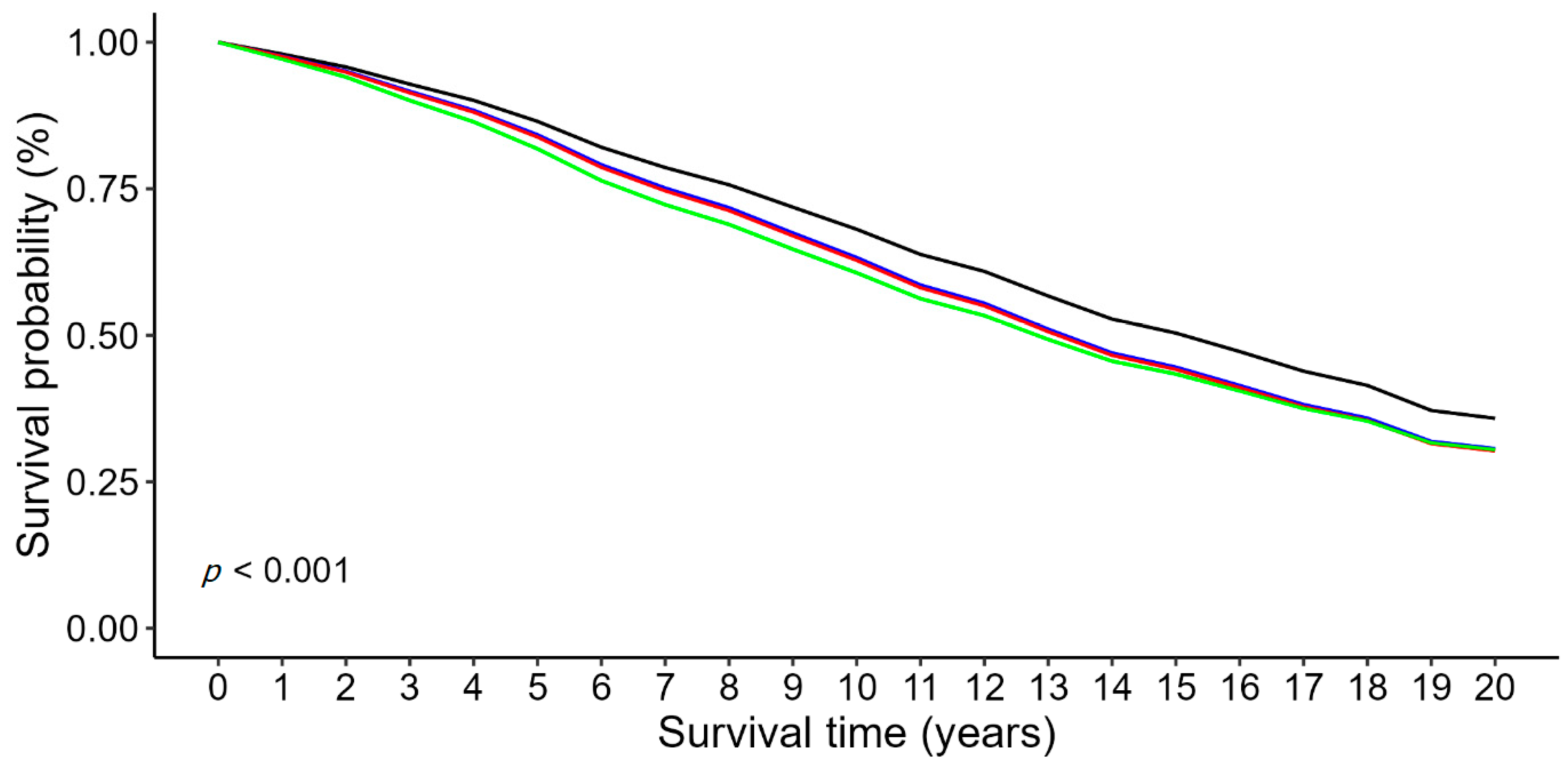
3.3. Associations Between the Hematologic Ratios and Mortality in Patients with IHD
3.4. ROC Curve Analysis of the Hematologic Ratios in Predicting Mortality in Patients with IHD
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Shahjehan, R.D.; Bhutta, B.S. Coronary artery disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Duggan, J.P.; Peters, A.S.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of coronary artery disease. Surg. Clin. N. Am. 2022, 102, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Thiele, H.; Haasenritter, J.; Wahlers, T.; Massberg, S.; Haverich, A. The treatment of coronary artery disease. Dtsch. Arztebl. Int. 2022, 119, 716–723. [Google Scholar] [PubMed]
- Fazmin, I.T.; Achercouk, Z.; Edling, C.E.; Said, A.; Jeevaratnam, K. Circulating microrna as a biomarker for coronary artery disease. Biomolecules 2020, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Ben Braiek, A.; Chahed, H.; Dumont, F.; Abdelhak, F.; Hichem, D.; Gamra, H.; Baudin, B. Identification of biomarker panels as predictors of severity in coronary artery disease. J. Cell. Mol. Med. 2021, 25, 1518–1530. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, L.; Wang, W.; Cui, H.; Zhang, Y.; Xu, J.; Zhang, W.; Zheng, T.; Yang, J. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: A retrospective cohort study. Cardiovasc. Diabetol. 2022, 21, 142. [Google Scholar] [CrossRef]
- Peters, S.A.; Singhateh, Y.; Mackay, D.; Huxley, R.R.; Woodward, M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 123–131. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Qin, L. Lipid profile and prognosis in patients with coronary heart disease: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2021, 21, 69. [Google Scholar] [CrossRef]
- Mørland, J.G.; Magnus, P.; Vollset, S.E.; Leon, D.A.; Selmer, R.; Tverdal, A. Associations between serum high-density lipoprotein cholesterol levels and cause-specific mortality in a general population of 345,000 men and women aged 20–79 years. Int. J. Epidemiol. 2023, 52, 1257–1267. [Google Scholar] [CrossRef]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Ko, Y.A.; Mehta, A.; Alkhoder, A.A.; Alras, Z.; Desai, S.R.; Patel, K.J.; Hooda, A.; et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 2022, 7, 672–680. [Google Scholar] [CrossRef]
- Calling, S.; Johansson, S.E.; Wolff, M.; Sundquist, J.; Sundquist, K. The ratio of total cholesterol to high density lipoprotein cholesterol and myocardial infarction in women’s health in the lund area (whila): A 17-year follow-up cohort study. BMC Cardiovasc. Disord. 2019, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, X.; Lo, K.; Huang, Y.; Feng, Y. The effect of total cholesterol/high-density lipoprotein cholesterol ratio on mortality risk in the general population. Front. Endocrinol. 2022, 13, 1012383. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; Chen, Y.S.; Ji, H.Y.; Xie, W.M.; Jiang, J.; Ran, L.S.; Zhang, C.T.; Quan, X.Q. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: A comparison study. Lipids Health Dis. 2020, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Jialal, G.; Adams-Huet, B. The platelet to high density lipoprotein-cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes Metab. Res. Rev. 2021, 37, e3403. [Google Scholar] [CrossRef]
- Gürsoy, M.O.; Yılmaz, C.; Bayam, E.; Güner, A.; Emren, S.V.; Kalkan, S.; Üzüm, Y.; Keleş, N.; Karagöz, A.; Özkan, M. Monocyte to hdl ratio may predict thrombosis in patients with mechanical mitral and aortic valve prosthesis. J. Artif. Organs 2023, 27, 117–124. [Google Scholar] [CrossRef]
- Yu, S.; Guo, X.; Li, G.; Yang, H.; Zheng, L.; Sun, Y. Lymphocyte to high-density lipoprotein ratio but not platelet to lymphocyte ratio effectively predicts metabolic syndrome among subjects from rural china. Front. Cardiovasc. Med. 2021, 8, 583320. [Google Scholar] [CrossRef]
- Nazir, S.; Jankowski, V.; Bender, G.; Zewinger, S.; Rye, K.A.; van der Vorst, E.P.C. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv. Drug Deliv. Rev. 2020, 159, 94–119. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Elfil, M.; Negida, A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emergency 2016, 4, 111–113. [Google Scholar]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and inflammation: Insights from the theory of general pathological processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Quispe, R.; Elshazly, M.B.; Zhao, D.; Toth, P.P.; Puri, R.; Virani, S.S.; Blumenthal, R.S.; Martin, S.S.; Jones, S.R.; Michos, E.D. Total cholesterol/hdl-cholesterol ratio discordance with ldl-cholesterol and non-hdl-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: The aric study. Eur. J. Prev. Cardiol. 2020, 27, 1597–1605. [Google Scholar] [CrossRef]
- Soares, A.A.S.; Carvalho, L.S.F.; Bonilha, I.; Virginio, V.W.; Nadruz Junior, W.; Coelho-Filho, O.R.; Quinaglia, E.S.J.C.; Petrucci Junior, O.; Sposito, A.C. Adverse interaction between hdl and the mass of myocardial infarction. Atherosclerosis 2019, 281, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; de Lima-Junior, J.C.; Moura, F.A.; Barreto, J.; Bonilha, I.; Santana, M.; Virginio, V.W.; Sun, L.; Carvalho, L.S.F.; Soares, A.A.S.; et al. Reciprocal multifaceted interaction between hdl (high-density lipoprotein) and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A.; Elmas, Ö.F.; Atasoy, M.; Türsen, Ü.; Lotti, T. Can monocyte to hdl cholesterol ratio and monocyte to lymphocyte ratio be markers for inflammation and oxidative stress in patients with vitiligo? A preliminary study. Arch. Dermatol. Res. 2021, 313, 491–498. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, Q.; Wu, S.; Wan, Y.; Lei, Y. Compared with the monocyte to high-density lipoprotein ratio (mhr) and the neutrophil to lymphocyte ratio (nlr), the neutrophil to high-density lipoprotein ratio (nhr) is more valuable for assessing the inflammatory process in parkinson’s disease. Lipids Health Dis. 2021, 20, 35. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, X.; Ban, J.; Yue, L.; Ren, L.; Chen, S. Association of neutrophil to high-density lipoprotein cholesterol ratio with cardiac ultrasound parameters and cardiovascular risk: A cross-sectional study based on healthy populations. J. Inflamm. Res. 2023, 16, 1853–1865. [Google Scholar] [CrossRef]
- Ozgeyik, M.; Ozgeyik, M.O. Long-term prognosis after treatment of total occluded coronary artery is well predicted by neutrophil to high-density lipoprotein ratio: A comparison study. Kardiologiia 2021, 61, 60–67. [Google Scholar] [CrossRef]
- Lamichhane, P.; Agrawal, A.; Abouainain, Y.; Abousahle, S.; Regmi, P.R. Utility of neutrophil-to-high-density lipoprotein-cholesterol ratio in patients with coronary artery disease: A narrative review. J. Int. Med. Res. 2023, 51, 3000605231166518. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, J.; Zou, H.; Li, M.; Su, Z.; Sun, W.; Kong, X. Prognostic role of neutrophil to high-density lipoprotein cholesterol ratio for all-cause and cardiovascular mortality in the general population. Front. Cardiovasc. Med. 2022, 9, 807339. [Google Scholar] [CrossRef]
- Liu, S.; Feng, B.; Song, Q.; Zhang, Y.; Wu, S.; Cai, J. Neutrophil to high-density lipoprotein cholesterol ratio predicts adverse cardiovascular outcomes in subjects with pre-diabetes: A large cohort study from China. Lipids Health Dis. 2022, 21, 86. [Google Scholar] [CrossRef]
- Guo, J.; Chen, M.; Hong, Y.; Huang, Y.; Zhang, H.; Zhou, Y.; Zhou, B.; Fu, M. Comparison of the Predicting Value of Neutrophil to high-Density Lipoprotein Cholesterol Ratio and Monocyte to high-Density Lipoprotein Cholesterol Ratio for in-Hospital Prognosis and Severe Coronary Artery Stenosis in Patients with ST-Segment Elevation Acute Myocardial Infarction Following Percutaneous Coronary Intervention: A Retrospective Study. J. Inflamm. Res. 2023, 16, 4541–4557. [Google Scholar]
- Gao, J.; Lu, J.; Sha, W.; Xu, B.; Zhang, C.; Wang, H.; Xia, J.; Zhang, H.; Tang, W.; Lei, T. Relationship between the neutrophil to high-density lipoprotein cholesterol ratio and severity of coronary artery disease in patients with stable coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 1015398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kang, Z.; Yin, D.; Gao, J. Role of neutrophils in different stages of atherosclerosis. Innate Immun. 2023, 29, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Nakajima, H.; Toh, R.; Hirata, K.I.; Ishida, T. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall, N = 2265 | Mortality Status | p-Value | |
|---|---|---|---|---|
| Alive, N = 1421 | Deceased, N = 844 | |||
| Age, years | 66.5 ± 12.7 | 62.7 ± 12.9 | 72.8 ± 9.5 | <0.001 |
| Age, category | <0.001 | |||
| 20–49 | 242 (11%) | 220 (15%) | 22 (2.6%) | |
| 50–59 | 318 (14%) | 255 (18%) | 63 (7.5%) | |
| 60–69 | 642 (28%) | 467 (33%) | 175 (21%) | |
| 70–79 | 610 (27%) | 315 (22%) | 295 (35%) | |
| 80+ | 453 (20%) | 164 (12%) | 289 (34%) | |
| Sex | 0.018 | |||
| Male | 1370 (60%) | 833 (59%) | 537 (64%) | |
| Female | 895 (40%) | 588 (41%) | 307 (36%) | |
| Race | <0.001 | |||
| Non-Hispanic White | 1311 (58%) | 734 (52%) | 577 (68%) | |
| Non-Hispanic Black | 366 (16%) | 249 (18%) | 117 (14%) | |
| Hispanic White | 162 (7.2%) | 132 (9.3%) | 30 (3.6%) | |
| Other/Unknown | 426 (19%) | 306 (22%) | 120 (14%) | |
| BMI, kg/m2 | 29.5(6.4) | 30.3 ± 6.7) | 28.1 ± 5.7 | <0.001 |
| BMI, category | <0.001 | |||
| <25.0 | 543 (24%) | 284 (20%) | 259 (31%) | |
| 25.0–29.9 | 812 (36%) | 501 (35%) | 311 (37%) | |
| ≥30 | 910 (40%) | 636 (45%) | 274 (32%) | |
| Years since CAD diagnosed | 11.4 ± 10.9 | 11.2 ± 10.7 | 11.5 ± 11.2 | 0.602 |
| <5 years | 718 (32%) | 457 (32%) | 261 (32%) | |
| ≥5 years | 1516 (68%) | 952 (68%) | 564 (68%) | 0.697 |
| Poverty income ratio | 0.04 | |||
| >1 | 1602 (78%) | 980 (76%) | 622 (80%) | |
| ≤1 | 456 (22%) | 303 (24%) | 153 (20%) | |
| Education level | <0.001 | |||
| Above high school | 1527 (68%) | 1015 (72%) | 512 (61%) | |
| Never attended high school | 732 (32%) | 404 (28%) | 328 (39%) | |
| Smoking status | <0.001 | |||
| Never | 871 (38%) | 575 (40%) | 296 (35%) | |
| Former | 950 (42%) | 550 (39%) | 400 (47%) | |
| Current | 444 (20%) | 296 (21%) | 148 (18%) | |
| DM | 804 (35%) | 485 (34%) | 319 (38%) | 0.078 |
| Hypertension | 1670 (75%) | 1014 (72%) | 656 (79%) | <0.001 |
| SBP, mmHg | 113.6 ± 22.0 | 130.5 ± 20.1 | 138.9 ± 24.0 | <0.001 |
| DBP, mmHg | 67.4 ± 15.5 | 69.2 ± 14.3 | 64.3 ± 16.9 | <0.001 |
| Chronic respiratory disease | 604 (27%) | 411 (29%) | 193 (23%) | 0.002 |
| History of stroke | 306 (14%) | 166 (12%) | 140 (17%) | <0.001 |
| History of cancer | 463 (20%) | 265 (19%) | 198 (23%) | 0.006 |
| CKD | 633 (28%) | 320 (23%) | 313 (37%) | <0.001 |
| Medication | ||||
| Beta Blocker | 1055 (47%) | 658 (46%) | 397 (47%) | 0.735 |
| ACEI/ARB | 1143 (50%) | 733 (52%) | 410 (49%) | 0.167 |
| Statin | 1267 (56%) | 820 (58%) | 447 (53%) | 0.028 |
| HDL, md/dL | 49.9 ± 15.4 | 49.6 ± 14.9 | 50.4 ± 16.2 | 0.713 |
| Total Cholesterol, md/dL | 184.5 ± 44.7 | 181.9 ± 43.3 | 188.7 ± 46.7 | 0.005 |
| Neutrophil, 103 cells/uL | 4.4 ± 1.7 | 4.3 ± 1.7 | 4.6 ± 1.8 | <0.001 |
| Monocyte, 103 cells/uL | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.043 |
| Platelet, 103 cells/uL | 232.1 ± 67.4 | 230.2 ± 64.1 | 235.3 ± 72.7 | 0.176 |
| Lymphocyte, 103 cells/uL | 2.1 ± 2.9 | 2.1 ± 2.0 | 2.1 ± 4.0 | <0.001 |
| Survival times, months | 80 (44,137) | 85 (44,143) | 75 (43,124.3) | <0.001 |
| Hematologic Indices | Overall, N = 2265 | Mortality Status | p-Value | |
|---|---|---|---|---|
| Alive, N = 1421 | Deceased, N = 844 | |||
| TC/HDL ratio, continuous | 3.967 ± 1.392 | 3.930 ± 1.391 | 4.030 ± 1.393 | 0.082 |
| TC/HDL ratio, quartiles | 0.27 | |||
| Q1 | 566 (25%) | 365 (26%) | 201 (24%) | |
| Q2 | 566 (25%) | 361 (25%) | 205 (24%) | |
| Q3 | 565 (25%) | 358 (25%) | 207 (25%) | |
| Q4 | 568 (25%) | 337 (24%) | 231 (27%) | |
| Neutrophil/HDL ratio, continuous | 0.097 ± 0.050 | 0.096 ± 0.051 | 0.100 ± 0.050 | 0.01 |
| Neutrophil/HDL ratio, quartiles | 0.036 | |||
| Q1 | 563 (25%) | 379 (27%) | 184 (22%) | |
| Q2 | 568 (25%) | 355 (25%) | 213 (25%) | |
| Q3 | 566 (25%) | 352 (25%) | 214 (25%) | |
| Q4 | 568 (25%) | 335 (24%) | 233 (28%) | |
| Monocyte/HDL ratio, continuous | 0.013 ± 0.007 | 0.013 ± 0.007 | 0.014 ± 0.007 | 0.219 |
| Monocyte/HDL ratio, quartiles | 0.799 | |||
| Q1 | 563 (25%) | 363 (26%) | 200 (24%) | |
| Q2 | 564 (25%) | 352 (25%) | 212 (25%) | |
| Q3 | 561 (25%) | 347 (24%) | 214 (25%) | |
| Q4 | 577 (25%) | 359 (25%) | 218 (26%) | |
| Platelet/HDL ratio, continuous | 5.039 ± 2.058 | 5.012 ± 1.980 | 5.084 ± 2.183 | 0.9 |
| Platelet/HDL ratio, quartiles | 0.907 | |||
| Q1 | 566 (25%) | 349 (25%) | 217 (26%) | |
| Q2 | 566 (25%) | 356 (25%) | 210 (25%) | |
| Q3 | 566 (25%) | 361 (25%) | 205 (24%) | |
| Q4 | 567 (25%) | 355 (25%) | 212 (25%) | |
| Lymphocyte/HDL ratio, continuous | 0.046 ± 0.064 | 0.047 ± 0.047 | 0.046 ± 0.085 | <0.001 |
| Lymphocyte/HDL ratio, quartiles | <0.001 | |||
| Q1 | 556 (25%) | 310 (22%) | 246 (29%) | |
| Q2 | 575 (25%) | 352 (25%) | 223 (26%) | |
| Q3 | 567 (25%) | 377 (27%) | 190 (23%) | |
| Q4 | 567 (25%) | 382 (27%) | 185 (22%) | |
| Hematologic Indices | Univariate | Multivariable Model 1 | Multivariable Model 2 | |||
|---|---|---|---|---|---|---|
| Crude HR (95%CI) | p-Value | aHR (95%CI) | p-Value | aHR (95%CI) | p-Value | |
| TC/HDL ratio, z-score | 0.91 (0.84, 0.97) | 0.007 | 1.07 (0.99, 1.15) | 0.075 | 1.01 (0.93, 1.10) | 0.8 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.81 (0.67, 0.99) | 0.038 | 0.89 (0.73, 1.08) | 0.2 | 0.84 (0.68, 1.04) | 0.11 |
| Q3 | 0.74 (0.61, 0.90) | 0.002 | 0.97 (0.79, 1.18) | 0.7 | 0.9 (0.73, 1.12) | 0.4 |
| Q4 | 0.75 (0.62, 0.90) | 0.003 | 1.13 (0.93, 1.38) | 0.2 | 0.99 (0.79, 1.23) | 0.9 |
| Neutrophil/HDL ratio, z-score | 1.09 (1.02, 1.16) | 0.015 | 1.18 (1.10, 1.28) | <0.001 | 1.17 (1.08, 1.28) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.06 (0.87, 1.29) | 0.584 | 0.95 (0.78, 1.17) | 0.7 | 0.99 (0.80, 1.24) | >0.9 |
| Q3 | 1.12 (0.92, 1.36) | 0.279 | 1.14 (0.93, 1.40) | 0.2 | 1.14 (0.92, 1.43) | 0.2 |
| Q4 | 1.26 (1.04, 1.53) | 0.019 | 1.44 (1.17, 1.76) | <0.001 | 1.41 (1.13, 1.77) | 0.003 |
| Monocyte/HDL ratio, z-score | 1.08 (1.01, 1.16) | 0.025 | 1.07 (0.99, 1.15) | 0.081 | 1.06 (0.98, 1.15) | 0.2 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.04 (0.86, 1.26) | 0.689 | 0.97 (0.79, 1.18) | 0.7 | 0.96 (0.77, 1.19) | 0.7 |
| Q3 | 1.09 (0.90, 1.32) | 0.389 | 1.14 (0.94, 1.40) | 0.2 | 1.11 (0.90, 1.38) | 0.3 |
| Q4 | 1.08 (0.89, 1.30) | 0.452 | 1.09 (0.89, 1.34) | 0.4 | 1.04 (0.83, 1.30) | 0.7 |
| Platelet/HDL ratio, z-score | 0.88 (0.82, 0.95) | <0.001 | 1.05 (0.97, 1.13) | 0.2 | 1.03 (0.95, 1.12) | 0.5 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.81 (0.67, 0.98) | 0.031 | 0.91 (0.75, 1.10) | 0.3 | 0.89 (0.72, 1.10) | 0.3 |
| Q3 | 0.69 (0.57, 0.83) | <0.001 | 0.89 (0.73, 1.08) | 0.3 | 0.83 (0.67, 1.03) | 0.089 |
| Q4 | 0.64 (0.53, 0.77) | <0.001 | 1.04 (0.85, 1.27) | 0.7 | 0.99 (0.79, 1.23) | 0.9 |
| Lymphocyte/HDL ratio, z-score | 0.99 (0.87, 1.13) | 0.878 | 1.05 (1.00, 1.10) | 0.05 | 1.05 (0.99, 1.10) | 0.084 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.83 (0.70, 1.00) | 0.051 | 0.87 (0.73, 1.05) | 0.14 | 0.91 (0.75, 1.11) | 0.3 |
| Q3 | 0.67 (0.56, 0.81) | <0.001 | 0.9 (0.74, 1.09) | 0.3 | 0.82 (0.67, 1.01) | 0.069 |
| Q4 | 0.62 (0.51, 0.75) | <0.001 | 0.96 (0.78, 1.17) | 0.7 | 0.91 (0.73, 1.13) | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Wu, C.-H.; Lee, C.-H.; Chen, T.-Y.; Cheng, C.-I. Prognostic Value of Neutrophil, Monocyte, Lymphocyte, and Platelet/High-Density Lipoprotein Ratios in Ischemic Heart Disease: An NHANES Analysis. Medicina 2024, 60, 2084. https://doi.org/10.3390/medicina60122084
Wu C-C, Wu C-H, Lee C-H, Chen T-Y, Cheng C-I. Prognostic Value of Neutrophil, Monocyte, Lymphocyte, and Platelet/High-Density Lipoprotein Ratios in Ischemic Heart Disease: An NHANES Analysis. Medicina. 2024; 60(12):2084. https://doi.org/10.3390/medicina60122084
Chicago/Turabian StyleWu, Chia-Chen, Chia-Hui Wu, Chien-Ho Lee, Tien-Yu Chen, and Cheng-I Cheng. 2024. "Prognostic Value of Neutrophil, Monocyte, Lymphocyte, and Platelet/High-Density Lipoprotein Ratios in Ischemic Heart Disease: An NHANES Analysis" Medicina 60, no. 12: 2084. https://doi.org/10.3390/medicina60122084
APA StyleWu, C.-C., Wu, C.-H., Lee, C.-H., Chen, T.-Y., & Cheng, C.-I. (2024). Prognostic Value of Neutrophil, Monocyte, Lymphocyte, and Platelet/High-Density Lipoprotein Ratios in Ischemic Heart Disease: An NHANES Analysis. Medicina, 60(12), 2084. https://doi.org/10.3390/medicina60122084






