Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review
Abstract
1. Introduction
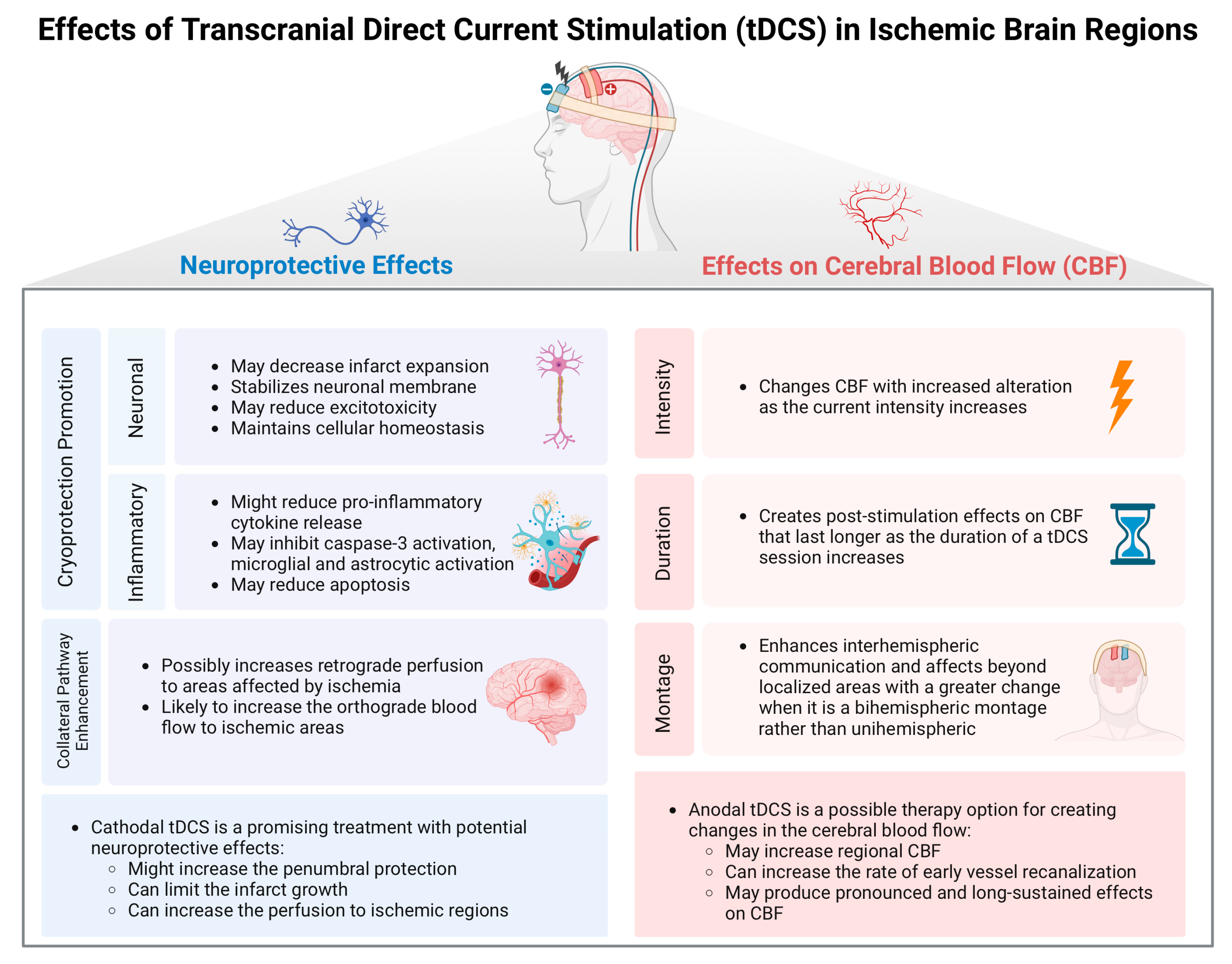
2. Neuroprotective Effects of tDCS in Acute Stroke
3. Effects of tDCS on Cerebral Blood Flow
4. Neuroimaging Biomarkers of CBF
5. Conclusions/Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Ratcliff, J.J.; Hall, A.J.; Saville, B.R.; Allen, J.W.; Paulon, G.; McGlothlin, A.; Lewis, R.J.; Fitzgerald, M.; Caveney, A.F.; et al. Trial of Early Minimally Invasive Removal of Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, M.E.; Bucak, B.; Keser, Z. Advances in stroke neurorehabilitation. J. Clin. Med. 2023, 12, 6734. [Google Scholar] [CrossRef]
- Baron, J.C. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat. Rev. Neurol. 2018, 14, 325–337. [Google Scholar] [CrossRef]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Monai, H.; Ohkura, M.; Tanaka, M.; Oe, Y.; Konno, A.; Hirai, H.; Mikoshiba, K.; Itohara, S.; Nakai, J.; Iwai, Y.; et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat. Commun. 2016, 7, 11100. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain. Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Ilyas, S.; Ali, H.; Ali, I.; Ullah, R.; Arshad, H.; Khalid, S.; Azim, M.E.; Liu, T.; Wang, J. Exploring the Differential Effects of Transcranial Direct Current Stimulation: A Comparative Analysis of Motor Cortex and Cerebellar Stimulation. Heliyon 2024, 10, e26838. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Vascular safety of brain plasticity induction via transcranial direct currents. Neurology 2015, 84, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Zaghi, S.; Lopes, M.; Fregni, F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur. J. Neurol. 2008, 15, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, L.; Fregni, F. Home-based transcranial direct current stimulation (tDCS) to prevent and treat symptoms related to stress: A potential tool to remediate the behavioral consequences of the COVID-19 isolation measures? Front. Integr. Neurosci. 2020, 14, 46. [Google Scholar] [CrossRef]
- Gunduz, M.E.; Pacheco-Barrios, K.; Bonin Pinto, C.; Duarte, D.; Vélez, F.G.S.; Gianlorenco, A.C.L.; Teixeira, P.E.P.; Giannoni-Luza, S.; Crandell, D.; Battistella, L.R.; et al. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: A randomized factorial trial. Neurorehabil. Neural Repair 2021, 35, 704–716. [Google Scholar] [CrossRef]
- Bahr Hosseini, M.; Hou, J.; Bikson, M.; Iacoboni, M.; Gornbein, J.; Saver, J.L. Central Nervous System Electrical Stimulation for Neuroprotection in Acute Cerebral Ischemia. Stroke 2019, 50, 2892–2901. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, K.; Zhao, Z.; Qu, Y. Neuroprotection by Transcranial Direct Current Stimulation in Rodent Models of Focal Ischemic Stroke: A Meta-Analysis. Front. Neurosci. 2021, 15, 761971. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Bikson, M. Neurovascular-modulation: A review of primary vascular responses to transcranial electrical stimulation as a mechanism of action. Brain Stimul. 2021, 14, 837–847. [Google Scholar] [CrossRef]
- Pruvost-Robieux, E.; Benzakoun, J.; Turc, G.; Marchi, A.; Mancusi, R.L.; Lamy, C.; Domigo, V.; Oppenheim, C.; Calvet, D.; Baron, J.C.; et al. Cathodal Transcranial Direct Current Stimulation in Acute Ischemic Stroke: Pilot Randomized Controlled Trial. Stroke 2021, 52, 1951–1960. [Google Scholar] [CrossRef]
- Bahr-Hosseini, M.; Nael, K.; Unal, G.; Iacoboni, M.; Liebeskind, D.S.; Bikson, M.; Saver, J.L.; Sanossian, N.; Wu, A.; Dobkin, B.; et al. High-definition Cathodal Direct Current Stimulation for Treatment of Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2319231. [Google Scholar] [CrossRef]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef]
- Paquette, C.; Sidel, M.; Radinska, B.A.; Soucy, J.P.; Thiel, A. Bilateral transcranial direct current stimulation modulates activation-induced regional blood flow changes during voluntary movement. J. Cereb. Blood Flow Metab. 2011, 31, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Merzagora, A.C.; Foffani, G.; Panyavin, I.; Mordillo-Mateos, L.; Aguilar, J.; Onaral, B.; Oliviero, A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage 2010, 49, 2304–2310. [Google Scholar] [CrossRef]
- Giovannella, M.; Ibañez, D.; Gregori-Pla, C.; Kacprzak, M.; Mitjà, G.; Ruffini, G.; Durduran, T. Concurrent measurement of cerebral hemodynamics and electroencephalography during transcranial direct current stimulation. Neurophotonics 2018, 5, 15001. [Google Scholar] [CrossRef]
- Takai, H.; Tsubaki, A.; Sugawara, K.; Miyaguchi, S.; Oyanagi, K.; Matsumoto, T.; Onishi, H.; Yamamoto, N. Effect of Transcranial Direct Current Stimulation Over the Primary Motor Cortex on Cerebral Blood Flow: A Time Course Study Using Near-Infrared Spectroscopy. In Oxygen Transport to Tissue XXXVII; Springer: Berlin/Heidelberg, Germany, 2016; pp. 335–341. [Google Scholar]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage 2011, 58, 26–33. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Meesen, R.L.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity-and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef]
- Mosayebi-Samani, M.; Jamil, A.; Salvador, R.; Ruffini, G.; Haueisen, J.; Nitsche, M.A. The impact of individual electrical fields and anatomical factors on the neurophysiological outcomes of tDCS: A TMS-MEP and MRI study. Brain Stimul. 2021, 14, 316–326. [Google Scholar] [CrossRef]
- Shinde, A.B.; Lerud, K.D.; Munsch, F.; Alsop, D.C.; Schlaug, G. Effects of tDCS dose and electrode montage on regional cerebral blood flow and motor behavior. Neuroimage 2021, 237, 118144. [Google Scholar] [CrossRef]
- Sherwood, M.S.; McIntire, L.; Madaris, A.T.; Kim, K.; Ranganath, C.; McKinley, R.A. Intensity-dependent changes in quantified resting cerebral perfusion with multiple sessions of transcranial DC stimulation. Front. Hum. Neurosci. 2021, 15, 679977. [Google Scholar] [CrossRef]
- Liu, M.L.; Karabanov, A.N.; Piek, M.; Petersen, E.T.; Thielscher, A.; Siebner, H.R. Short periods of bipolar anodal TDCS induce no instantaneous dose-dependent increase in cerebral blood flow in the targeted human motor cortex. Sci. Rep. 2022, 12, 9580. [Google Scholar] [CrossRef]
- Sacca, V.; Maleki, N.; Wen, Y.; Hodges, S.; Kong, J. Modulation effects of repeated transcranial direct current stimulation at the dorsolateral prefrontal cortex: A pulsed continuous arterial spin labeling study. Brain Sci. 2023, 13, 395. [Google Scholar] [CrossRef]
- Stagg, C.J.; Lin, R.L.; Mezue, M.; Segerdahl, A.; Kong, Y.; Xie, J.; Tracey, I. Widespread Modulation of Cerebral Perfusion Induced during and after Transcranial Direct Current Stimulation Applied to the Left Dorsolateral Prefrontal Cortex. J. Neurosci. 2013, 33, 11425. [Google Scholar] [CrossRef]
- Muccio, M.; Masters, L.W.; Pilloni, G.; He, P.; Krupp, L.; Datta, A.; Bikson, M.; Charvet, L.; Ge, Y. Cerebral metabolic rate of oxygen (CMRO2) changes measured with simultaneous tDCS-MRI in healthy adults. Brain Res. 2022, 1796, 148097. [Google Scholar] [CrossRef]
- Stefano, L.H.; Favoretto, D.B.; Nascimento, D.C.; Santos, L.R.; Louzada, F.; Bikson, M.; Leite, J.P.; Pontes-Neto, O.M.; Edwards, D.J.; Edwards, T.G. Middle cerebral artery blood flow stability in response to high-definition transcranial electrical stimulation: A randomized sham-controlled clinical trial. Clin. Neurol. Neurosurg. 2022, 220, 107345. [Google Scholar] [CrossRef]
- Giorli, E.; Tognazzi, S.; Briscese, L.; Bocci, T.; Mazzatenta, A.; Priori, A.; Orlandi, G.; Del Sette, M.; Sartucci, F. Transcranial direct current stimulation and cerebral vasomotor reserve: A study in healthy subjects. J. Neuroimaging 2015, 25, 571–574. [Google Scholar] [CrossRef]
- Razza, L.B.; da Silva, P.H.R.; Busatto, G.F.; Duran, F.L.D.S.; Pereira, J.; De Smet, S.; Klein, I.; Zanão, T.A.; Luethi, M.S.; Baeken, C.; et al. Brain Perfusion Alterations Induced by Standalone and Combined Non-Invasive Brain Stimulation over the Dorsolateral Prefrontal Cortex. Biomedicines 2022, 10, 2410. [Google Scholar] [CrossRef]
- Jeong, H.; Song, I.U.; Chung, Y.A.; Kim, D.; Na, S.; Lee, S.H. Changes of regional cerebral blood flow after repeated transcranial direct current stimulation in healthy participants: A pilot study. Acta Radiol. 2023, 64, 2590–2593. [Google Scholar] [CrossRef]
- Dutta, A.; Jacob, A.; Chowdhury, S.R.; Das, A.; Nitsche, M.A. EEG-NIRS based assessment of neurovascular coupling during anodal transcranial direct current stimulation-a stroke case series. J. Med. Syst. 2015, 39, 36. [Google Scholar] [CrossRef]
- Iyer, P.C.; Rosenberg, A.; Baynard, T.; Madhavan, S. Influence of neurovascular mechanisms on response to tDCS: An exploratory study. Exp. Brain Res. 2019, 237, 2829–2840. [Google Scholar] [CrossRef]
- Klomjai, W.; Aneksan, B.; Chotik-Anuchit, S.; Jitkaew, P.; Chaichanudomsuk, K.; Piriyaprasarth, P.; Vachalathiti, R.; Nilanon, Y.; Hiengkaew, V. Effects of different montages of transcranial direct current stimulation on haemodynamic responses and motor performance in acute stroke: A randomized controlled trial. J. Rehabil. Med. 2022, 54, 3208. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Ramakrishnan, V.; Kautz, S.; George, M.S.; Adams, R.J.; Feng, W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose–Response Relationship. Brain Stimul. 2016, 9, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Martin, W.R.W.; Herscovitch, P.; Mintun, M.A.; Markham, J. Brain Blood Flow Measured with Intravenous H2(15)O.: II. Implementation and Validation. J. Nucl. Med. 1983, 24, 790–798. [Google Scholar] [PubMed]
- Fan, A.P.; Jahanian, H.; Holdsworth, S.J.; Zaharchuk, G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J. Cereb. Blood Flow Metab. 2016, 36, 842–861. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; George, M.S. Optimized APPS-tDCS electrode position, size, and distance doubles the on-target stimulation magnitude in 3000 electric field models. Sci. Rep. 2022, 12, 20116. [Google Scholar] [CrossRef]
- Caulfield, K.A.; Badran, B.W.; DeVries, W.H.; Summers, P.M.; Kofmehl, E.; Li, X.; Borckardt, J.J.; Bikson, M.; George, M.S. Transcranial electrical stimulation motor threshold can estimate individualized tDCS dosage from reverse-calculation electric-field modeling. Brain Stimul. 2020, 13, 961–969. [Google Scholar] [CrossRef]
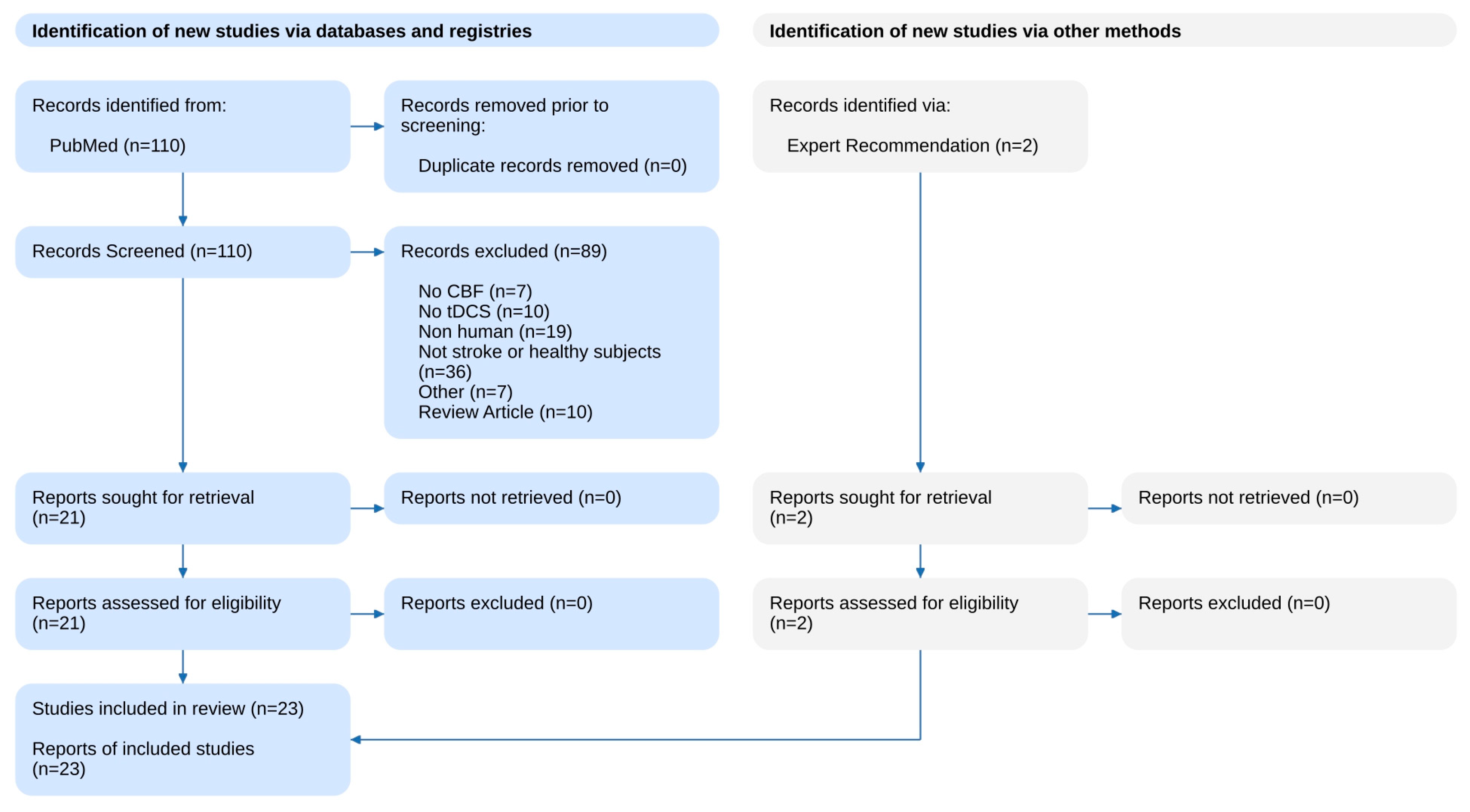
| Author | Sample Size/Population | Stimulation Method | Control | Outcome | Main Results |
|---|---|---|---|---|---|
| Studies on healthy participants | |||||
| Lang et al. (2005) [21] | 16 healthy right-handed male (mean age 36 ± 9.7) | Left M1, 1 mA, 10 min, anodal and cathodal | Sham stimulation | H215O positron emission tomography of regional CBF at rest and during finger movements | Anodal and cathodal tDCS induced widespread increases and decreases in rCBF in cortical and subcortical areas compared to sham; These changes persisted throughout the 50-minute period of PET scanning. |
| Paquette et al. (2011) [22] | 9 healthy (mean age: 28 years; 3 males) | Bilateral M1 (anodal on dominant side, cathodal on non-dominant), 2 mA, 4 min | Sham stimulation | Positron emission tomography (PET) | tDCS with active movement; changes in rCBF were significantly lower on the cathodal than the anodal side compared with sham stimulation. Bilateral tDCS stimulation can induce changes in brain activation during movement and the cathode induces stronger effects. |
| Merzagora et al. (2010) [23] | 12 healthy (mean age 29.5 ± 3.9; 6 males) | Left frontal (FP1), 1 mA, 10 min, anodal | Sham stimulation | Functional near-infrared spectroscopy (fNIRS) | Anodal tDCS significantly increased oxyhemoglobin (HbO2) concentration compared to sham stimulation; Changes lasted longer with increased stimulation time. |
| Giovanella et al. (2018) [22,24] | 20 healthy (11 males) | Left frontal lobe (AF7) 1 mA, 10 min, anodal or cathodal | Sham stimulation | Functional diffuse correlation spectroscopy (fDCS) and time-resolved functional near-infrared spectroscopy (TR-fNIRS) | CBF increased in both anodal (10%) and cathodal (11%) stimulation but not the sham stimulation; Changes were observed in the ipsilateral hemisphere only; Increase in CBF was constant during the stimulation period and lasted at least 30 min after. |
| Takai H. et al. (2016) [25] | 7 healthy (mean age: 22.8 ± 2.0) | Right M1, 1 mA, 20 min, anodal and cathodal | Sham stimulation | Diachronic intracranial hemodynamic changes using near-infrared spectroscopy | Both anodal and cathodal tDCS led to significantly lower concentrations of O2Hb in the contralateral premotor cortex, supplementary motor area, and M1, suggesting widespread changes in cerebral blood flow. |
| Zheng X. et al. (2011) [26] | 14 healthy (mean age 25.7 ± 5.7; 9 males). | Right M1, average 1.4 mA, anodal and cathodal | No | Arterial spin labeling in the MRI scanner with an alternating off–on tDCS sampling paradigm | tDCS increases rCBF and is selectively based on the polarity of the stimulation, with a secondary impact from the stimulation strength; The correlation between change in rCBF and current strength was positive in anodal stimulation and negative in cathodal stimulation; Effects were seen in network activity directly beneath stimulating electrode and between functionally connected brain regions |
| Jamil A. et al. (2020) [27] | 29 healthy (mean age 25.0 ± 4.4; 16 males) | Left M1; 0.5–1.0–1.5–2.0 mA, 15 min, anodal or cathodal | Sham stimulation | Resting-state arterial spin labeling (ASL-MRI) and compared changes to TMS-induced cortical excitability | Both anodal and cathodal tDCS showed intensity- and polarity-dependent changes in CBF compared to sham; The 2.0 mA intensity led to the greatest blood flow alterations compared to lower intensities of 0.5–1.5 mA; Changes lasted 60–75 min for 0.5–1.5 mA and entire 2 hr post-treatment evaluation period for 2.0 mA anodal tDCS; At all levels of intensity, anodal electrode led to higher changes in perfusion compared to cathode; Correlations between changes in CBF and motor-cortical excitability measured using TMS. |
| Mosayebi-Samani et al. (2021) [28] | 29 healthy right-handed participants (mean age 25.0 ± 4.44; 16 males) | Left M1; 0.5–1.0–1.5–2.0 mA, 15 min, anodal or cathodal | Sham stimulation | Functional magnetic resonance imaging (MRI) via arterial spin labeling (ASL) | Anodal tDCS increased CBF under electrode at all intensities, mostly in the early stages; Cathodal tDCS decreased CBF, except for the 0.5 mA condition in both polarity conditions, especially in the late stages. |
| Shinde A. et al. (2021) [29] | 32 healthy (mean age 34.2 ± 13.5; 15 males) | Unihemispheric (anode on C4, cathode on Fp1) and bihemispheric (anode on C4, cathode on C3) montages, 2 mA or 4 mA, 10 min | Sham stimulation | Functional magnetic resonance imaging (MRI) via arterial spin labeling (ASL) | CBF in the right hemispheric peri-rolandic area increased with dose under the anodal electrode, while showing trend to increase with dose and significant effect of montage in left hemispheric peri-rolandic area; The bihemispheric montage showed additional rCBF increases in frontomesial regions in the 4 mA condition but not in the 2 mA condition. |
| Sherwood et al. (2021) [30] | 47 healthy (mean age 27.9 ± 4.85; 38 males) | Left prefrontal cortex, 1 or 2 mA, 30 min, three days, anodal | Sham stimulation | Pseudo-continuous arterial spin labeling (pcASL) | Anodal tDCS showed increased CBF in multiple regions, persist for up to 24 h following stimulation. |
| Liu et al. (2022) [31] | 9 young healthy individuals (mean age 31.22; SD 4.55; 5 males) | Left M1, 0.5–1.0–1.5–2.0 mA, 30 s, anodal | No | Magnetic resonance imaging (MRI) combined with pseudo-continuous arterial spin labeling (pcASL MRI) in targeted left M1 hand area | pcASL MRI did not consistently show immediate effects of short-duration anodal tDCS on CBF. |
| Sacca et al. (2023) [32] | 37 healthy females (mean age 27.4 ± 6.4) | Right DLPFC, 2 mA, 20 min, 3 consecutive days, anodal and cathodal | Sham stimulation | Cerebral blood flow using pulsed continuous arterial spin labeling (pCASL) | Anodal tDCS increased CBF in bilateral thalamus, insula, lateral prefrontal cortex, midcingulate cortex, occipital lobe, and cerebellum; Increased CBF in the right insula in both the cathodal and sham tDCS groups. |
| Stagg et al. (2013) [33] | 24 healthy (mean age 26.7; 6 females) | Left DLPFC, 1 mA, 10 min, anodal or cathodal | Sham stimulation | Pseudo-continuous arterial spin labeling (pcASL) | Polarity-dependent CBF changes in regions structurally connected to left DLPFC (thalami) with anodal (increased) and cathodal (decreased) stimulation. |
| Muccico et al. (2022) [34] | 23 healthy (mean age = 35.6 ± 15; 10 males) | Left anodal dorsolateral prefrontal cortex, 2 mA, 15 min, anodal or cathodal | No | Cerebral blood flow (CBF), venous blood oxygenation (Yv), and cerebral metabolic rate of oxygen (CMRO2) | Global CBF, from bilateral internal carotid and vertebral arteries, was significantly greater during stimulation and remained elevated post-tDCS; Increased CMRO2 levels with CBF during tDCS and remained increased post-tDCS; interconnection between the neuronal and hemodynamic properties; Venous blood oxygenation levels were similar during tDCS and post-tDCS. |
| Stefano et al. (2022) [35] | 11 right-handed healthy (mean age 31 ± 5.6; 5 males) | Right temporo-parietal junction, 1, 2, and 3 mA, anodal and cathodal | Sham stimulation | Mean middle cerebral artery blood flow velocity (MCA-BFv) bilaterally using transcranial doppler ultrasound during and after stimulation | None of the tDCS stimulation showed significant changes in MCA-BFv in ipsilateral or the contralateral stimulation side. |
| Giorli et al. (2014) [32,36] | 25 healthy (mean age 26.43 ± 6.63; 19 females) | Right M1, 1 mA, 15 min, anodal or cathodal | Sham stimulation | Cerebral vasomotor reserve (VMR) by transcranial color-coded sonography | tDCS induced polarity-specific alterations in VMR; Anodal tDCS decreased mean flow velocity (MFV) in MCA, whereas cathodal tDCS increased MFV; No significant changes in sham stimulation. |
| Razza et al. (2022) [37] | 23 healthy (mean age 28.7 ± 7; 15 females) | Factorial 2 × 2 design tDCS and iTBS, 2 mA, 4 sessions (1 per week), 20 min, anodal or cathodal | Sham stimulation | Single-Photon Emission Computed Tomography (SPECT) | Polarity-dependent effect; cathodal and anodal tDCS increased and decreased DLPFC CBF, respectively. |
| Jeong et al. (2023) [38] | 5 healthy | Bifrontal tDCS over DLPFC 1 mA, 30 min, 20 sessions | No | Single-Photon Emission Computed Tomography (SPECT) | Increased right superior frontal gyrus CBF at the follow-up. |
| Studies on stroke patients | |||||
| Dutta A. et al. (2015) [39] | 4 chronic (> 6 months) ischemic stroke patients (aged 31 to 76; 3 males) | Over Cz, ON–OFF 30 s repeated 15 times, current density of 0.526 A/m2, anodal | Sham stimulation | Changes in oxyhemoglobin (HbO2) and deoxyhemoglobin (Hb) concentrations using near-infrared spectroscopy (NIRS) | Rapid alterations in regional tissue concentrations of oxygenated (HbO2) and deoxygenated (Hb) hemoglobin within the first 60 s with anodal tDCS. |
| Iyer et al. (2019) [40] | 20 chronic stroke patients (aged 45–72 years; 13 male) | M1 (lower limb), 1 mA, 15 min, anodal | Sham stimulation | Middle cerebral artery (MCA) cerebral blood velocity (CBv), cerebrovascular resistance (CVRi), and other cerebral hemodynamic-related variables using transcranial doppler | No change was found in CBv with anodal tDCS; At baseline, responders demonstrated lower corticomotor excitability, lower CBv, and higher CVRi compared to non-responders. |
| Estelle Pruvost-Robieux et al. (2021) [19] | 45 acute ischemic stroke patients in the MCA territory, NIHSS score between 4 and 25, and eligible for reperfusion therapies | Affected M1, 1.5 mA, 20-min epochs, delivered every hour over 6 h period, cathodal | Sham stimulation | Infarct growth (primary outcome), arterial recanalization | Cathodal tDCS is safe and feasible in acute MCA-territory stroke; No significant difference between active and sham groups. Potential benefits of C-tDCS in patients with NIHSS >10 or large vessel occlusion. |
| Klomjai et al. (2022) [41] | 82 eligible acute stroke participants | Ipsilesional M1, 1.5 mA, 20 min for 5 consecutive days, anodal, cathodal, bihemispheric | Sham stimulation | Cerebral mean blood flow velocity (MFV) using transcranial color-coded Doppler in each MCA | None of the groups showed significant changes in the MFV in the lesioned or non-lesioned hemispheres post-intervention or at 1 month; Significantly greater clinical improvement in bihemispheric group. |
| Bahr-Hosseini M. et al. (2023) [20] | 10 acute ischemic stroke (ineligible for reperfusion therapies) patients within 24 h from onset (7 active, 3 sham). Mean age 75 ± 10; 6 females | 6 predefined montages according to the location of the large vessel occlusion, 3 + 3 dose escalation plan, cathodal | Sham stimulation | (1) Improved perfusion (reduction in hypoperfusion region volume); (2) collateral enhancement (increase in quantified relative cerebral blood volume [qrCBV]); (3) penumbral tissue salvage (tissue at risk not progressing to infarction) at 2 to 4 h (early time point) and 24 to 30 h (late time point) | Higher rates of penumbral tissue salvage and alleviation of hypoperfused ischemic regions with active stimulation compared with sham; Enhancement of rCBV and a higher rate of early recanalization post-stimulation in active stimulation; The increase in post-stimulation qrCBV showed a dose-response trend. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunduz, M.E.; Kocahasan, M.; Keser, Z. Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review. Medicina 2024, 60, 2061. https://doi.org/10.3390/medicina60122061
Gunduz ME, Kocahasan M, Keser Z. Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review. Medicina. 2024; 60(12):2061. https://doi.org/10.3390/medicina60122061
Chicago/Turabian StyleGunduz, Muhammed Enes, Melike Kocahasan, and Zafer Keser. 2024. "Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review" Medicina 60, no. 12: 2061. https://doi.org/10.3390/medicina60122061
APA StyleGunduz, M. E., Kocahasan, M., & Keser, Z. (2024). Transcranial Direct Current Stimulation to Provide Neuroprotection and Enhance Cerebral Blood Flow in Stroke: A Comprehensive Review. Medicina, 60(12), 2061. https://doi.org/10.3390/medicina60122061






