The Saphenous Vein Graft: Can a Frog Become a Princess?
Abstract
1. Introduction
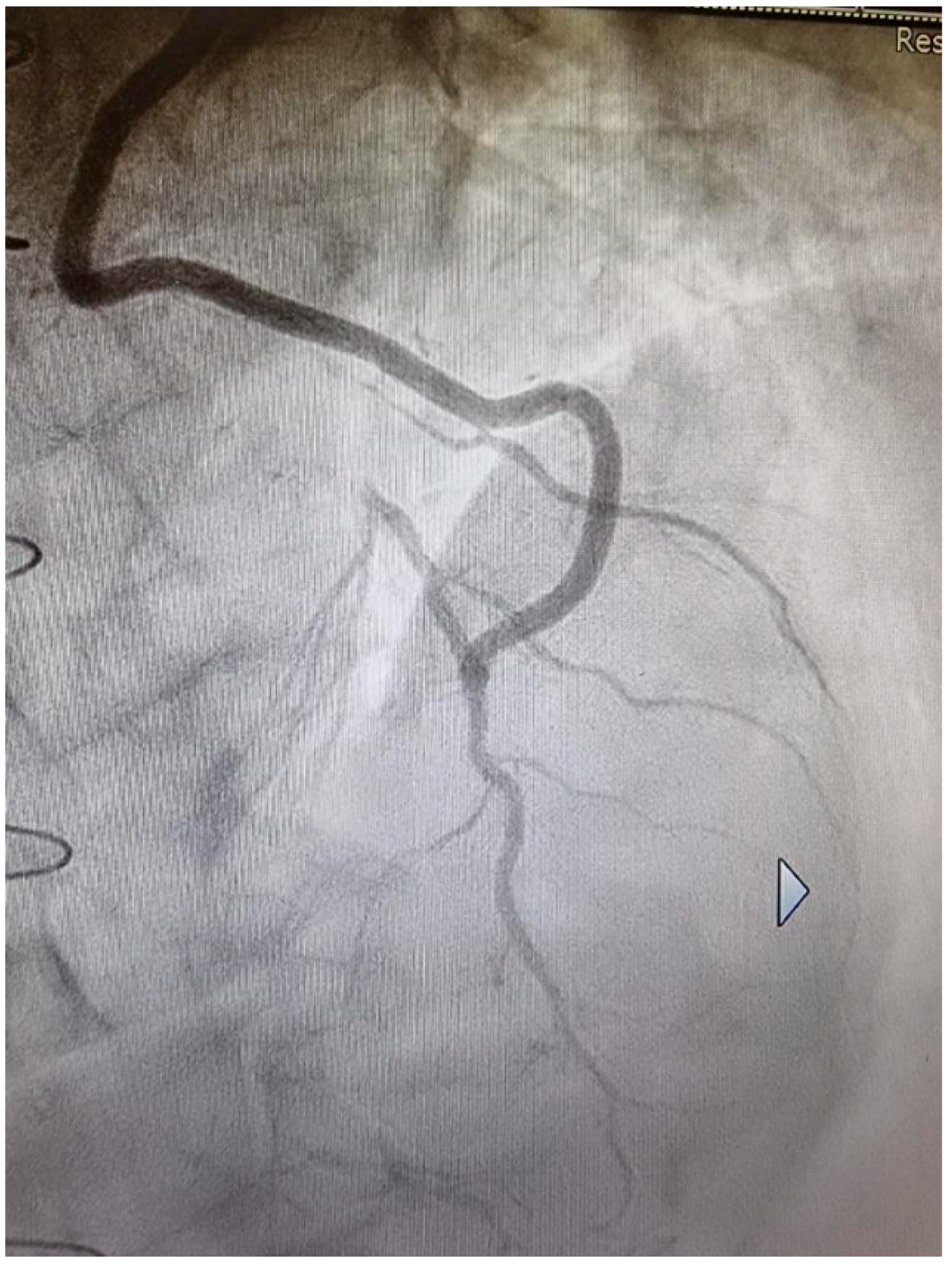
2. Surgical Strategies to Improve the Outcome of Saphenous Vein Graft
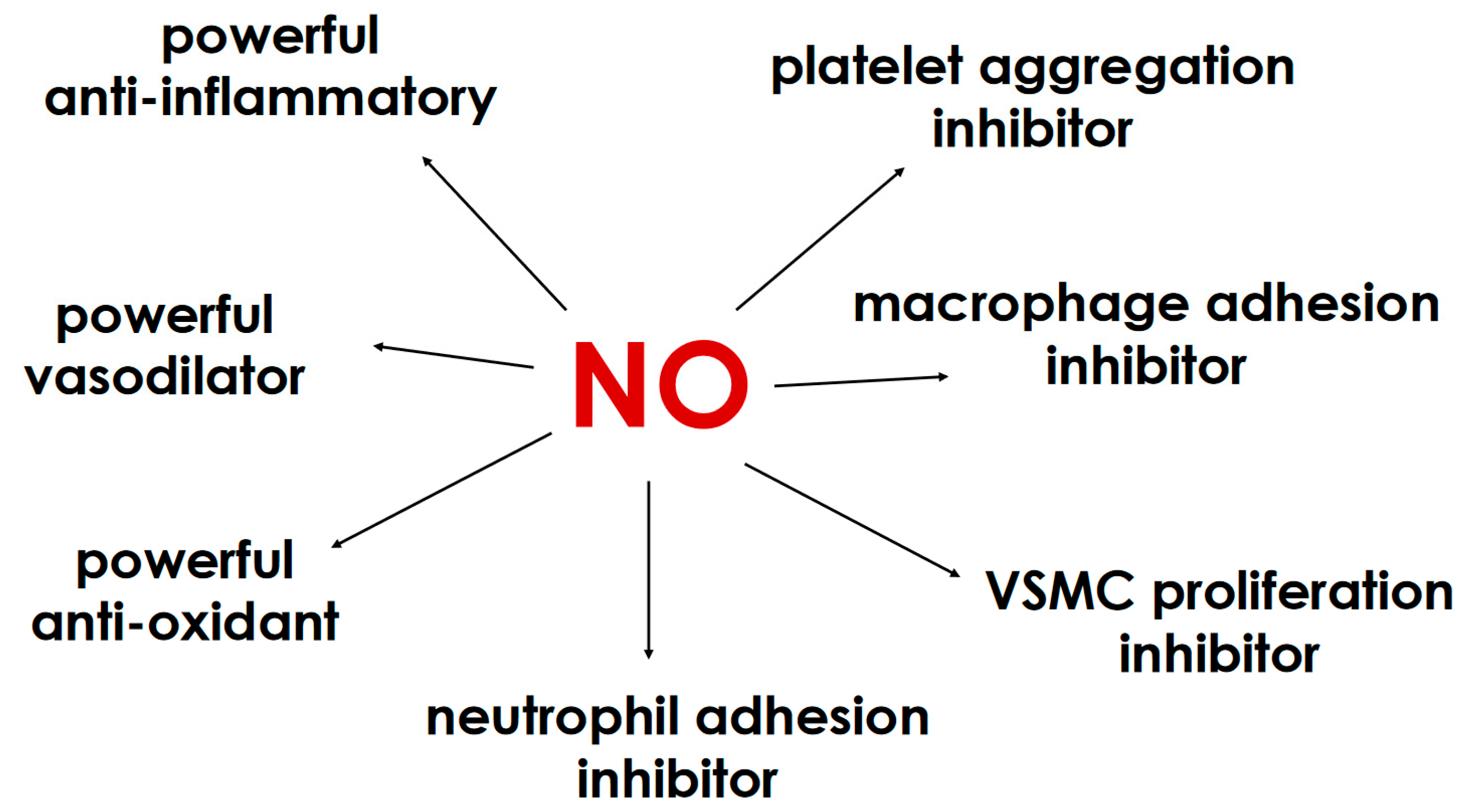
3. The Unifying Theory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FitzGibbon, G.M.; Burton, J.R.; Leach, A.J. Coronary bypass graft fate: Angiographic grading of 1400 consecutive grafts early after operation and of 1132 after one year. Circulation 1978, 57, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Lytle, B.W.; Loop, F.D.; Cosgrove, D.M.; Ratliff, N.B.; Easley, K.; Taylor, P.C. Long-term (5 to 12 years) serial studies of internal mammary artery and saphenous vein coronary bypass grafts. J. Thorac. Cardiovasc. Surg. 1985, 89, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 380, 437–446. [Google Scholar] [CrossRef]
- Souza, D. A new no-touch preparation technique: Technical notes. Scand. J. Thorac. Cardiovasc. Surg. 1996, 30, 41–44. [Google Scholar] [CrossRef]
- Dreifaldt, M.; Souza, D.S.; Loesch, A.; Muddle, J.R.; Karlsson, M.G.; Filbey, D.; Bodin, L.; Norgren, L.; Dashwood, M.R. The “no-touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J. Thorac. Cardiovasc. Surg. 2011, 141, 145–150. [Google Scholar] [CrossRef]
- Samano, N.; Souza, D.; Pinheiro, B.B.; Kopjar, T.; Dashwood, M. Twenty-Five Years of No-Touch Saphenous Vein Harvesting for Coronary Artery Bypass Grafting: Structural Observations and Impact on Graft Performance. Braz. J. Cardiovasc. Surg. 2020, 35, 91–99. [Google Scholar] [CrossRef]
- Samano, N.; Geijer, H.; Liden, M.; Fremes, S.; Bodin, L.; Souza, D. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2015, 150, 880–888. [Google Scholar] [CrossRef]
- Souza, D.S.; Johansson, B.; Bojö, L.; Karlsson, R.; Geijer, H.; Filbey, D.; Bodin, L.; Arbeus, M.; Dashwood, M.R. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: Results of a randomized longitudinal trial. J. Thorac. Cardiovasc. Surg. 2006, 132, 373–378. [Google Scholar] [CrossRef]
- Kim, K.B.; Hwang, H.Y.; Hahn, S.; Kim, J.S.; Oh, S.J. A randomized comparison of the Saphenous Vein Versus Right Internal Thoracic Artery as a Y-Composite Graft (SAVE RITA) trial: One-year angiographic results and mid-term clinical outcomes. J. Thorac. Cardiovasc. Surg. 2014, 148, 901–907; discussion 7–8. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Lee, Y.; Sohn, S.H.; Choi, J.W.; Kim, K.B. Equivalent 10-year angiographic and long-term clinical outcomes with saphenous vein composite grafts and arterial composite grafts. J. Thorac. Cardiovasc. Surg. 2021, 162, 1535–1543.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, K.B. Saphenous Vein Versus Right Internal Thoracic Artery as a Y-Composite Graft: Ten-Year Angiographic and Long-Term Clinical Results of the SAVE RITA Trial. Circulation 2021, 144, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- He, G.W.; Fan, L.; Grove, K.L.; Furnary, A.; Yang, Q. Expression and function of endothelial nitric oxide synthase messenger RNA and protein are higher in internal mammary than in radial arteries. Ann. Thorac. Surg. 2011, 92, 845–850. [Google Scholar] [CrossRef]
- Aksungar, F.B.; Moini, H.; Unal, M.; Yilmaz, O.; Sonmez, B.; Bilsel, S. Nitric oxide, endothelin-1, and superoxide production in arterial bypass grafts. Tex. Heart Inst. J. 2006, 33, 294–299. [Google Scholar]
- Saito, T.; Kurazumi, H.; Suzuki, R.; Matsunaga, K.; Tsubone, S.; Lv, B.; Kobayashi, S.; Nagase, T.; Mizoguchi, T.; Samura, M.; et al. Perivascular Adipose Tissue Is a Major Source of Nitric Oxide in Saphenous Vein Grafts Harvested via the No-Touch Technique. J. Am. Heart Assoc. 2022, 11, e020637. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Endothelial Nitric Oxide Synthase in the Perivascular Adipose Tissue. Biomedicines 2022, 10, 1754. [Google Scholar] [CrossRef]
- Prapas, S.; Katsavrias, K.; Gaudino, M.; Puskas, J.D.; Di Mauro, M.; Zografos, P.; Guarracini, S.; Linardakis, I.; Panagiotopoulos, I.; Di Marco, M.; et al. Saphenous vein to the right coronary system from the right thoracic artery or the aorta. LONG term propensity matched results of two groups. Eur. J. Cardiothorac. Surg. 2024, 65, ezae060. [Google Scholar] [CrossRef]
- Tarr, F.I.; Sasvari, M.; Tarr, M.; Racz, R. Evidence of nitric oxide produced by the internal mammary artery graft in venous drainage of the recipient coronary artery. Ann. Thorac. Surg. 2005, 80, 1728–1731. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Koo, B.K.; Yeom, S.Y.; Kim, T.K.; Kim, K.B. Endothelial Shear Stress of the Saphenous Vein Composite Graft Based on the Internal Thoracic Artery. Ann. Thorac. Surg. 2018, 105, 564–571. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Koo, B.K.; Oh, S.J.; Kim, K.B. Morphologic changes of the saphenous vein Y-composite graft based on the left internal thoracic artery: 1-year intravascular ultrasound study. J. Thorac. Cardiovasc. Surg. 2015, 149, 487–493. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calafiore, A.M.; Prapas, S.; Condello, I.; Katsavrias, K.; Nasso, G.; Gaudino, M. The Saphenous Vein Graft: Can a Frog Become a Princess? Medicina 2024, 60, 1915. https://doi.org/10.3390/medicina60121915
Calafiore AM, Prapas S, Condello I, Katsavrias K, Nasso G, Gaudino M. The Saphenous Vein Graft: Can a Frog Become a Princess? Medicina. 2024; 60(12):1915. https://doi.org/10.3390/medicina60121915
Chicago/Turabian StyleCalafiore, Antonio Maria, Sotirios Prapas, Ignazio Condello, Konstantinos Katsavrias, Giuseppe Nasso, and Mario Gaudino. 2024. "The Saphenous Vein Graft: Can a Frog Become a Princess?" Medicina 60, no. 12: 1915. https://doi.org/10.3390/medicina60121915
APA StyleCalafiore, A. M., Prapas, S., Condello, I., Katsavrias, K., Nasso, G., & Gaudino, M. (2024). The Saphenous Vein Graft: Can a Frog Become a Princess? Medicina, 60(12), 1915. https://doi.org/10.3390/medicina60121915







