The MARVIN Hypothesis: Linking Unhealthy Lifestyles to Intracranial Aneurysm Rupture Risk and Clinical Prognosis
Abstract
1. Introduction
2. Materials and Methods
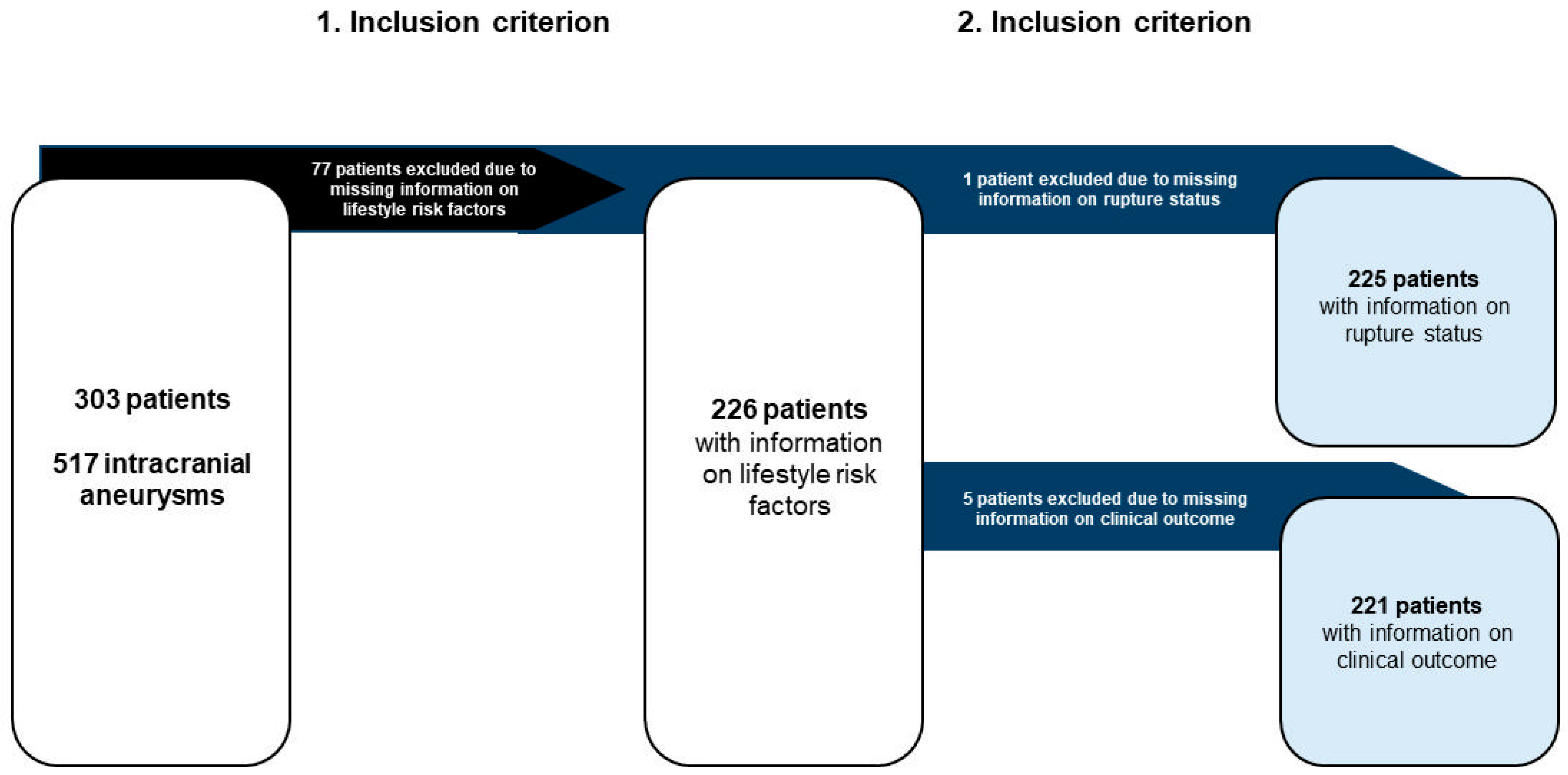
2.1. Data Acquisition
2.2. Statistical Analysis
3. Results
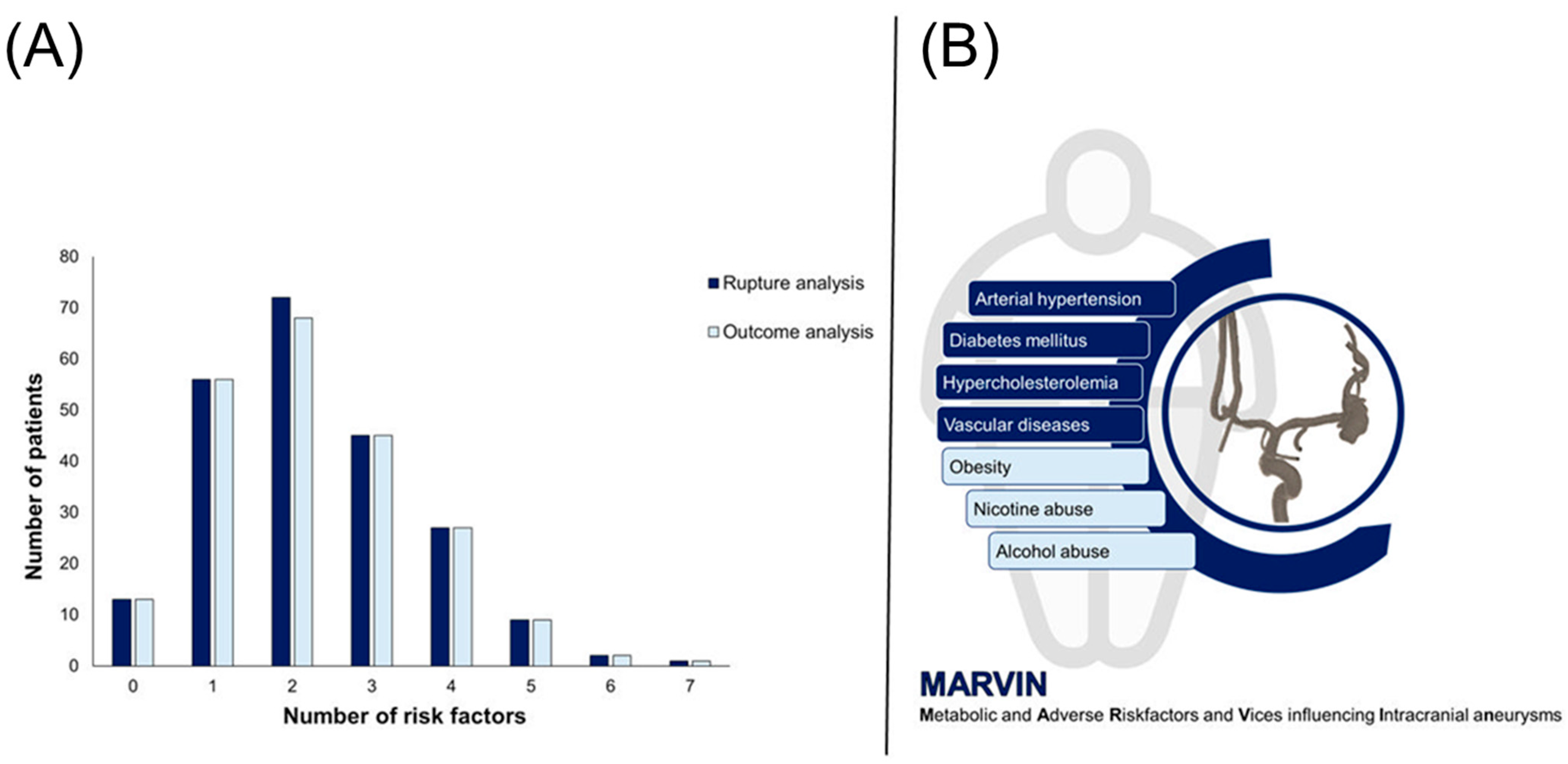
3.1. Cohort Overview
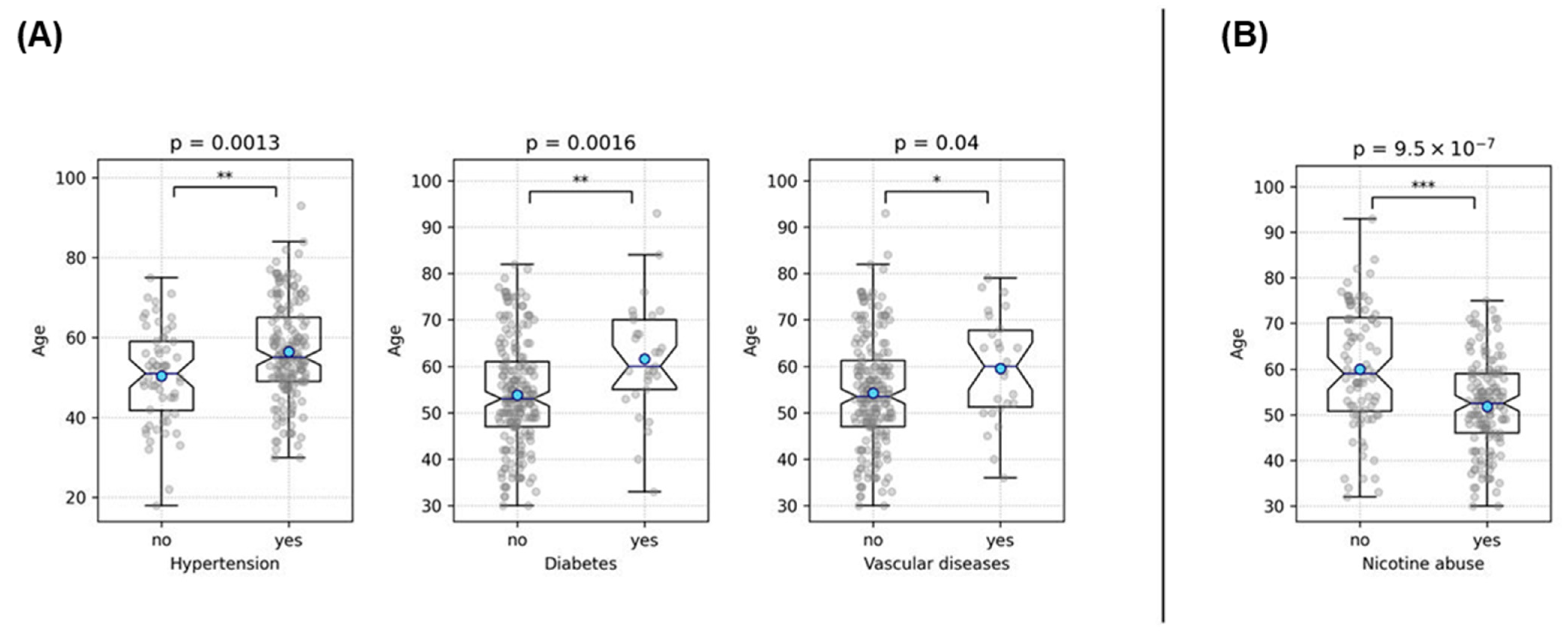
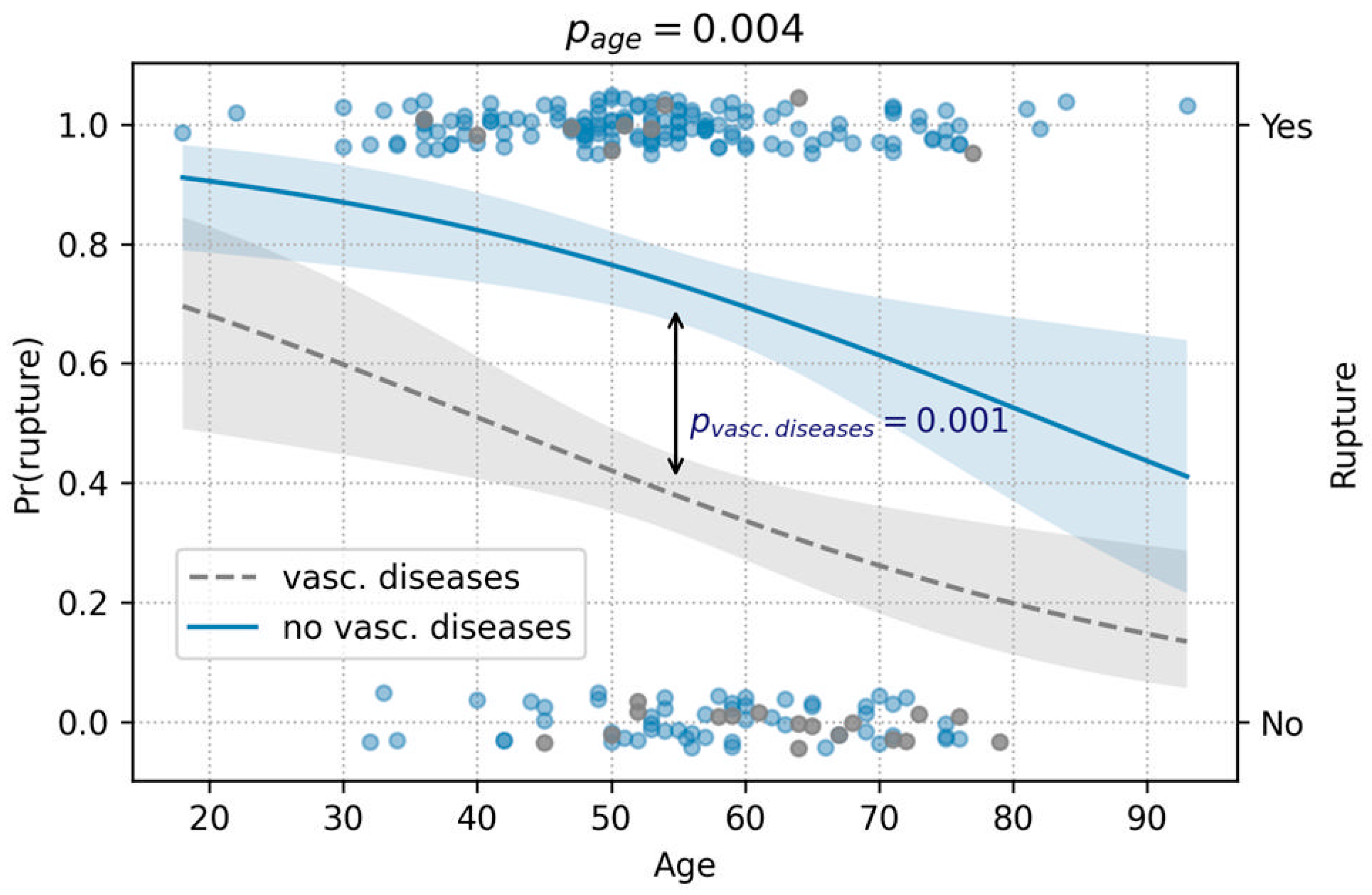
3.1.1. Rupture Analysis
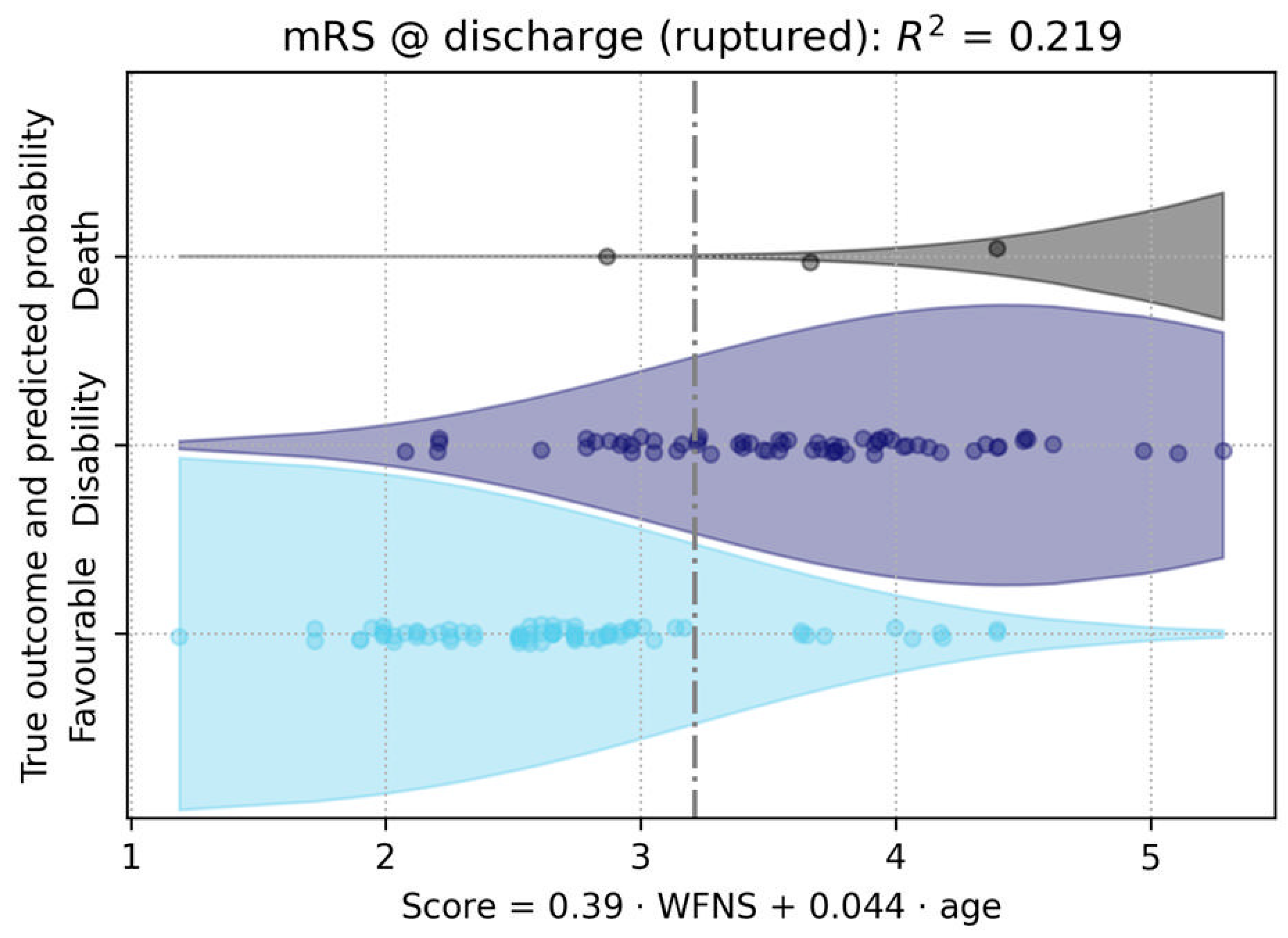
3.1.2. Outcome Analysis
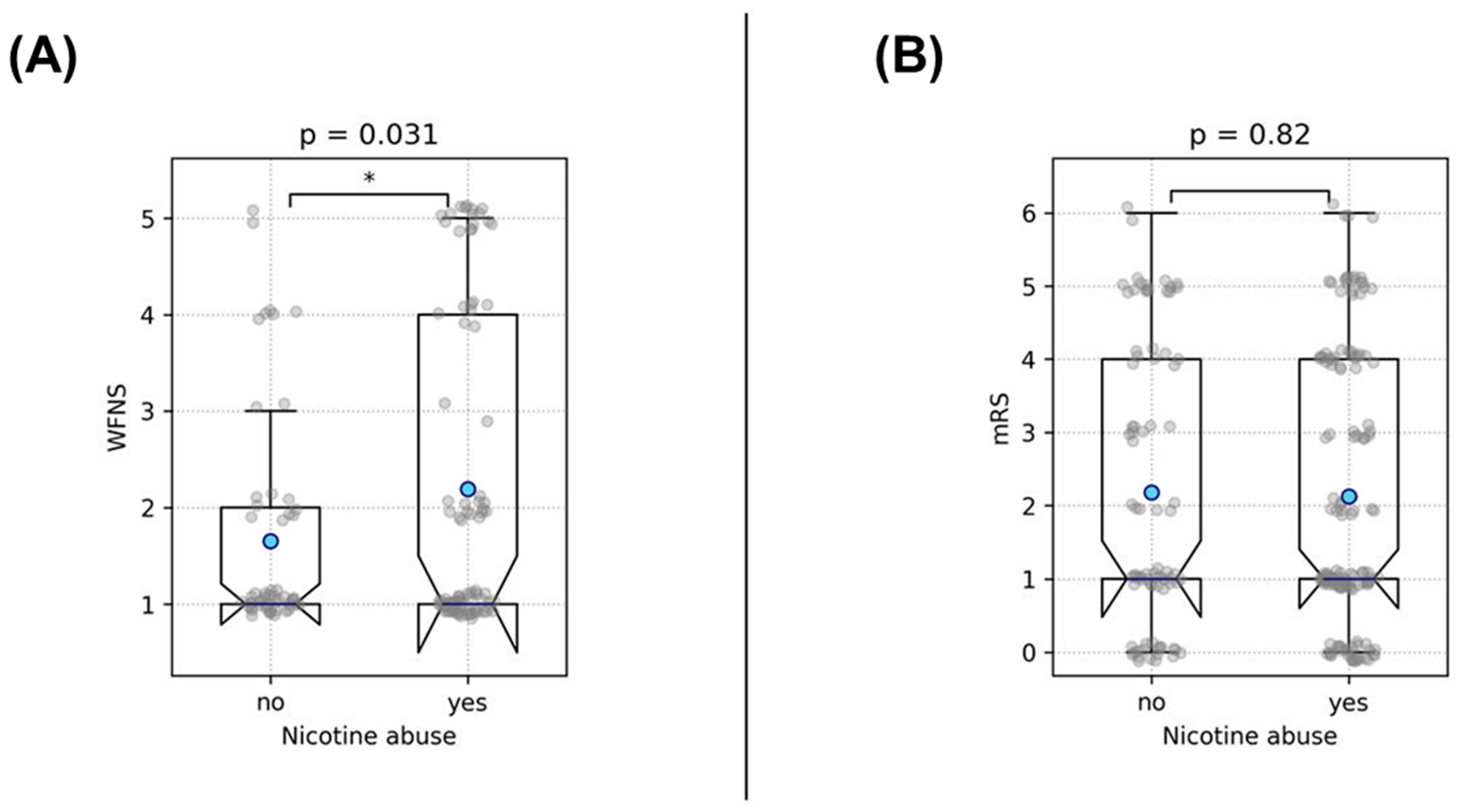
3.2. Analysis of Clinical Outcome and Rupture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, J.D.; Bond, K.M.; Mekary, R.A.; Dewan, M.C.; Rattani, A.; Baticulon, R.; Kato, Y.; Azevedo-Filho, H.; Morcos, J.J.; Park, K.B. Estimating the Global Incidence of Aneurysmal Subarachnoid Hemorrhage: A Systematic Review for Central Nervous System Vascular Lesions and Meta-Analysis of Ruptured Aneurysms. World Neurosurg. 2018, 115, 430–447.e7. [Google Scholar] [CrossRef] [PubMed]
- Ingall, T.; Asplund, K.; Mähönen, M.; Bonita, R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000, 31, 1054–1061. [Google Scholar] [CrossRef]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef]
- Etminan, N.; Brown, R.D.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; Torner, J.C.; Derdeyn, C.P.; Raabe, A.; Mocco, J.; et al. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology 2015, 85, 881–889. [Google Scholar] [CrossRef]
- Alleyne, C.H. Aneurysmal subarachnoid hemorrhage: Have outcomes really improved? Neurology 2010, 74, 1486–1487. [Google Scholar] [CrossRef]
- Petridis, A.K.; Kamp, M.A.; Cornelius, J.F.; Beez, T.; Beseoglu, K.; Turowski, B.; Steiger, H.-J. Aneurysmal Subarachnoid Hemorrhage. Dtsch. Aerzteblatt Online 2017, 114, 226–236. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of Hemodynamic Characteristics and Cerebral Aneurysm Rupture. Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wallin, A.; Wolk, A.; Markus, H.S. Differing association of alcohol consumption with different stroke types: A systematic review and meta-analysis. BMC Med. 2016, 14, 178. [Google Scholar] [CrossRef]
- Karhunen, V.; Bakker, M.K.; Ruigrok, Y.M.; Gill, D.; Larsson, S.C. Modifiable Risk Factors for Intracranial Aneurysm and Aneurysmal Subarachnoid Hemorrhage: A Mendelian Randomization Study. J. Am. Heart Assoc. 2021, 10, e022277. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.; Söderholm, M.; Söderberg, S.; Alfredsson, L.; Andersson, M.; Bellocco, R.; Björck, M.; Broberg, P.; Eriksson, M.; Forsberg, B.; et al. Risk factors for subarachnoid haemorrhage: A nationwide cohort of 950 000 adults. Int. J. Epidemiol. 2019, 48, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Rinkel, G.J.E.; Lawes, C.M.M.; Algra, A.; Bennett, D.A.; van Gijn, J.; Anderson, C.S. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke 2005, 36, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Greving, J.P.; Wermer, M.J.H.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- Kroll, M.E.; Green, J.; Beral, V.; Sudlow, C.L.M.; Brown, A.; Kirichek, O.; Price, A.; Yang, T.O.; Reeves, G.K.; For the Million Women Study Collaborators. Adiposity and ischemic and hemorrhagic stroke: Prospective study in women and meta-analysis. Neurology 2016, 87, 1473–1481. [Google Scholar] [CrossRef]
- Can, A.; Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; Cai, T.; et al. Lipid-Lowering Agents and High HDL (High-Density Lipoprotein) Are Inversely Associated with Intracranial Aneurysm Rupture. Stroke 2018, 49, 1148–1154. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Thefeld, W. Verbreitung der Herz-Kreislauf-Risikofaktoren Hypercholesterinämie, Übergewicht, Hypertonie und Rauchen in der Bevölkerung. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2000, 43, 415–423. [Google Scholar] [CrossRef]
- Jabbarli, R.; Dinger, T.F.; Oppong, M.D.; Pierscianek, D.; Dammann, P.; Wrede, K.H.; Kaier, K.; Köhrmann, M.; Forsting, M.; Kleinschnitz, C.; et al. Risk Factors for and Clinical Consequences of Multiple Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Stroke 2018, 49, 848–855. [Google Scholar] [CrossRef]
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 1988, 68, 985–986. [CrossRef]
- Fisher, C.M.; Kistler, J.P.; Davis, J.M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S. Treatment Scoring of Unruptured Intracranial Aneurysms. Stroke 2019, 50, 2344–2350. [Google Scholar] [CrossRef]
- Juvela, S. PHASES score and treatment scoring with cigarette smoking in the long-term prediction of rupturing of unruptured intracranial aneurysms. J. Neurosurg. 2022, 136, 156–162. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Grundy, S.M.; Pasternak, R.; Greenland, P.; Smith, S.; Fuster, V. Assessment of Cardiovascular Risk by Use of Multiple-Risk-Factor Assessment Equations: A Statement for Healthcare Professionals from the American Heart Association and the American College of Cardiology. Circulation 1999, 100, 1481–1492. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Xanthakis, V.; Enserro, D.M.; Murabito, J.M.; Polak, J.F.; Wollert, K.C.; Januzzi, J.L.; Wang, T.J.; Tofler, G.; Vasan, R.S. Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014, 130, 1676–1683. [Google Scholar] [CrossRef]
- Spring, B.; Moller, A.C.; Colangelo, L.A.; Siddique, J.; Roehrig, M.; Daviglus, M.L.; Polak, J.F.; Reis, J.P.; Sidney, S.; Liu, K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 2014, 130, 10–17. [Google Scholar] [CrossRef]
- Yang, Q.; Cogswell, M.E.; Flanders, W.D.; Hong, Y.; Zhang, Z.; Loustalot, F.; Gillespie, C.; Merritt, R.; Hu, F.B. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 2012, 307, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Jiang, M.; Fan, Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: A meta-analysis. Int. J. Cardiol. 2016, 214, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Enserro, D.M.; Vasan, R.S.; Xanthakis, V. Cardiovascular Health Status and Incidence of Heart Failure in the Framingham Offspring Study. Circ. Heart Fail. 2016, 9, e002416. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Rupture Analysis (n = 225) | Outcome Analysis (n = 221) |
|---|---|---|
| Sex | 75 male; 150 female | 76 male; 145 female |
| Age at diagnosis (mean) | 54.8 (±12.5) years | 54.9 (±12.5) years |
| Arterial hypertension | 165 (73.3%) | 163 (73.8%) |
| Diabetes mellitus Type 2 | 29 (13%) | 29 (13.1%) |
| Hypercholesterolemia | 45 (20%) | 45 (20.4%) |
| Vascular diseases | 26 (11.6%) | 26 (11.8%) |
| Nicotine abuse | 141 (62.7%) | 138 (62.4%) |
| Alcohol abuse | 36 (16%) | 36 (16.3%) |
| Obesity | 67 (30%) | 64 (29.0%) |
| Ruptured aneurysms | 154 (68.4%) | 152 (68.8%) |
| Multiple aneurysms | 88 (39.1%) | 87 (39.4%) |
| Aneurysm size (mm, mean) | 7.4 (±3.0) | 7.4 (±3.0) |
| Aneurysm localization | ||
| ACA | 2 (0.9%) | 2 (0.8%) |
| ACOM | 105 (46.7%) | 103 (46.6%) |
| Pericallosal artery | 2 (0.9%) | 2 (0.8%) |
| MCA | 45 (20%) | 45 (20.4%) |
| ICA | 28 (12.4%) | 27 (12.2%) |
| PCOM | 3 (1.3%) | 3 (1.4%) |
| Anterior choroidal artery | 2 (0.9%) | 2 (0.8%) |
| Basilar artery | 25 (11.1%) | 24 (10.9%) |
| Vertebral artery | 3 (1.3%) | 3 (1.4%) |
| PICA | 7 (3.1%) | 7 (3.2%) |
| SCA | 3 (1.3%) | 3 (1.4%) |
| WFNS score | ||
| 1 | 86 (55.8%) | 86 (56.6%) |
| 2 | 23 (14.9%) | 23 (15.1%) |
| 3 | 4 (2.6%) | 4 (2.6%) |
| 4 | 21 (13.6%) | 21 (14.8%) |
| 5 | 18 (11.7%) | 18 (11.8%) |
| No information | 2 (1.3%) | 0 (0%) |
| Fisher grade | ||
| 1 | 4 (2.6%) | 4 (2.6%) |
| 2 | 5 (3.3%) | 5 (3.3%) |
| 3 | 55 (35.7%) | 54 (35.5%) |
| 4 | 89 (55.8%) | 89 (56.6%) |
| No information | 1 (0.7%) | 0 (0%) |
| Variable 1 | Variable 2 | p-Value | Odds Ratio |
|---|---|---|---|
| Hypertension | Nicotine abuse | 0.034 | 0.5 |
| Hypertension | Hypercholesterolemia | 0.001 | 6.6 |
| Hypertension | Obesity | 0.002 | 3.6 |
| Hypertension | Vascular diseases | 0.011 | 10.5 |
| Hypertension | Diabetes | 0.059 | 3.5 |
| Nicotine abuse | Alcohol abuse | 0.003 | 4.4 |
| Hypercholesterolemia | Vascular diseases | 1.86 × 10−6 | 7.4 |
| Obesity | Diabetes | 0.033 | 2.5 |
| Variable | Univariate Analysis (p-Value) | Multivariate Analysis (p-Value) |
|---|---|---|
| Age | 0.003 | <10⁻⁵ |
| Sex | 0.6 | - |
| WFNS Score | <0.001 | <10⁻⁶ |
| Hypertension | 0.6 | - |
| Nicotine abuse | 0.8 | - |
| Diabetes | 0.1 | - |
| Hypercholesterolemia | 0.4 | - |
| Obesity | 0.1 | - |
| Alcohol abuse | 0.7 | - |
| Vascular diseases | 0.5 | - |
| Variable | Univariate Analysis (p-Value) | Multivariate Analysis (p-Value) |
|---|---|---|
| Age | 0.001 | 0.004 |
| Sex | 0.3 | - |
| Hypertension | 0.5 | - |
| Nicotine abuse | 0.3 | - |
| Diabetes | 0.7 | - |
| Hypercholesterolemia | 0.09 | - |
| Obesity | 1 | - |
| Alcohol abuse | 0.9 | - |
| Vascular diseases | 0.0002 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiatek, V.M.; Fischer, I.; Khajuria, R.; Amini, A.; Steinkusch, H.; Rashidi, A.; Stein, K.-P.; Dumitru, C.A.; Sandalcioglu, I.E.; Neyazi, B. The MARVIN Hypothesis: Linking Unhealthy Lifestyles to Intracranial Aneurysm Rupture Risk and Clinical Prognosis. Medicina 2024, 60, 1813. https://doi.org/10.3390/medicina60111813
Swiatek VM, Fischer I, Khajuria R, Amini A, Steinkusch H, Rashidi A, Stein K-P, Dumitru CA, Sandalcioglu IE, Neyazi B. The MARVIN Hypothesis: Linking Unhealthy Lifestyles to Intracranial Aneurysm Rupture Risk and Clinical Prognosis. Medicina. 2024; 60(11):1813. https://doi.org/10.3390/medicina60111813
Chicago/Turabian StyleSwiatek, Vanessa M., Igor Fischer, Rajiv Khajuria, Amir Amini, Hannah Steinkusch, Ali Rashidi, Klaus-Peter Stein, Claudia A. Dumitru, I. Erol Sandalcioglu, and Belal Neyazi. 2024. "The MARVIN Hypothesis: Linking Unhealthy Lifestyles to Intracranial Aneurysm Rupture Risk and Clinical Prognosis" Medicina 60, no. 11: 1813. https://doi.org/10.3390/medicina60111813
APA StyleSwiatek, V. M., Fischer, I., Khajuria, R., Amini, A., Steinkusch, H., Rashidi, A., Stein, K.-P., Dumitru, C. A., Sandalcioglu, I. E., & Neyazi, B. (2024). The MARVIN Hypothesis: Linking Unhealthy Lifestyles to Intracranial Aneurysm Rupture Risk and Clinical Prognosis. Medicina, 60(11), 1813. https://doi.org/10.3390/medicina60111813






