Multisystem Inflammatory Syndrome in Children (MIS-C) in a Lithuanian Paediatric Tertiary Care Center
Abstract
1. Introduction
2. Materials and Methods
- -
- basic characteristics (age, gender, race, height, weight, date of hospitalization);
- -
- epidemiological data (known previous COVID-19 infection, vaccination status against SARS-CoV-2);
- -
- clinical data (gastrointestinal, mucocutaneous, cardiovascular, respiratory, renal, neurological and other symptoms);
- -
- comorbidities;
- -
- laboratory data (SARS-CoV-2 RT-PCR or serology, full blood count (FBC), C-reactive protein (CRP), procalcitonin (PCT), interleukin 6 (IL-6), ferritin, troponin I, B-type natriuretic peptide (BNP), D-Dimer, albumin and estimated glomerular filtration rate (eGFR, which was based on the revised Schwartz equation [20]);
- -
- echocardiographic initial findings and early outcome on discharge (left ventricular ejection fraction (LVEF), coronary artery involvement, valvular regurgitation, pericardial effusion);
- -
- electrocardiographic (ECG) findings (conduction abnormalities such as atrioventricular (AV) block, bundle branch blocks; tachyarrhythmias, including premature beats; significant repolarization abnormalities, including ST deviation > 1 mm, negative T waves at I, II, aVF, V5-6; QTc prolongation);
- -
- treatment (oxygen therapy, vasoactive drugs, intravenous immunoglobulin (IVIG), glucocorticoids, other immunomodulators);
- -
- outcome data (duration of hospitalization, admissions to ICU, recovered/deceased).
3. Results
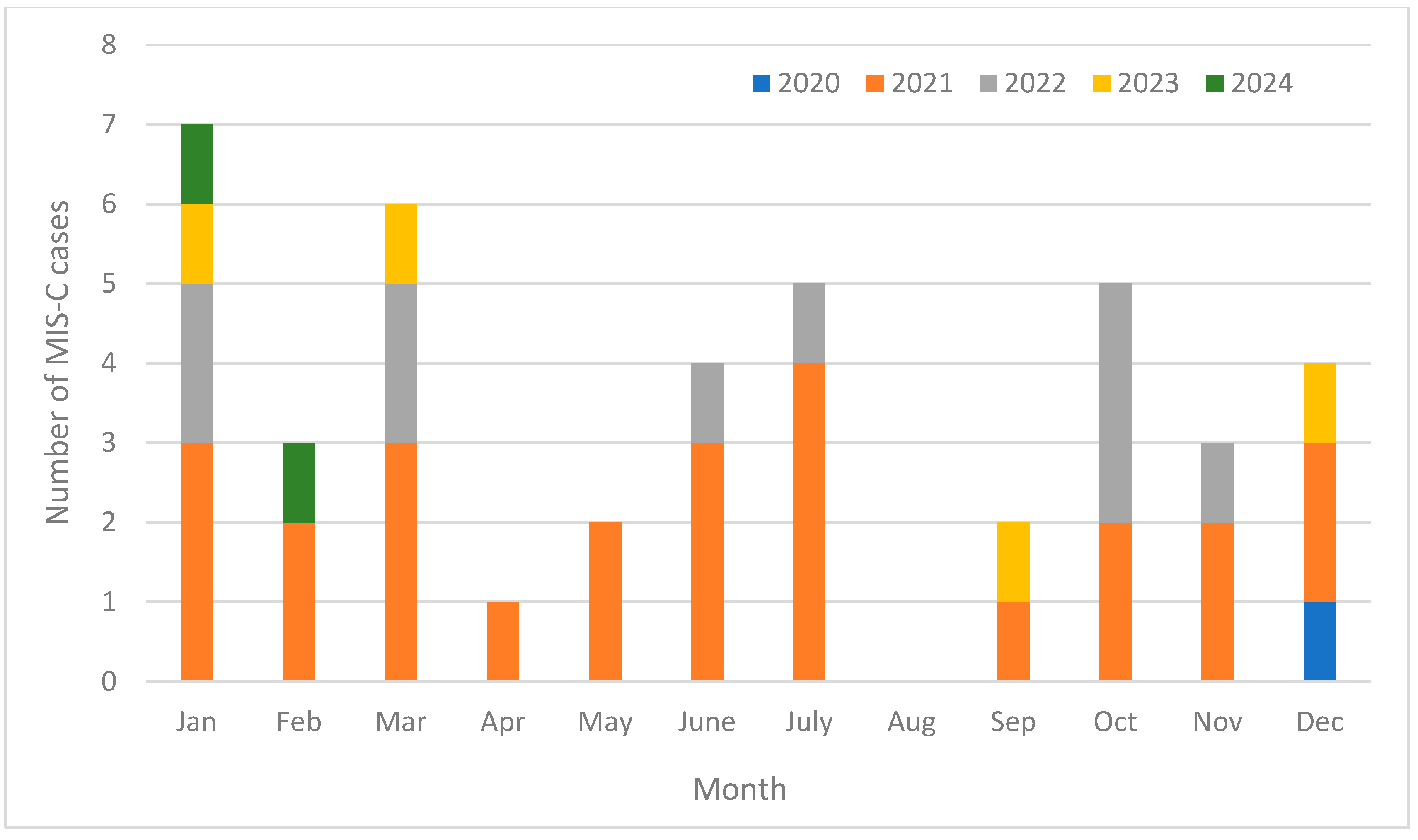
3.1. Study Sample
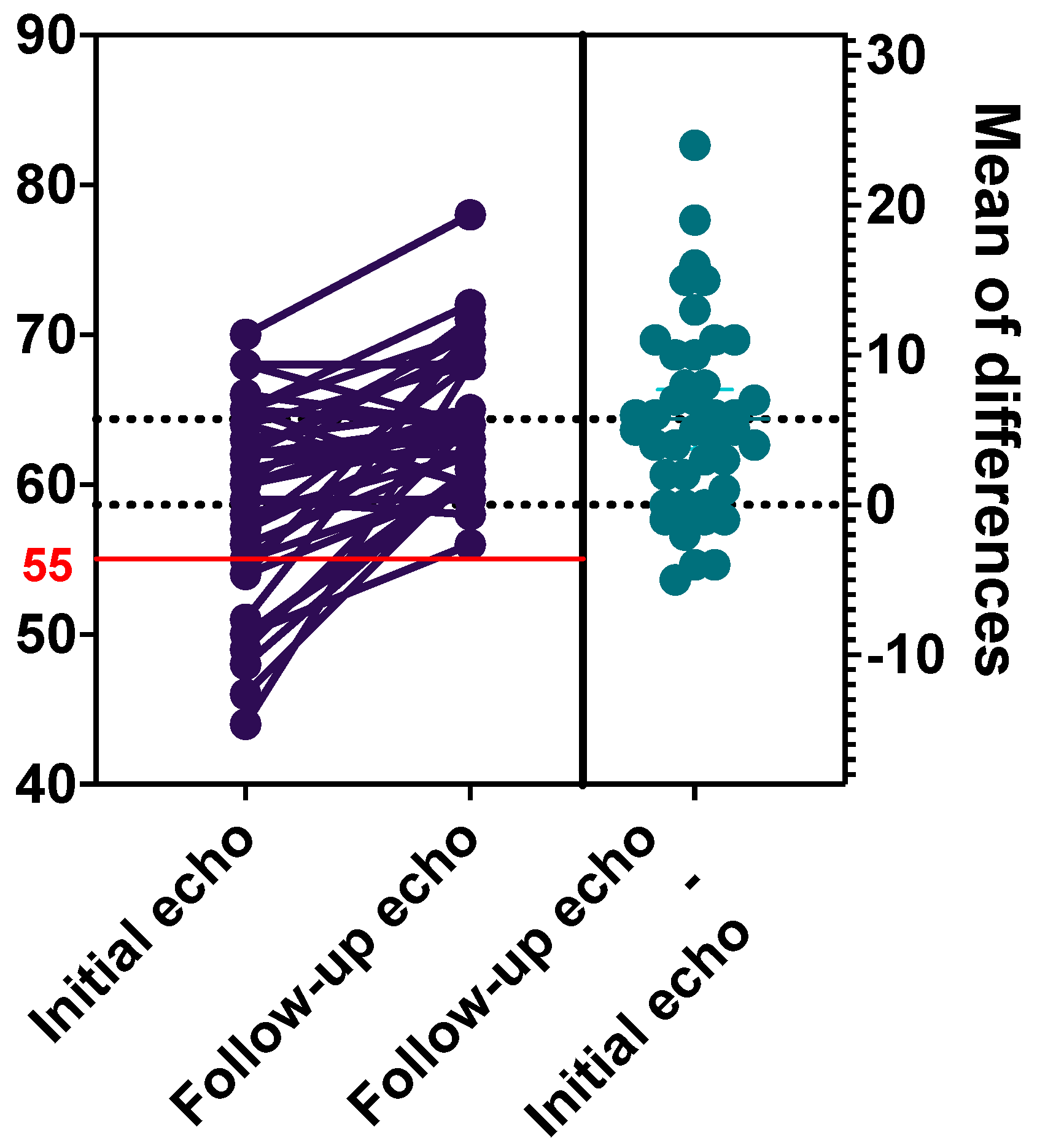
3.2. Echocardiography Findings
3.3. Treatment and Outcome Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 22 August 2024).
- Molloy, M.J.; Auger, K.A.; Hall, M.; Shah, S.S.; Schondelmeyer, A.C.; Parikh, K.; Kazmier, K.M.; Katragadda, H.; Jacob, S.A.; Jerardi, K.E.; et al. Epidemiology and Severity of Illness of MIS-C and Kawasaki Disease During the COVID-19 Pandemic. Pediatrics 2023, 152, e2023062101. [Google Scholar] [CrossRef] [PubMed]
- Higienos Instituto Duomenys, Paskaičiuoti iš Privalomojo Sveikatos Draudimo Informacinės Sistemos. Available online: https://stat.hi.lt/ (accessed on 16 August 2024).
- World Health Organization (WHO). Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 26 August 2024).
- Centers for Disease Control and Prevention (CDC). Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 Infection 2023 Case Definition. Available online: https://ndc.services.cdc.gov/case-definitions/multisystem-inflammatory-syndrome-in-children-mis-c-2023/ (accessed on 26 August 2024).
- Alvarado-Gamarra, G.; del Aguila, O.; Dominguez-Rojas, J.; Chonlon-Murillo, K.; Atamari-Anahui, N.; Borcic, A.; Sánchez, S.; Huamani-Echaccaya, P.; Garcés-Ghilardi, R.; Estupiftan-Vigil, M.; et al. Clinical phenotypes of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Andes Pediatr. 2022, 93, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.; Balajthy, A.; Biró, E.; Bíró, B.; Reiger, Z.; Szikszay, E.; Mogyorósy, G.; Káposzta, R.; Szabó, T. Multicolored MIS-C, a single-centre cohort study. BMC Pediatr. 2023, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Ali, H.; Amjad, F.; Hussain, M.Z.H.; Rahman, A.R.; Khan, M.H.; Padhani, Z.A.; Abbas, F.; Imam, D.; Alikhan, Z.; et al. Clinical presentation, diagnosis and management of multisystem inflammatory syndrome in children (MIS-C): A systematic review. BMJ Paediatr. Open 2024, 8, e002344. [Google Scholar] [CrossRef]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef]
- Uka, A.; Bressieux-Degueldre, S.; Buettcher, M.; Kottanattu, L.; Plebani, M.; Niederer-Loher, A.; Schöbi, N.; Hofer, M.; Tomasini, J.; Trück, J.; et al. Cardiac involvement in children with paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Data from a prospective nationwide surveillance study. Swiss Med. Wkly. 2023, 153, 40092. [Google Scholar] [CrossRef]
- Ludwikowska, K.M.; Moksud, N.; Tracewski, P.; Sokolski, M.; Szenborn, L. Cardiac Involvement in Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) in Poland. Biomedicines 2023, 11, 1251. [Google Scholar] [CrossRef]
- Friedman, K.G.; Harrild, D.M.; Newburger, J.W. Cardiac Dysfunction in Multisystem Inflammatory Syndrome in Children. J. Am. Coll. Cardiol. 2020, 76, 1962–1964. [Google Scholar] [CrossRef]
- Rhedin, S.; Lundholm, C.; Horne, A.; Smew, A.I.; Osvald, E.C.; Haddadi, A.; Alfvén, T.; Kahn, R.; Król, P.; Brew, B.H.; et al. Risk factors for multisystem inflammatory syndrome in children—A population-based cohort study of over 2 million children. Lancet Reg. Health Eur. 2022, 19, 100443. [Google Scholar] [CrossRef]
- Kapoor, R.; Chandra, T.; Singh, C.P.; Singh, R.; Pandey, I. Multisystem Inflammatory Syndrome in Children (MIS-C) Related to SARS-CoV-2 and 1-Year Follow-up. Indian. J. Pediatr. 2023, 90, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Oster, M.E.; Godfred-Cato, S.E.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; Tsang, C.A.; Pierce, T.J.; Kennedy, J.L.; et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child. Adolesc. Health 2021, 5, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sarmiento, J.; Acevedo, L.; Niño-Serna, L.F.; Boza, R.; García-Silva, J.; Yock-Corrales, A.; Yamazaki-Nakashimada, M.A.; Faugier-Fuentes, E.; Del Águila, O.; Camacho-Moreno, G.; et al. Risk Factors Associated with Intensive Care Admission in Children with Severe Acute Respiratory Syndrome Coronavirus 2-Related Multisystem Inflammatory Syndrome (MIS-C) in Latin America: A Multicenter Observational Study of the REKAMLATINA Network. J. Intensive Care Med. 2024, 39, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.M.; Pham, D.V.; Cao, T.V.; Hoang, C.N.; Nguyen, H.T.T.; Nguyen, G.D.; Le, C.N.; Thieu, Q.Q.; Ta, T.A.; Dau, H.V.; et al. Severity predictors for multisystemic inflammatory syndrome in children after SARS-CoV-2 infection in Vietnam. Sci. Rep. 2024, 14, 15810. [Google Scholar] [CrossRef] [PubMed]
- Lietuvos sveikatos mokslų universiteto Medicinos akademijos Vaikų ligų klinika, Vilniaus universiteto Medicinos fakulteto Klinikinės medicinos instituto Vaikų ligų klinika. Vaikų COVID-19 infekcija: Diagnostikos ir gydymo metodinės rekomendacijos. Kaunas. 2022, pp. 1–38. Available online: https://sam.lrv.lt/uploads/sam/documents/files/2022%20Vaiku%20COVID-19%20infekcija%20-%20diag-ir-gyd-metod-rekom_Uzs79%20--%20eISBN%20(lock).pdf (accessed on 26 August 2024).
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Ptak, K.; Szymońska, I.; Olchawa-Czech, A.; Kukla, K.; Cisowska, M.; Kwinta, P. Comparison of the course of multisystem inflammatory syndrome in children during different pandemic waves. Eur. J. Pediatr. 2023, 182, 1647–1656. [Google Scholar] [CrossRef]
- Yousaf, A.R.; Lindsey, K.N.; Wu, M.J.; Shah, A.B.; Free, R.J.; Simeone, R.M.; Zambrano, L.D.; Campbell, A.P. Notes from the Field: Surveillance for Multisystem Inflammatory Syndrome in Children—United States, 2023. MMWR Morb Mortal Wkly Rep 2024, 73, 225–228. [Google Scholar] [CrossRef]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef]
- Ooms, C.; Mossong, J.; Vergison, A.; Biver, A.; Wagner, K.; Niel, O.; Parrish, A.; Abdelrahman, T.T.; de la Fuente Garcia, I. Multisystem inflammatory syndrome in children during the first two years of the COVID-19 pandemic in Luxembourg. Front. Pediatr. 2023, 11, 1141074. [Google Scholar] [CrossRef]
- Pino, R.; Antoñanzas, J.M.; Paredes-Carmona, F.; Perramon, A.; Rivière, J.G.; Coma, M.; Martínez-Mejías, A.; Ripoll, F.; López, N.; Conti, R.; et al. Multisystem inflammatory syndrome in children and SARS-CoV-2 variants: A two-year ambispective multicentric cohort study in Catalonia, Spain. Eur. J. Pediatr. 2023, 182, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Hamad Saied, M.; van der Griend, L.; van Straalen, J.W.; Wulffraat, N.M.; Vastert, S.; Jansen, M.H.A. The protective effect of COVID-19 vaccines on developing multisystem inflammatory syndrome in children (MIS-C): A systematic literature review and meta-analysis. Pediatr. Rheumatol. Online J. 2023, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- La Torre, F.; Elicio, M.P.; Monno, V.A.; Chironna, M.; Moramarco, F.; Campanozzi, A.; Civino, A.; Cecinati, V.; Vairo, U.; Giordano, M.; et al. Incidence and Prevalence of Multisystem Inflammatory Syndrome in Children (MIS-C) in Southern Italy. Children 2023, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Fliesler, N. MIS-C: New Findings Bring COVID-Related Syndrome into Focus. Available online: https://answers.childrenshospital.org/mis-c-case-series/ (accessed on 20 July 2024).
- Nygaard, U.; Holm, M.; Hartling, U.B.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Matthesen, A.T.; Linstow, M.L.; von Espenhain, L. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: A Danish nationwide prospective cohort study. Lancet Child. Adolesc. Health 2022, 6, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Stacevičienė, I.; Ivaškevičienė, I.; Burokienė, S.; Steponavičienė, A.; Vaičiūnienė, D.; Tarutytė, G.; Jankauskienė, A. Epidemiological changes of acute respiratory infections in children: A single-center experience after COVID-19 lockdown. PLoS ONE 2024, 19, e0300877. [Google Scholar] [CrossRef]
- Stierman, B.; Abrams, J.Y.; Godfred-Cato, S.E.; Oster, M.E.; Meng, L.; Yip, L.; Patel, P.; Balachandran, N.; Prezzato, E.; Pierce, T.; et al. Racial and Ethnic Disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pediatr. Infect. Dis. J. 2021, 40, e400–e406. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Kumar, N.P.; Venkataraman, A.; Varadarjan, P.; Selladurai, E.; Sankaralingam, T.; Thiruvengadam, K.; Selvam, R.; Thimmaiah, A.; Natarajan, S.; et al. Sex-specific differences in systemic immune responses in MIS-C children. Sci. Rep. 2024, 14, 1720. [Google Scholar] [CrossRef]
- Stewart, D.J.; Mudalige, N.L.; Johnson, M.; Shroff, R.; du Pré, P.; Stojanovic, J. Acute kidney injury in paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) is not associated with progression to chronic kidney disease. Arch. Dis. Child. 2022, 107, e21. [Google Scholar] [CrossRef]
- Yeom, J.S.; Woo, H.O.; Park, J.S.; Park, E.S.; Seo, J.H.; Youn, H.S. Kawasaki disease in infants. Korean J. Pediatr. 2013, 56, 377–382. [Google Scholar] [CrossRef]
- Das, N.; Hill, R.; Trivedi, M.; Kenkre, T.S.; Alsaied, T.; Feingold, B.; Harris, T.H.; Christopher, A.B. Longitudinal Assessment of Cardiac Function Following Multisystem Inflammatory Syndrome in Children Associated with COVID-19. Pediatr. Cardiol. 2023, 44, 607–617. [Google Scholar] [CrossRef] [PubMed Central]
- Arslan, S.Y.; Bal, Z.S.; Bayraktaroglu, S.; Ozenen, G.G.; Bilen, N.M.; Levent, E.; Ay, O.; Ozkaya, P.Y.; Ozkinay, F.; Cicek, C.; et al. Cardiac Assessment in Children with MIS-C: Late Magnetic Resonance Imaging Features. Pediatr. Cardiol. 2023, 44, 44–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All Cases | <5 Years | 5–11 Years | ≥12 Years | p-Value | |
|---|---|---|---|---|---|
| Counts (%) | 42 (100) | 18 (42.9) | 18 (42.9) | 6 (14.3) | |
| Male sex | 31 (73.8) | 13 (72.2) | 13 (72.2) | 5 (83.3) | 0.858 |
| Comorbidities | 9 (21.4) | 3 (16.7) | 3 (16.7) | 3 (50.0) | 0.193 |
| A known history of symptomatic COVID-19 | 18 (42.9) | 5 (27.8) | 8 (44.4) | 5 (83.3) | 0.058 |
| Signs and symptoms | |||||
| Gastrointestinal symptoms | 32 (76.2) | 14 (77.8) | 12 (66.7) | 6 (100) | 0.260 |
| Mucocutaneous symptoms | 36 (85.7) | 16 (88.9) | 18 (100) | 2 (33.3) | 0.000 |
| Cardiovascular symptoms | 37 (88.1) | 17 (94.4) | 17 (94.4) | 3 (50.0) | 0.006 |
| Respiratory symptoms | 18 (42.9) | 7 (38.9) | 9 (50.0) | 2 (33.3) | 0.716 |
| Neurological symptoms | 13 (31.0) | 7 (38.9) | 4 (22.2) | 2 (33.3) | 0.571 |
| Renal symptoms | 8 (19.0) | 4 (22.2) | 3 (16.7) | 1 (16.7) | 0.909 |
| Other symptoms * | 3 (7.1) | 2 (11.1) | 1 (5.6) | 0 (0) | 0.638 |
| MIS-C phenotype | |||||
| Kawasaki-like disease | 31 (73.8) | 15 (83.3) | 14 (77.8) | 2 (33.3) | 0.047 |
| MIS-C with shock; | 5 (11.9) | 0 (0) | 4 (22.2) | 1 (16.7) | 0.116 |
| Undifferentiated MIS-C | 6 (14.3) | 3 (16.7) | 0 (0) | 3 (50.0) | 0.007 |
| Echocardiography and electrocardiography at admission | |||||
| Abnormal echocardiogram | 30 (71.4) | 13 (72.2) | 15 (83.3) a | 2 (33.3) a | 0.064 |
| Abnormal ECG | 22 (52.4) | 9 (50.0) | 10 (55.6) | 3 (50.0) | 0.943 |
| Laboratory test results at admission (normal range), peak value: median (IQR) | |||||
| White blood cell count (4.5–12.0 × 109/L; n = 42) | 15.4 (10.9–20.1) | 16.8 (11.7–25.1) | 14.5 (10.9–17.7) | 15.1 (8.2–16.8) | 0.801 |
| Absolute lymphocyte count (1.5–6.8 × 109/L; n = 42) | 2.2 (1.2–4.7) | 3.9 (2.0–9.2) | 1.3 (0.9–3.1) | 1.3 (0.8–4.6) | 0.008 |
| Absolute neutrophil count (1.5–7.5 × 109/L; n = 42) | 10.9 (7.7–14.1) | 11.1 (8.1–14.1) | 11.0 (7.7–15.5) | 9.3 (7.0–14.8) | 0.641 |
| Platelets (140–450 × 109/L; n = 42) | 352.5 (137.3–612.8) | 442.5 (138.0–657.0) | 371.0 (137.3–570.0) | 162.5 (100.3–367.0) | 0.169 |
| C-reactive protein (<5 mg/L; n = 42) | 158.6 (100.7–228.5) | 148.7 (115.9–230.9) | 170.9 (61.6–218.5) | 165.5 (87.5–256.4) | 0.801 |
| Procalcitonin (<0.05 ng/mL; n = 41) | 3.4 (1.6–7.4) | 3.5 (1.4–7.1) | 3.4 b (1.4–5.1) | 12.0 b (1.6–31.0) | 0.983 |
| Interleukin–6 (<5.9 ng/L; n = 23) | 103.0 (28.6–238.0) | 142.0 (34.8–395.8) | 46.0 (20.5–190.8) | 49.3 (49.3–49.3) | 0.142 |
| Troponin I (<16 ng/L; n = 40) | 12.5 (2.0–70.3) | 2.5 (1.0–13.0) | 50.0 (6.0–225.5) | 14.0 (4.0–439.0) | 0.036 |
| B-type natriuretic peptide (<100 ng/L; n = 35) | 207.3 (71.1–960.2) | 113.4 (17.5–794.9) | 611.4 c (88.5–965.5) | 141.9 c (47.1–434.8) | 0.276 |
| Ferritin (7–140 μg/L; n = 41) | 375.5 (252.7–501.7) | 289.0 (212.6–386.9) | 384.6 (252.4–846.6) | 481.6 (361.5–1247.8) | 0.038 |
| D-Dimer (45–280 μg/L; n = 42) | 2102.5 (1283.8–3170.0) | 2007.5 (1502.5–2721.3) | 2142.5 (907.5–4906.3) | 1990.0 (1113.8–7519.5) | 0.801 |
| Albumin (38–54 g/L, n = 34) | 29.64 (25.6–34.3) | 28.4 (26.5–35.5) | 29.0 (24.4–34.2) | 32.7 (29.5–38.4) | 0.567 |
| Estimated glomerular filtration rate (n = 41) | 108.4 (83.5–123.8) | 114.4 (95.5–129.6) | 110.3 (88.1–121.7) | 56.2 (42.9–94.0) | 0.012 |
| Management | |||||
| Intravenous immunoglobulin | 39 (92.9) | 17 (94.4) | 17 (94.4) | 5 (83.3) | 0.638 |
| Glucocorticoids | 34 (81.0) | 13 (72.2) | 16 (88.9) | 5 (83.3) | 0.458 |
| Oxygen therapy | 11 (26.2) | 3 (16.7) | 7 (38.9) | 1 (16.7) | 0.284 |
| Vasoactive drugs | 5 (11.9) | 0 (0) | 4 (22.2) | 1 (16.7) | 0.116 |
| ICU treatment | 28 (66.7) | 11 (61.1) | 13 (72.2) | 4 (66.7) | 0.792 |
| Echocardiographic Findings | Initial Findings, n (%) | Early Outcome on Discharge, n (%) | p-Value |
|---|---|---|---|
| Left ventricular ejection fraction < 55% | 11 (26.2) | 0 (0) | N/A |
| Coronary artery dilation | 1 (2.4) | 0 (0) | N/A |
| Mitral valve regurgitation | 13 (31.0) | 4 (9.5) | 0.012 |
| Tricuspid valve regurgitation | 13 (31.0) | 3 (7.1) | 0.013 |
| Pericardial effusion | 27 (64.3) | 6 (14.3) | 0.000 |
| Non-ICU Patients | ICU Patients | p-Value | |
|---|---|---|---|
| Counts (%) | 14 (33.3) | 28 (66.7) | |
| <5 years | 7 (50.0) | 11 (39.3) | 0.369 |
| 5–11 years | 5 (35.7) | 13 (46.4) | 0.373 |
| ≥12 years | 2 (14.3) | 4 (14.3) | 0.666 |
| Male sex | 10 (71.4) | 21 (75.0) | 0.541 |
| Comorbidities | 3 (21.4) | 6 (21.4) | 1.000 |
| A known history of symptomatic COVID-19 | 3 (21.4) | 15 (53.6) | 0.047 |
| Signs and symptoms | |||
| Gastrointestinal symptoms | 12 (85.7) | 20 (71.4) | 0.267 |
| Mucocutaneous symptoms | 12 (85.7) | 24 (85.7) | 1.000 |
| Cardiovascular symptoms | 10 (71.4) | 27 (96.4) | 0.035 |
| Respiratory symptoms | 3 (21.4) | 15 (53.6) | 0.047 |
| Neurological symptoms | 4 (28.6) | 9 (32.1) | 0.553 |
| Renal symptoms | 2 (14.3) | 6 (21.4) | 0.457 |
| Other symptoms * | 2 (14.3) | 1 (3.6) | 0.254 |
| MIS-C phenotype | |||
| Kawasaki-like disease | 11 (78.6) | 20 (71.4) | 0.459 |
| MIS-C with shock | 0 (0) | 5 (17.9) | 0.116 |
| Undifferentiated MIS-C | 3 (21.4) | 3 (10.7) | 0.311 |
| Echocardiography and electrocardiography at admission | |||
| Abnormal echocardiogram | 9 (64.3) | 21 (75.0) | 0.353 |
| Abnormal ECG | 7 (50.0) | 15 (53.6) | 0.543 |
| Laboratory test results, peak value: median (IQR) | |||
| White blood cell count (n = 42) | 15.0 (10.2–18.0) | 15.4 (11.4–21.7) | 0.545 |
| Absolute lymphocyte count (n = 42) | 1.9 (1.3–3.6) | 2.2 (1.2–5.5) | 0.901 |
| Absolute neutrophil count (n = 42) | 10.2 (7.6–13.1) | 11.0 (8.8–15.2) | 0.289 |
| Platelets (n = 42) | 457 (236.8–587.3) | 252 (127.5–614.3) | 0.712 |
| C-reactive protein (n = 42) | 97.0 (28.6–145.1) | 196.8 (138.2–249.8) | 0.000 |
| Procalcitonin (n = 41) | 1.5 (0.7–3.1) | 4.0 (2.6–15.4) | 0.003 |
| Interleukin-6 (n = 23) | 49.2 (24.6–298.6) | 117.5 (28.8–245.8) | 0.656 |
| Troponin I (n = 40) | 3.0 (1.3–27.0) | 13.5 (2.3–79.3) | 0.466 |
| B-type natriuretic peptide (n = 35) | 82.4 (10.7–418.8) | 351.9 (95.0–965.4) | 0.041 |
| Ferritin (n = 41) | 313.5 (202.6–382.3) | 390.6 (266.6–635.9) | 0.125 |
| D-Dimer (n = 42) | 1900 (600.0–2302.5) | 2190 (1367.5–5058.8) | 0.316 |
| Albumin (n = 34) | 38.3 (34.0–40.0) | 27.6 (24.4–32.0) | 0.000 |
| Estimated glomerular filtration rate (n = 41) | 110.7 (92.1–126.1) | 103.8 (103.8–123.0) | 0.118 |
| Management | |||
| Intravenous immunoglobulin | 11 (78.6) | 28 (100) | 0.032 |
| Glucocorticoids | 11 (78.6) | 23 (82.1) | 0.543 |
| Oxygen therapy | 0 (0) | 11 (39.3) | 0.005 |
| Vasoactive drugs | 0 (0) | 5 (17.9) | 0.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stacevičienė, I.; Ivaškevičienė, I.; Kinčinienė, O.; Kilaitė, L.; Jankauskienė, A. Multisystem Inflammatory Syndrome in Children (MIS-C) in a Lithuanian Paediatric Tertiary Care Center. Medicina 2024, 60, 1774. https://doi.org/10.3390/medicina60111774
Stacevičienė I, Ivaškevičienė I, Kinčinienė O, Kilaitė L, Jankauskienė A. Multisystem Inflammatory Syndrome in Children (MIS-C) in a Lithuanian Paediatric Tertiary Care Center. Medicina. 2024; 60(11):1774. https://doi.org/10.3390/medicina60111774
Chicago/Turabian StyleStacevičienė, Indrė, Inga Ivaškevičienė, Odeta Kinčinienė, Loriana Kilaitė, and Augustina Jankauskienė. 2024. "Multisystem Inflammatory Syndrome in Children (MIS-C) in a Lithuanian Paediatric Tertiary Care Center" Medicina 60, no. 11: 1774. https://doi.org/10.3390/medicina60111774
APA StyleStacevičienė, I., Ivaškevičienė, I., Kinčinienė, O., Kilaitė, L., & Jankauskienė, A. (2024). Multisystem Inflammatory Syndrome in Children (MIS-C) in a Lithuanian Paediatric Tertiary Care Center. Medicina, 60(11), 1774. https://doi.org/10.3390/medicina60111774






